Abstract
Wetlands offer many ecosystem services, including the long-term sequestering of carbon (C) in soil. Here we present a study of C sequestration rates in a relatively undisturbed wetland landscape of southwest Florida. Accordingly, carbon sequestration was determined in four wetland plant communities and an adjacent hydric pine flatwood community that represent a gradient of inundation extent. Going from the wettest to the driest, communities were designated as: deep slough, bald cypress (Taxodium distichum), wet prairie and pond cypress (Taxodium distichum var. imbricarium). An adjacent hydric pine flatwood community was also included in the study as a reference upland site. Three soil cores were collected from each of these communities and were analyzed for total C content. Core samples were also analyzed for 137Cs and 210Pb activity to estimate accretion rates. C sequestration rates (g-C m−2 yr−1) were the highest in the deep slough (98 ± 9) and bald cypress (98 ± 5) followed by the pond cypress (64 ± 7), wet prairie (39 ± 1) and pine flatwood (22 ± 5). These results suggest that impediment of decomposition by anaerobic conditions caused by prolonged wet cycles, may not account for all the variability in C sequestration rates observed in this subtropical setting. Instead, this variability could also be attributed to other factors like the quantity and chemical composition of the organic material reaching the soil. When methane emissions are taken into account, cypress-dominated (bald and pond cypress) and the deep slough communities act as net carbon sinks.
Introduction
Climate regulation through carbon (C) sequestration in wetlands soils may be one of the most important ecosystem services of wetlands in the long term. Carbon is sequestered in wetlands when C inputs (productivity and/or sedimentation) surpasses C outputs (decomposition and C exports) and the remaining organic material, mostly senesced plant material, is accumulated in the wetland's anaerobic sediment layer as a mat of partially decayed organic material, or peat. A fraction of this organic matter may also be incorporated into the mineral fraction of the soil as soil organic C. Globally, it is estimated that 455–700 Pg-C (1 Pg = 1015 g) of carbon in organic form is stored in wetlands (Mitsch & Gosselink Citation2015). By comparison, Lal (Citation2008) estimates that there is 1550 Pg-C stored in the earth's soil organic C pool. This pool includes various forms of organic C, from highly active humus to relatively inert charcoal (Lal Citation2008). Considering that wetlands occupy only 5–8% of the terrestrial land surface (Mitsch & Gosselink Citation2015), these global estimates rank them as the terrestrial ecosystems with the highest C density (Kayranli et al. Citation2010), leading scientists from different disciplines to emphasize the key role that wetland ecosystems may play in the Earth's radiative forcing despite their relatively low percent coverage of the terrestrial world (Whiting & Chanton Citation2001; Frolking et al. Citation2006; Page et al. Citation2011; Mitsch et al. Citation2013).
Values for C fluxes in wetlands, however, are far from definitive (Roulet Citation2000). For instance, early estimates of C sequestration rates for North America (52.7 Tg-C yr−1) and the world (137 Tg-C yr−1) had an uncertainty of more than 100% according to Bridgham et al. (Citation2006). More recently, Mitsch et al. (Citation2013) revised this number and after including a revised area for tropical wetlands and their sequestration rates, they estimated that the worldwide sequestration may be around 830 Tg-C yr−1. Much of our current knowledge of wetlands as carbon sources and sinks comes from extensively studied northern peatlands (Gorham Citation1991; Maltby & Immirzi Citation1993). In general, wetlands in boreal and subarctic biomes experience low temperatures that are partially responsible for inhibit organic matter decomposition and also limit productivity (Clymo Citation1984; Roulet et al. Citation2007). In the case of wetlands in warm subtropical and tropical climates, this temperature effect seems to be more complex and less understood. On the one hand, wetlands are generally more productive in lower latitudes. On the other hand, higher temperatures in these regions could lead to a rate of decomposition that exceeds that of productivity (Franzluebbers et al. Citation2001; Mitsch et al. Citation2010).
Beyond the controlling effect that macroclimate may have on productivity and decomposition, C sequestration seems also to differ according to the wetland type or hydrogeomorphic setting and the plant communities therein. For example, in a study of temperate wetlands in different hydrogeomorphic settings, Bernal and Mitsch (Citation2012) found depressional and isolated wetlands to sequester two times more C than riverine flow-through wetlands (317 versus 140 g-C m−2 yr−1, respectively). Conversely, in a follow-up study in the tropics, the same authors found that C sequestration rates in tropical, slow-flow-through wetlands were as much as three to four times higher than in tropical, depressional and seasonal riverine wetlands (Bernal & Mitsch Citation2013). They attributed the observed differences in C sequestration in these studies to site-specific factors such as the extent of inundation or the form of organic matter (recalcitrant versus labile) entering the systems. The effect of these factors has also been noted in other studies of tropical peatland ecosystems (Chimner & Ewel Citation2005; Hirano et al. Citation2009).
Despite the apparent benefit that wetland ecosystems have on the reduction of Earth's radiative forcing through C sequestration, possible feedbacks to the atmosphere of C in the form of methane (CH4) are a real concern (Gedney et al. Citation2004; Bridgham et al. Citation2006; Kayranli et al. Citation2010; Bastviken et al. Citation2011). CH4 is a greenhouse gas (GHG) produced in wetlands by organic matter decomposition under anaerobic conditions (Whalen Citation2005). Once in the atmosphere, CH4 has an adverse effect on the radiation budget of earth because of its global warming potential (GWP) that is 25 times greater than the potential of the same mass of carbon dioxide (CO2) over 100 years (Forster et al. Citation2007). Calculations made in 2005 based on carbon equivalents (i.e. taking into account the GWP of CH4) of the global wetland area and its organic C stock suggested that these ecosystems should be regarded as a relatively small net source of GHG (Mitra et al. Citation2005). More recently, Mitsch et al. (Citation2013) used a dynamic model of C fluxes from 21 wetlands in different climates to estimate that wetlands in the world may be currently acting as net C sinks of about 830 Tg yr−1, with an average of 118 g-C m−2 yr−1 of net C retention.
Accurate estimation of C sequestration in wetlands across different landscapes is critical for developing better C budgets that will ultimately help us understand the role of wetlands as GHG sinks or sources in future climate scenarios. Here, we present a study of C sequestration rates from different wetland plant communities and an adjacent hydric pine flatwood community that are characteristics of southwest Florida. These wetland plant communities are situated in a single hydrogeomorphic setting, but represent a gradient of inundation. We expected a gradient in C sequestration of the wetland plant communities that will follow the gradient of inundation. Then, we compare the C sequestration rates with previously published CH4 emissions rates for these same plant communities to assess their role as sources or sinks of C GHG. We follow this with a discussion of possible causes for the variability in C sequestration among these wetland communities and speculate about their impact on radiative forcing, as possible net GHG sinks.
Methods
Study site
This study was conducted in Corkscrew Swamp Sanctuary in southwest Florida (26° N 23ʹ W, 81° N 35ʹ W). This nature preserve, within the Corkscrew Regional Ecosystem Watershed, is a collection of relatively undisturbed freshwater wetlands characteristic of southwest Florida (). Climate in this portion of Florida is characterized by very warm and wet summers, mild winters with occasional light frost and spring droughts. Mean annual precipitation and temperature in the headwater of the watershed are 1201 mm and 23.2°C, respectively (values from 35-yr records since 1971, Station COOP: 084210, Immokalee, FL, South Florida Water Management District). A complete ecological description of Corkscrew Swamp is presented by Duever et al. (Citation1984). Briefly, Corkscrew Swamp is a riverine cypress strand on a relatively small and flat watershed (i.e. 32,030 ha). Low erosive force of the waterways allows the development of vegetation and accumulation of peat in what would normally be the main stream channel, leading to a diffuse system of shallow irregular channels. Mineral substrate profiles, consisting mainly of sands overlying limestone, decline along a line perpendicular to the general flow direction, from the surrounding pinelands to the deepest channels in the strand. Ground surface, however, is relatively flat because organic soils have accumulated in low-lying areas, creating a generally level topography. The most significant factor affecting the distribution of the plant communities is the hydroperiod (i.e. inundation extent) that can last for more than 250 days in deep sloughs, depending on the amount and distribution of yearly rainfall. Maximum wet season water levels and, to a lesser extent, minimum dry season water levels can also be important in determining plant community zonation (Duever et al. Citation1984).
Figure 1. Location of the study sites corresponding to four different wetland plant communities and an adjacent upland community in southwest Florida. The black circles indicate the sites from which soil cores were extracted. DS = deep slough, BC = bald cypress, WP = wet prairie, PC = pond cypress and PF = pine flatwood (upland).
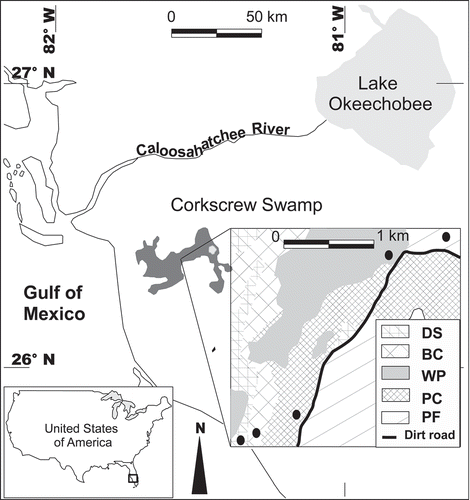
We selected four distinct wetland plant communities across the gradient of inundation in the Corkscrew Swamp landscape. The plant communities investigated were designated as: (1) Deep slough, mostly bare soil with sparse emergent macrophytes like Peltandra virginica, Thalia geniculata and Pontederia cordata, a subcanopy dominated by Annona glabra, Fraxinus caroliniana and Cephalanthus occidentalis and an open canopy of tall Taxodium distichum, (2) Bald cypress, also bare soil with a sparse understory dominated by Osmunda regalis in small mounds created by dead trees and roots and Crinum americanum in small depressions; both under a canopy and subcanopy similar in composition to that of deep slough, (3) Wet prairie, co-dominated by Cladium jamaicense, P. cordata, Ludwigia sp., Alisma subcordatum Raf. with no bare soil, and (4) pond cypress, a dense stand of Taxodium distichum var. imbricarium relatively smaller than the trees in the bald cypress community but with a relatively closed canopy, with Ludwigia sp. and Sagittaria graminea primarily covering the forest floor. A hydric pine flatwood (Pinus elliottii, Serenoa repens and Aristida stricta) community that was never flooded during the study period was also included as a reference upland community. Inundation during 2011 and 2012 had a duration in days of 264 and 198; 181 and 149; 138 and 117; and 125 and 67 for deep slough, bald cypress, wet prairie and pond cypress, respectively (Villa Citation2014).
Sample collection and preparation
Three 6.5-cm-diameter cores were collected in each plant community (15 cores total) using a universal core head sediment sampler (WaterMark) equipped with ~60-cm polycarbonate barrels. Cores in deep slough, wet prairie and pond cypress were collected between June and September 2011. Cores in bald cypress and pine flatwood were collected between March and June 2012. Distance between cores was less than 1 m, except for those from the bald cypress community, in which one core was collected about 5 m apart from the other two. When sites had standing water, surface water in the corer was siphoned off before processing the soil core. Processing in the field consisted of sectioning the core into 2-cm depth intervals, then packaging and sealing them in separate plastic bags. During sectioning, we cleaned the work area after packaging each sample to avoid any possible soil mixing.
Once in the lab, samples corresponding to each triplicate depth interval were dried at 55°C for 48 hr. After drying, samples were weighed to calculate the soil bulk density. Then, debris (small branches) and roots, when present, were carefully removed and all samples were ground to a 2-mm particle size, homogenized and stored in sealed bags until analysis. Core depths varied between and within sites. Only depth intervals that had the three replicates were used in the analyses and calculations. Maximum analyzed core depths (cm) were 32, 48, 32, 26 and 14 for deep slough, bald cypress, wet prairie, pond cypress and pine flatwood, respectively.
Accretion rates
Accretion rates were determined non-destructively by measuring 137Cs and 210Pb activity in each 2-cm soil interval (Craft & Richardson Citation1993; Bernal & Mitsch Citation2013). Composite subsamples (~10 g) corresponding to each depth interval at each plant community were run in a high-efficiency Germanium Detector (GL 2820R, Canberra). 137Cs is a man-made radionuclide distributed worldwide primarily as the consequence of atmospheric deposition after nuclear weapons testing (Smith et al. Citation2000). Depositional patterns of this isotope normally exhibit a distinct peak in the activity in the soil profile that corresponds to year 1964, one year after the Test Ban Treaty (Ritchie & McHenry Citation1990). Thus, by knowing the depth interval with the peak in 137Cs activity, the average accretion rate can be estimated as the depth of the interval with the peak in 137Cs activity divided by the time from 1964 to the year of sampling.
210Pb is a naturally occurring radioisotope. 210Pb can be formed in wetlands from in situ decay of 226Ra (supported 210Pb). 210Pb can also be deposited in wetlands from the decay of 222Rn in the atmosphere, or indirectly via the water column (unsupported 210Pb) (MacKenzie et al. Citation2011). This unsupported component of the 210Pb inventory can be used to establish chronologies of wetland sediments or peat because once it is incorporated in the soils it decays exponentially with time in accordance with its half-life (22.2 yr) (Oldfield & Appleby Citation1984). To establish peat chronologies in our plant communities, we assumed a constant rate of supply of unsupported 210Pb and applied the constant-rate-of-supply (CRS) model described by Appleby and Oldfield (Citation1978, Citation1983) and Oldfield and Appleby (Citation1984). The average of the relatively constant down-core total 210Pb activity in the soil profile was assumed to represent the supported 210Pb activity (Craft & Richardson Citation1998; Brenner et al. Citation2001).
Soil analyses and carbon sequestration rates
Duplicate samples corresponding to each core 2-cm depth interval in each plant community were analyzed for total carbon (TC%) and inorganic carbon (IC%) in a Total Carbon Analyzer for soil samples (TOC-V series, SSM-5000A; Shimadzu Corporation, Kyoto, Japan). TC was determined by total combustion at 900°C, whereas IC was determined by digestion with 10 mol L−1 H3PO4 at 200°C. The organic carbon (OC) fraction per depth was calculated as the difference between TC and IC. The soil bulk density at each depth interval was calculated with the dry weight and the volume.
Soil TC concentration (g-C kg−1) of each depth interval was obtained by multiplying the percentage value of C by 10. TC was then multiplied by the corresponding dry weight to obtain the mass of TC per interval. The mass of TC was integrated in the profile down to the age of the peat that was estimated with 137Cs and 210Pb. As these depths did not coincide with the 2-cm intervals, we calculated the dry weight in each bottommost interval by multiplying its height by the corresponding bulk density and dividing by the area of the core. Then, the integrated mass of C and the integrated dry weights were divided by the area of the core and the number of years from the age of the peat to the year of sampling to estimate the C sequestration rates (g-C m−2 yr−1) and mass accretion rates (g m−2 yr−1), respectively.
Carbon sequestration versus methane emissions
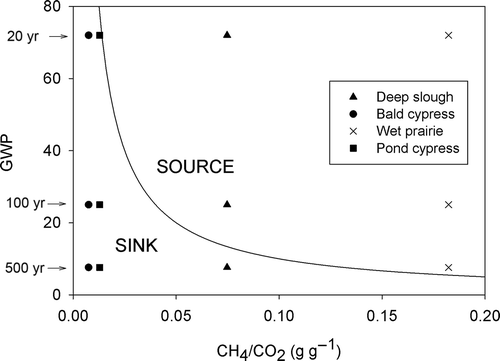
To assess the role that wetland plant communities in southwest Florida may be playing in a climate change context, we related the CH4/CO2 ratio to the GWPs of CH4 in an approach similar to that presented by Whiting and Chanton (Citation2001). The CH4/CO2 ratio was calculated using the average 2-yr CH4 emissions measured by Villa and Mitsch (Citation2014) (26.9 g CH4 m−2 yr−1 in deep slough, 2.7 g CH4 m−2 yr−1 in bald cypress, 25.9 g CH4 m−2 yr−1 in wet prairie and 3 g CH4 m−2 yr−1 in pond cypress). The C sequestration rates in this study assumed as the net atmospheric CO2 assimilated by the system (e.g. Mitsch et al. Citation2013). The pine flatwood community was excluded from this analysis because it acted as a net sink for CH4 during the study period considered in Villa and Mitsch (Citation2014). The GWPs used were the three reported in Forster et al. (Citation2007) (i.e. 72 for 20 yr, 25 for 100 yr and 7.6 for 500 yr). This GWP is an emission metric proposed by the Intergovernmental Panel on Climate Change to assess the overall climate response associated with a forcing agent (i.e. GHGs). It compares integrated radiative efficiencies of GHGs to that of CO2, assumed as the standard gas, over specified time periods. Decrease over time of the GWPs results from the reduced radiative forcing of CH4 given its lifetime of 12 yr (Forster et al. Citation2007).
To determine if the soil in a plant community was acting as a source or sink of C GHG we established a GHG compensation boundary, in which the GWP multiplied by CH4/CO2 ratio was equal to 1 [GWP (CH4/CO2) = 1] (Whiting & Chanton Citation2001). This boundary was constructed by plotting first the three GWPs (y-axis) versus their respective compensation boundary value [GWP = (CO2/CH4)] and then tracing an empirical best fit line for these three points. Then, we plotted the CH4/CO2 ratios calculated for our plant communities versus the GWP of CH4 for 20, 100 and 500 yr. Accordingly, given a specific GWP, a system would be acting as a net GHG source if a system has a CH4/CO2 ratio that falls in the area above and to the right of this boundary. Conversely, it would be acting as a GHG sink if the ratio falls into the area below and to the left of this boundary.
Results
Soil profiles
The variation of soil bulk density and carbon concentrations with depth for each plant community is shown in (a and b respectively). The cores extracted at the bald cypress community had a thick root zone between 20 and 28 cm depth that was not included in the analyses. Bulk density remained low through most of the depth sampled in deep slough and bald cypress (i.e. <1 g cm−3), averaging 0.30 and 0.10 g cm−3 respectively. In turn, bulk density showed a significant increase with depth in the wet prairie, pond cypress and pine flatwood communities as the peat layer was gradually replaced by sand. Accordingly, bulk density (g cm−3) in these communities went from 0.39, 0.17 and 0.63 in the surface, 2-cm depth interval, to values over 1.00 at the 8, 10 and 4 cm depth intervals, respectively.
Figure 2. Soil profile at each wetland plant community showing: (a) bulk density and (b) carbon concentration.
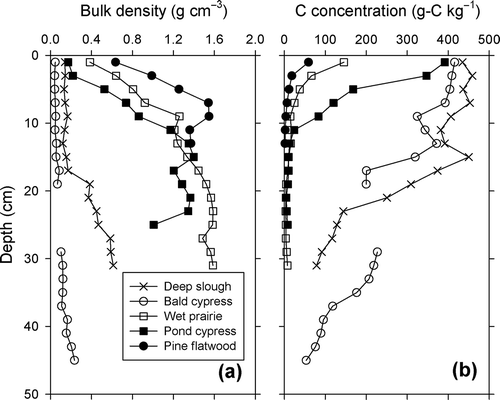
Most of the C measured in the different communities was in organic form (>99%). Soil TC concentrations decreased with depth in all wetland communities and the upland site. In the deep slough and bald cypress, this decrease was less pronounced than in the other communities, going from 435.8 and 415.6 g-C kg−1 at the soil surface to 76.5 and 35 g-C kg−1 at the deepest depth interval, respectively. TC concentrations decreased sharply from the soil surface in the wet prairie and pond cypress communities and remained low throughout the sandy layer. In the hydric pine flatwood community, where the soil consisted primarily of fine sand, TC concentration was low throughout the profile. Values (g-C kg−1) in these three communities (wet prairie, pond cypress and pine flatwood) went from 145.3, 391.8 and 5.9 at the soil surface to 10.8, 3.81 and <0.01, respectively.
Soil accretion and carbon sequestration rates
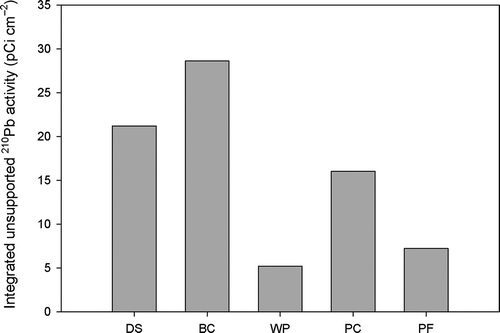
137Cs activity showed distinct peaks in the wet prairie, pond cypress and pine flatwood community. Peaks in the deep slough and bald cypress communities were not clearly identifiable. High 137Cs activity in the profile of these two communities was rather evenly distributed across different depth intervals (, ). Therefore we used only 210Pb to determine the accretion rates in the different communities. Total integrated unsupported 210Pb ranged from 5.2 pCi cm−2 in wet prairie to 28.6 pCi cm−2 in bald cypress and had a mean ± standard error of 15.6 ± 4.4 pCi cm−2 (). The distribution of this unsupported 210Pb suggests that there is preferential deposition towards the forested communities dominated by cypress (deep slough, bald and pond), whereas the wet prairie and the pine flatwood tend to be more subject to erosive and re-distribution processes.
Table 1. Mean accretion rates using the 137Cs peak activity and the CRS model (210Pb), net and mass accretion rates, and the mean (range) of carbon concentration since 1950 for the soils in each community.
Figure 3. 137Cs and 210Pb activity in four different wetland plant communities (a, b, c and d) and an adjacent upland site (e). Left and center columns represent total 137Cs and 210Pb activity through the soil profile, respectively. Dates corresponding to the peak of 137Cs and those obtained using the constant rate of supply of unsupported 210Pb (CRS) model are presented in the second y-axis. Right column contains 210Pb as a function of cumulative mass in log scale.
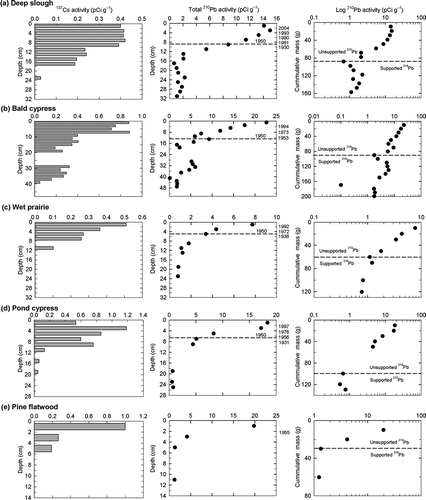
Figure 4. Integrated unsupported 210Pb activity for four different wetland plant communities and an adjacent upland community. DS = deep slough, BC = bald cypress, WP = wet prairie, PC = pond cypress and PF = pine flatwood (upland).
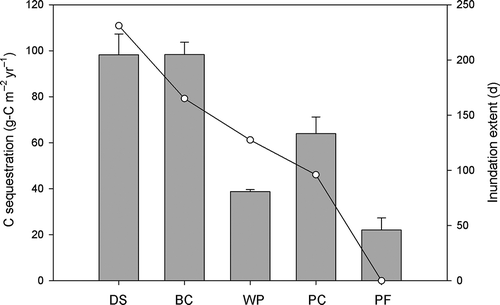
The CRS model applied to these unsupported inventories dated soil intervals back to 1897 in the deep slough community (). However, the mean ± standard error of the minimum detection limits (MDL) for the unsupported 210Pb measurements in all communities, including the pine flatwood community, was 1.4 ± 0.03 pCi g−1. According to MacKenzie et al. (Citation2011), MDL of 0.27 pCi g−1 lead to potential bias towards erroneous old values for ages older than about 80 yr. To avoid bias induced by the MDL in our measurements, we calculate carbon sequestration rates since ~1950 (i.e. ~60 yr) in the four wetland communities. In the pine flatwood, only the top interval could be dated to 1956 with the CRS model and therefore C sequestration and bulk accretion were determined in this community since that year. summarizes mean accretion rates using the 137Cs peak activity and the CRS model, as well as the net and mass accretion rates and the carbon concentration since 1950. The accretion rates estimated with 137Cs were fairly similar to those estimated using the 210Pb CRS model in the pond cypress and pine flatwood. However, in the case of the wet prairie, the rate from the 137Cs peak was considerably lower. Carbon sequestration rates (mean ± standard error) in wetland plant communities ranged from 98 ± 9 g-C m−2 yr−1 in deep slough and bald cypress 98 ± 5, to 39 ± 1 g-C m−2 yr−1 in wet prairie. Carbon sequestration in the upland pine flatwood community was 22 ± 5 g-C m−2 yr−1, representing a more than 4-fold increase from the surrounding upland communities to the wettest wetland communities ().
Figure 5. Carbon sequestration and inundation extent in wetland plant communities of southwest Florida. Bars represents mean carbon sequestration rates (n = 3) for: DS = deep slough, BC = bald cypress, WP = wet prairie, PC = pond cypress and PF = pine flatwood. Error bars denote the standard error of the mean. Circles represent the annual average 2011 and 2012 inundation extent for each corresponding plant community.
Carbon sequestration versus methane emissions
Our assessment indicated that soil in the bald and pond cypress communities function as a net C GHG sink, independent of the time horizon for which uptake and emissions are considered. The deep slough community acts as a net GHG source when considered on a 20- and 100-yr horizon, but it switches to a net sink when the analysis is considered for 500 yr. The wet prairie community remained a GHG source regardless of the time horizon used in the analysis ().
Figure 6. Wetland plant communities in southwest Florida as sinks or sources of greenhouse gases (i.e. CH4 and CO2) evaluated for three different time horizons. The curved line represents the greenhouse gas compensation boundary and is an empirical best fit of three global warming potentials contemplated in Forster et al. (Citation2007). Values below or left of the compensation boundary indicate a net sink of GHG, whereas values above and right of the boundary net source of GHG.
Discussion
Soil accretion rates
The estimation of soil accretion using the 137Cs peak activity in the soil profile was not a reliable method for the deep slough and bald cypress communities. The absence of 137Cs-binding clay particles (Schell et al. Citation1989; MacKenzie et al. Citation1997; Brenner et al. Citation2001) and possibly active uptake of 137Cs by plants (Oldfield et al. Citation1979) could explain the profiles with evenly distributed 137Cs near the topsoil depth intervals. Moreover, 137Cs activity in these two communities was measured at depths that, according to our 210Pb dating, were decades older than the start of atomic bomb testing. This is not an unusual finding in wetland environments of Florida (e.g. Brenner et al. Citation2001) and supports the idea of post-depositional mobility of this radionuclide through the soil profile. For the other three communities (wet prairie, pond cypress and pine flatwood), 137Cs peaks were measured, yet the accretion rates estimated with the two methods (137Cs peak and 210Pb CRS model) were somehow different, leading to further discrepancies in the calculation of the C sequestration rates. Moreover, the use of 137Cs in environments where sands dominate the profile, as is the case in these three communities, must be regarded with caution because an increase in sand-size particles in soil profiles will cause a decrease in the activity of 137Cs that cannot be related to the atmospheric fallout rates of 137Cs (Ritchie & McHenry Citation1990). Altogether, our results highlight the potential value of using 210Pb as an alternative, yet independent method to date soils using 137Cs in wetlands with high organic matter and low clay content or those with profiles that are dominated by sands, like the ones found in this study.
Mean accretion rates since 1950 in the cypress-dominated communities were similar to those reported for ~100 yr in different cypress communities in Georgia by Craft and Casey (Citation2000) (i.e. 0.8–2.2 cm yr−1). We could not find in the literature accretion rates for sites with plant communities similar to the wet prairie and pine flatwood communities considered in this study. However, the accretion rate in the wet prairie was lower than those reported for ~30 yr by Craft and Richardson (Citation1993) in unenriched marshes of the Everglades with short inundation periods (i.e. 1.6–2.4 cm yr−1). The mean accretion rates in the different communities followed closely the trend of the distribution of unsupported 210Pb in the soil profiles (, ). This distribution of unsupported 210Pb also suggests that particles from wet prairie and pine flatwood (lower values) are being either eroded and deposited in the adjacent forested communities (higher values) or re-deposited within the same community. More 210Pb profiles in different sites within each community should help determine which of these two processes is dominant. Also, despite the fact that wet prairie had low integrated unsupported 210Pb in the soil profile, the specific mass accretion rate was more than double the rate of the other communities. We can only explain this by a relative higher proportion of sand in the profile of this particular site that has accumulated since 1950.
Carbon sequestration in the different wetland plant communities
High C sequestration rates in tropical wetlands of warm and wet climates have been attributed primarily to the low decomposition rates in such environments (Chimner & Ewel Citation2005; Jauhiainen et al. Citation2005; Hirano et al. Citation2009). Prolonged cycles with standing water above the soil surface or waterlogged soils may well enhance C accumulation by impeding aerobic decomposition and attenuating warm air temperatures. Our results, which show the variation in C sequestration along a gradient of inundation in a single hydrogeomorphic setting, partially support this claim. We found a general trend in the inundation gradient with lower C sequestration rates corresponding with communities with shorter inundation periods, confirming our initial prediction (). However, this gradient was not straightforward. The deep slough community and bald cypress had very similar C sequestration rates regardless of having different inundation periods. Also, the wet prairie community had lower C sequestration rates than the pond cypress community despite having a longer inundation period. Therefore, the slowing of decomposition by prolonged inundation periods can only explain to some extent the differences in C sequestration between the plant communities studied in Corkscrew Swamp.
Differences in C sequestration rates between wetlands in tropical and subtropical wetlands can be attributed to the quality of the organic matter entering the system. For instance, Bernal and Mitsch (Citation2013) speculated about the effect of recalcitrant matter in the higher C sequestration rates observed in sites dominated by forested communities when compared to macrophyte-dominated sites in different wetlands of Costa Rica and Botswana. Day (Citation1982), in a study in the Great Dismal Swamp, Virginia, attributed differences in the decay rates to the chemical characteristics of the litter, rather than to the environmental conditions resulting from flooding. Specifically, increases in decay rates were the result of relatively higher nutrient content (nitrogen and phosphorus), and low lignin and tannic acid content, and C:N ratios. These litter composition differences could help explain why, in our study, C sequestration rates in the wet prairie were lower when compared with the adjacent cypress-dominated communities. In the first place, leachates from cypress leaves, the dominant taxa and main input of organic matter in bald cypress and pond cypress communities, have higher percent lignin content than leachates from co-dominant species in the deep slough and wet prairie like T. geniculata and C. jamaicense, respectively (i.e. 17% versus 6.9% and 9.8%, respectively), and also has higher C:N ratios (i.e. 51.5 versus 14.1 versus 24.1, respectively) (Osborne et al. Citation2007). Secondly, decomposition rates, and therefore C turnover, are up to one order of magnitude higher in other co-dominant plant species of the wet prairie when compared with that of cypress (Deghi et al. Citation1980; Battle & Golladay Citation2001; Chimney & Pietro Citation2006).
Differences measured in C sequestration rates of Corkscrew plant communities can also be attributed to the quantity of the organic matter entering the system. For instance, Cohen (Citation1973) estimated that 40% of the peat in the Okefenokee Swamp, southern Georgia, was produced in situ by roots. Cypress-dominated communities in Corkscrew have a belowground productivity in the top 30 cm ranging from 1633 to 1946 g m−2 (Duever et al. Citation1984), whereas belowground productivity in communities dominated by sawgrass, one of the co-dominant species in the wet prairie, is around 390 g m−2 (Miao et al. Citation1997).
Despite existing differences in quality of the organic matter litter composition and quantity of organic matter being incorporated into the soils, the fate of the organic matter that reaches the soil surface is not clear yet in this dynamic environment of contrasting dry and wet conditions (i.e. aerobic versus anaerobic processes). Future research should focus on the synergistic effects that alternating aerobic and anaerobic conditions (McLatchey & Reddy Citation1998; Chimner & Ewel Citation2005), timing of litter fall, quantity and quality of litter (Pettit et al. Citation2011; Chow et al. Citation2013), and microbial and fungal community activity (Pettit et al. Citation2011; Todd-Brown et al. Citation2012) may be playing in the decomposition of organic matter and hence in the variability observed in the C sequestration rates.
Carbon sequestration in southwest Florida wetland ecosystems
To better assess the role that wetland ecosystems of southwest Florida may be playing in the sequestering of carbon at a wider scale, we compared the rates measured in this study to those reported in previous studies from tropical and subtropical zones of America and Africa (). To avoid confusion introduced by the use of different methodologies in the estimation of the carbon sequestration rates, we only selected studies which used radiometric dating, either with 137Cs or with 210Pb. We then arranged wetlands by geomorphic setting (Brinson Citation1993). According to these studies, C sequestration in tropical and subtropical zones ranges between 18 and 232 g-C m−2 yr−1. Also, rates (mean ± standard error) tend to be higher in riverine, low-gradient alluvial (100 ± 25 g-C m−2 yr−1), than in depressional (62 ± 18 g-C m−2 yr−1) or riverine, low-gradient, non-alluvial wetlands (56 ± 12 g-C m−2 yr−1). The rate for the latter geomorphic setting, excluding the data in this study, is 57 ± 17 g-C m−2 yr−1.
Table 2. Mean carbon sequestration rates from tropical and subtropical wetlands of America and Africa featuring their geomorphic setting, type, dominant plant species and general location. The rates were calculated using 137Cs to estimate the accretion rates or 210Pb (*). Values in parentheses indicate reported ranges. ND = Not described.
The C sequestration in the wetland communities that we studied ranged from 39 to 98 g-C m−2 yr−1, in the lower middle portion of the rates observed along the tropical/subtropical latitudinal range. Bernal and Mitsch (Citation2013) in a study of 12 freshwater wetland communities in contrasting wet and dry tropical climates found a Shelford-type nonlinear relationship between C sequestration rates and the P/T ratio (ratio of mean annual precipitation and air temperature, 10−2 mm yr−1/°C). According to this study, this ratio, a proxy for water availability, suggests a midpoint of the P/T ratio at which C sequestration in wetlands from lower latitudes seems to be enhanced. Based on the finding in their study, this point is around a P/T ratio of 1.2 with minimum and maximum sequestration rates at ratios near 0.2 and 1.8, respectively. However, regardless of the P/T ratios, the same authors also noted that the hydrogeomorphic type was a key factor in determining the carbon sequestration capacity of their different wetland soils. Located in one single hydrogeomorphic setting, our wetland communities have a P/T ratio of 0.5 (35-yr average), suggesting that wetlands in southwest Florida may not be at the optimal climatic location for sequestering carbon when compared with other tropical and subtropical freshwater wetlands. However, the lack of organic matter-binding parent materials in the soils of Corkscrew, where organic soils develop on top of a mineral substrate consisting typically on sands (Duever et al. Citation1984), may also be a factor determining the comparatively lower carbon sequestration rates (Trumbore & Harden Citation1997). Nonetheless, the role that these wetland communities are playing in carbon sequestration at a landscape scale should not be undervalued. Our results indicate that wetlands in southwest Florida can sequester up to four times more C in the soils than adjacent pine flatwood upland communities.
Implication of the balance between carbon sequestration and methane emissions
In its present state, the Corkscrew Swamp watershed represents a relatively undisturbed mosaic of wetland ecosystems that have developed in response to long-term climatic, hydrologic, edaphic and fire influences (Duever et al. Citation1984). Therefore, the 500-yr time horizon (GWP = 7.6) used in our analysis may be more realistically describing the emissions/uptake status of GHG in the Corkscrew wetland plant communities studied. Any possible positive radiative strength caused by CH4 emissions during the first stages of peat formation in the deep slough in the past, represented by the CH4/CO2 ratios in the 20- and 100-yr horizons, might have been offset by now by its long-term C sequestration (e.g. Frolking et al. Citation2006; Frolking & Roulet Citation2007). Accordingly, conservation strategies to maintain and enhance the wetland GHG sinking potential should focus on the cypress-dominated and deep slough communities.
Current efforts to restore the historical hydrological flows in the Everglades region (southern portion of Florida) features the redirection of unused freshwater for restoration purposes and human demands (Chimney & Goforth Citation2001; Perry Citation2004). Under this scenario, it is reasonable to expect that the inundation cycles will be modified in the different wetland ecosystems of the entire region including Corkscrew, either as a restoration measure or for water diversion. Considering the importance that the duration of inundation and the maximum and minimum water levels have on the plant community zonation (Duever et al. Citation1984), it is also reasonable to expect a shift in the plant communities. In general, changes favoring the areal expansion of cypress-dominated forest could enhance the net sink of GHG of the whole landscape. However, the practice of predicting the trajectory of plant communities in Corkscrew is rather challenging. Despite the fact that inundation is the main variable in the distribution of the plant communities, the isolated effects of this single variable could be hard to predict.
Some studies on the successional dynamics of pond cypress-dominated communities in depressional swamps in central Florida (Casey & Ewel Citation2006) and riparian forests in South Carolina (Giese et al. Citation2000) suggest that increased inundation periods and water levels may induce a shift in the pond cypress community towards a plant community dominated by species of hardwood forest like Nyssa spp., Salix spp., Gordonia lasianthus and Cephalanthus occidentalis. Whether or not bald cypress stands will develop later in the succession is still to be determined, but regardless, the new community will likely continue to function as a GHG sink. Conversely, dryer conditions with shorter inundation periods and lower water levels may lead to a dominance of pine flatwood communities, that could possibly lead also to the establishment of sedge and grass-dominated communities (Marois & Ewel Citation1983), altogether leading to a net increase in GHG emissions.
Conclusions
In this study, C sequestration in the soil of four different major wetland communities of southwest Florida was investigated. Sequestration rates were the highest in the community with the longest inundation, but did not follow a discernible pattern with the duration of inundation for the rest of communities considered across the landscape. Overall, the slowed decomposition caused by prolonged anaerobic periods brought about by high water levels and poor drainage observed in other tropical wetlands could only partially explain the rates of C accumulation found in this subtropical setting. Rather, the rates observed in these communities that alternate between prolonged wet and dry cycles may also be determined to some extent by the chemical composition of the organic matter reaching the soil. Over an extended time period (500 yr), the long-term C sequestration of the cypress-dominated (bald and pond) and deep slough communities outweighs their CH4 emissions. These communities should therefore be the focus of conservation strategies to enhance ecosystem service of climate regulation offered by wetlands in south Florida.
Acknowledgments
This study was conducted first at the Olentangy Wetland Research Park at The Ohio State University (mid 2010–mid 2012) and later at the Everglades Wetland Research Park at Florida Gulf Coast University (mid 2012–mid 2014). Authors wish to acknowledge the numerous volunteers that helped with field and laboratory work. Also, to Blanca Bernal for her help in the initial stages of this research. Anonymous reviewers provided key directions for the data analysis and discussion of results that considerably improved the final version of this manuscript. Director Jason Lauritsen, Dr. Shawn Liston and Dr. Michael Knight provided permission and help with logistics at the Corkscrew Swamp Sanctuary.
Additional information
Funding
References
- Appleby PG, Oldfield F. 1978. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena. 5:1–8.
- Appleby PG, Oldfield F. 1983. The assessment of 210Pb data from sites with varying sediment accumulation rates. Hydrobiologia. 103:29–35.
- Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. 2011. Freshwater methane emissions offset the continental carbon sink. Science. 331:50.
- Battle JM, Golladay SW. 2001. Hydroperiod influence on breakdown of leaf litter in Cypress-gum wetlands. Am Midl Nat. 146:128–145.
- Bernal B, Mitsch WJ. 2012. Comparing carbon sequestration in temperate freshwater wetland communities. Glob Change Biol. 18:1636–1647.
- Bernal B, Mitsch WJ. 2013. Carbon sequestration in freshwater wetlands in Costa Rica and Botswana. Biogeochemistry. 115:77–93.
- Brenner M, Schelske CL, Keenan LW. 2001. Historical rates of sediment and nutrient accumulation in marshes of the Upper St. Johns River Basin, Florida, U.S.A. J Paleolimnol. 26:241–257.
- Bridgham S, Megonigal J, Keller J, Bliss N, Trettin C. 2006. The carbon balance of North American wetlands. Wetlands. 26:889–916–916.
- Brinson MM. 1993. A hydrogemorphic classification for wetlands. Washington (DC): Wetland Research Program, US Army Corps of Engineers.
- Casey WP, Ewel KC. 2006. Patterns of succession in forested depressional wetlands in north Florida, USA. Wetlands. 26:147–160.
- Chimner RA, Ewel KC. 2005. A tropical freshwater wetland: II. Production, decomposition, and peat formation. Wetl Ecol Manag. 13:671–684.
- Chimney MJ, Goforth G. 2001. Environmental impacts to the Everglades ecosystem: a historical perspective and restoration strategies. Water Sci Technol. 44:93–100.
- Chimney MJ, Pietro KC. 2006. Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecol Eng. 27:301–321.
- Chow AT, Dai J, Conner WH, Hitchcock DR, Wang J-J. 2013. Dissolved organic matter and nutrient dynamics of a coastal freshwater forested wetland in Winyah Bay, South Carolina. Biogeochemistry. 112:571–587.
- Clymo RS. 1984. The limits to peat bog growth. Philos Trans R Soc Lond B Biol Sci. 303:605–654.
- Cohen AD. 1973. Petrology of some Holocene peat sediments from the Okefenokee swamp-marsh complex of southern Georgia. Geol Soc Am Bull. 84:3867–3878.
- Craft C, Washburn C, Parker A. 2008. Latitudinal trends in organic carbon accumulation in temperate freshwater peatlands. In: Vymazal J, editor. Wastewater treatment, plant dynamics and management in constructed and natural wetlands. New York (NY): Springer Science and Business Media B. V.; p. 23–31.
- Craft CB, Casey WP. 2000. Sediment and nutrient accumulation in floodplain and depressional freshwater wetlands of Georgia, USA. Wetlands. 20:323–332.
- Craft CB, Richardson CJ. 1993. Peat accretion and N, P and organic C accumulation in nutrient-enriched and unenriched Everglades peatlands. Ecol Appl. 3:446–458.
- Craft CB, Richardson CJ. 1998. Recent and long-term organic soil accretion and nutrient accumulation in the Everglades. Soil Sci Soc Am J. 62:834–843.
- Day Jr. FP. 1982. Litter decomposition rates in the seasonally flooded Great Dismal Swamp. Ecology. 63:670–678.
- Deghi GS, Ewel KC, Mitsch WJ. 1980. Effects of sewage effluent application on litter fall and litter decomposition in cypress swamps. J Appl Ecol. 17:397–408.
- Duever MJ, Carlson JE, Riopelle LA. 1984. Corkscrew Swamp: a virgin cypress strand. In: Ewel KC, Odum HT, editors. Cypress Swamps. Gainsville (FL): University Presses of Florida; p. 334–348.
- Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Faney DW, Haywood J, Lean J, Lowe DC, Myhre G, et al. 2007. Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin M, Manning D, Chen Z, Marquis M, Averyt KB, Tignor M, Mill HL, editors. Climate change 2007: the physical science basis. Cambridge: Cambridge University Press; p. 129–234.
- Franzluebbers AJ, Haney RL, Honeycutts CW, Arshad MA, Schomberg HH, Hons FM. 2001. Climatic influences on active fractions of soil organic matter. Soil Biol Biochem. 33:1103–1111.
- Frolking S, Roulet N, Fuglestvedt J. 2006. How northern peatlands influence the Earth’s radiative budget: sustained methane emission versus sustained carbon sequestration. J Geophys Res. 111:G01008.
- Frolking S, Roulet NT. 2007. Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions. Glob Change Biol. 13:1079–1088.
- Gedney N, Cox PM, Huntingford C. 2004. Climate feedback from wetland methane emissions. Geophys Res Lett. 31:L20503.
- Giese LA, Aust WM, Trettin CC, Kolka RK. 2000. Spatial and temporal patterns of carbon storage and species richness in three South Carolina coastal plain riparian forests. Ecol Eng. 15:S157–S170.
- Gorham E. 1991. Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl. 1:182–195.
- Hirano T, Jauhiainen J, Inoue T, Takahashi H. 2009. Controls on the carbon balance of tropical peatlands. Ecosystems. 12:873–887.
- Jauhiainen J, Takahashi H, Heikkinen JEP, Martikainen PJ, Vasander H. 2005. Carbon fluxes from a tropical peat swamp forest floor. Glob Change Biol. 11:1788–1797.
- Kayranli B, Scholz M, Mustafa A, Hedmark Å. 2010. Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands. 30:111–124.
- Lal R. 2008. Carbon sequestration. Philos Trans R Soc B Biol Sci. 363:815–830.
- MacKenzie AB, Farmer JG, Sugden CL. 1997. Isotopic evidence of the relative retention and mobility of lead and radiocaesium in Scottish ombrotrophic peats. Sci Total Environ. 203:115–127.
- MacKenzie AB, Hardie SML, Farmer JG, Eades LJ, Pulford ID. 2011. Analytical and sampling constraints in 210Pb dating. Sci Total Environ. 409:1298–1304.
- Maltby E, Immirzi P. 1993. Carbon dynamics in peatlands and other wetland soils regional and global perspectives. Chemosphere. 27:999–1023.
- Marois KC, Ewel KC. 1983. Natural and management-related variation in cypress domes. For Sci. 29:627–640.
- McLatchey GP, Reddy KR. 1998. Regulation of organic matter decomposition and nutrient release in a wetland soil. J Environ Qual. 27:1268–1274.
- Miao SL, Miao SL, Sklar FH. 1997. Biomass and nutrient allocation of sawgrass and cattail along a nutrient gradient in the Florida Everglades. Wetl Ecol Manag. 5:245–264.
- Mitra S, Wassmann R, Vlek PLG. 2005. An appraisal of global wetland area and its organic carbon stock. Curr Sci. 88:25–35.
- Mitsch WJ, Bernal B, Nahlik A, Mander Ü, Zhang L, Anderson C, Jørgensen S, Brix H. 2013. Wetlands, carbon, and climate change. Landsc Ecol. 28:583–597.
- Mitsch WJ, Gosselink JG. 2015. Wetlands. 5th ed. Hoboken (NJ): John Wiley & Sons.
- Mitsch WJ, Nahlik A, Wolski P, Bernal B, Zhang L, Ramberg L. 2010. Tropical wetlands: seasonal hydrologic pulsing, carbon sequestration, and methane emissions. Wetl Ecol Manag. 18:573–586.
- Oldfield F, Appleby PG. 1984. Empirical testing of 210Pb-dating models for lakes sediments. In: Haworth EY, Lund JWG, editors. Lake sediments environmental history. Minneapolis (MN): University of Minnesota Press; p. 93–124.
- Oldfield F, Appleby PG, Cambray RS, Eakins JD, Barber KE, Battarbee RW, Pearson GR, Williams JM. 1979. 210Pb, 137Cs and 239Pu profiles in ombrotrophic peat. Oikos. 33:40–45.
- Osborne TZ, Inglett PW, Reddy KR. 2007. The use of senescent plant biomass to investigate relationships between potential particulate and dissolved organic matter in a wetland ecosystem. Aquat Bot. 86:53–61.
- Page SE, Rieley JO, Banks CJ. 2011. Global and regional importance of the tropical peatland carbon pool. Glob Change Biol. 17:798–818.
- Perry W. 2004. Elements of South Florida’s comprehensive Everglades restoration plan. Ecotoxicology. 13:185–193.
- Pettit N, Davies T, Fellman J, Grierson P, Warfe D, Davies P. 2011. Leaf litter chemistry, decomposition and assimilation by macroinvertebrates in two tropical streams. Hydrobiologia. 1–15. doi:10.1007/s10750–011–0903–1.
- Reddy KR, DeLaune RD, DeBusk WF, Koch MS. 1993. Long term nutrient accumulation rates in the Everglades. Soil Sci Soc Am J. 57:1147–1155.
- Ritchie JC, McHenry JR. 1990. Application of radioactive fallout Cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: a review. J Environ Qual. 19:215–233.
- Roulet NT. 2000. Peatlands, carbon storage, greenhouse gases, and the Kyoto Protocol: prospects and significance for Canada. Wetlands. 20:605–615.
- Roulet NT, Lafleur PM, Richard PJH, Moore TR, Humphreys ER, Bubier J. 2007. Contemporary carbon balance and late Holocene carbon accumulation in a northern peatland. Glob Change Biol. 13:397–411.
- Schell WR, Tobin MJ, Massey CD. 1989. Evaluation of trace metal deposition history and potential element mobility in selected cores from peat and wetland ecosystems. Trace Met Lakes. 87–88:19–42.
- Smith JT, Clarke RT, Saxén R. 2000. Time-dependent behaviour of radiocaesium: a new method to compare the mobility of weapons test and Chernobyl derived fallout. J Environ Radioact. 49:65–83.
- Todd-Brown KO, Hopkins F, Kivlin S, Talbot J, Allison S. 2012. A framework for representing microbial decomposition in coupled climate models. Biogeochemistry. 109:19–33.
- Trumbore SE, Harden JW. 1997. Accumulation and turnover of carbon in organic and mineral soils of the BOREAS northern study area. J Geophys Res. 102:28817–28830.
- Villa JA. 2014. Carbon dynamics of subtropical wetland communities in south Florida [ Ph.D. Dissertation]. Columbus (OH): The Ohio State University.
- Villa JA, Mitsch WJ. 2014. Methane emissions from five wetland plant communities with different hydroperiods in the Big Cypress Swamp region of Florida Everglades. Ecohydrol Hydrobiol. doi:10.1016/j.ecohyd.2014.07.005
- Whalen SC. 2005. Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci. 22:73–94.
- Whiting GJ, Chanton JP. 2001. Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus B. 53:521–528.
