ABSTRACT
We assessed the impact of anthropogenic activities such as selective felling and resource extraction on plant diversity and forest structure in the broad-leaved forests of Bhutan. The forest area was grouped into three zones according to human influence: settlement-agriculture, semi-disturbed, and natural forest. A total of 140 plant species were identified. Maximum species richness of trees (8 species/plot) was recorded in natural forests and least in the settlement-agriculture zone (3 species/plot). Shannon (1.73 ± 0.62) and Simpson diversity (5.49 ± 2.97) indices were highest for the natural forest zone as compared to the semi-disturbed (0.92 ± 0.74, 2.70 ± 2.05) and settlement-agriculture zones (0.74 ± 0.74, 2.24 ± 2.13). The density and basal area of trees decreased from 373 N ha−1 and 37.9 m2 ha−1 in the natural forest zone to 114 N ha−1 and 5.7 m2 ha−1 in the settlement-agriculture zone. The diameter distribution revealed a lack of commercial tree species in the higher dbh classes from the settlement-agriculture and semi-disturbed zones due to selective felling. Increasing accessibility and anthropogenic activities caused the reduction in biodiversity of the watershed. Selective felling and resource extraction created gaps which were colonized by non-timber species and this may change the forest structure.
EDITED BY John Parrotta
1. Introduction
Watersheds and their forests are rich repositories of biodiversity. They are important for providing services to society such as the production function (e.g., timber and non-timber), protection (e.g., against soil erosion, avalanches), human welfare (e.g., CO2 sequestration, air, and water quality), and recreational function (Führer Citation2000). Today, the three important components of a watershed (land, water, and vegetation) are increasingly degraded (Sundriyal & Sharma Citation1996), leading to a loss in biodiversity (Pereira et al. Citation2010; Rands et al. Citation2010; Haddad et al. Citation2015). The relationship between biodiversity and disturbances (natural and anthropogenic) has been studied extensively (Connell Citation1978; Khan et al. Citation1987; Sundriyal & Sharma Citation1996; Cannon et al. Citation1998; Masaki et al. Citation1999; Bengtsson et al. Citation2000; Peltzer et al. Citation2000; McKinney Citation2002; Lorimer & White Citation2003; Hitimana et al. Citation2004; Kumar & Ram Citation2005; Rahman et al. Citation2009; Seidl et al. Citation2011; Wangchuk et al. Citation2014; Covey et al. Citation2015; Haddad et al. Citation2015; Pedro et al. Citation2015; Thom & Seidl Citation2015). All these studies report a strong impact on biodiversity by influencing forest conditions and forests succession dynamics.
Natural disturbances consist of wildfires, floods, pest calamities, lightning, wind throw, rock fall, and ungulate browsing (Ulanova Citation2000; Chazdon Citation2003; Hanewinkel et al. Citation2008; Seidl et al. Citation2011; Thom & Seidl Citation2015), while anthropogenic disturbances include activities such as logging, fodder, fuel wood and leaf litter extraction, agriculture clearing, and the introduction of non-native tree species (Bengtsson et al. Citation2000; Drapeau et al. Citation2000; Franklin et al. Citation2000; Chazdon Citation2003; Lorimer & White Citation2003; Onaindia et al. Citation2004; Wangda et al. Citation2009). Natural disturbances, which vary in size and frequency (Hiura et al. Citation1996), effect biodiversity and are an integral part of ecosystems (Dale et al. Citation2000; Franklin et al. Citation2002). In combination with stand and/or tree age they influence forest succession dynamics, which suggests that forest vegetation is never stable (Bengtsson et al. Citation2000). Anthropogenic disturbances due to management may have a higher impact on changes in biodiversity than what would occur under natural conditions (Kumar & Ram Citation2005). As a result anthropogenic disturbances may lead to less diverse ecosystems and less abundance with lower levels of spatial heterogeneity versus those associated with natural disturbances (Franklin et al. Citation2000; Mitchell et al. Citation2002). Anthropogenic impacts are expected to increase with the continued demand for forest resources (Husch et al. Citation2002; Rands et al. Citation2010; Hasenauer et al. Citation2012). It can be expected that this will change old growth forest ecosystems into manmade landscapes (Rahman et al. Citation2009), leading to habitat fragmentation and declining species richness (McKinney Citation2002).
Global changes in temperature and carbon dioxide concentration are also predicted to affect biodiversity by changing the competitive situation of tree species within forest stands (Dale et al. Citation2000; Hillebrand et al. Citation2008). Thom and Seidl (Citation2015) suggest that increasing disturbances under the expected climate change may lead to higher biological diversity but will negatively affect the ecosystem functions (McKinney Citation2002; Kumar & Ram Citation2005). Forest management practices also affect the future development of the forest diversity and the related ecosystem functions (Bengtsson et al. Citation2000; Peltzer et al. Citation2000). The impact may vary by region due to the species composition and the applied silvicultural concept (Fredericksen & Mostacedo Citation2000). Any harvesting or silvicultural operations may lead to a decrease (Cannon et al. Citation1998; Wangda et al. Citation2009), an increase (Magnusson et al. Citation1999; Paillet et al. Citation2010; Covey et al. Citation2015), limited (Pelissier et al. Citation1998) or no effect (Hall et al. Citation2003; Moktan et al. Citation2009b; Schweitzer & Dey Citation2011) on species richness, biodiversity or any other ecosystem service.
Forests in Bhutan, which are almost all owned by the state, can be categorized as either ‘natural forests’ or ‘semi-disturbed’, based on the anthropogenic ‘disturbances’ and ‘accessibility’. The ‘natural forests’, which are usually not under any form of management, may be seen as undisturbed or old growth. In these forests, there are either no anthropogenic disturbances or very limited disturbances in the form of harvesting of non-timber forest products and forest grazing. They are difficult to access and farther away from the human settlements (Dhital Citation2009). The ‘semi-disturbed’ forests are accessible and nearer to the communities and are preferred for extraction of timber and other resources. These forests are either managed through forest management plans or without management plans (Moktan et al. Citation2009a). Currently, only about 9% of the forest area is under regular sustainable forest management in Bhutan (DoFPS Citation2014). The remaining forest area is under tremendous pressure since the major part of the increasingly demanded resources comes from these forests (FRDD Citation2005b; FRMD Citation2013).
About 69% of the total population of Bhutan lives in rural areas (NSB Citation2005) where they are dependent on agriculture and forest resources. With the growth in population the demand for such resources is continuously increasing (DoFPS Citation2013). The major drivers of deforestation and forest degradation in Bhutan are developmental activities, livestock grazing, forest fire, timber, fuel wood, and non-timber forest product extraction (WMD Citation2013). Although a loss of forest diversity is expected due to these activities, only very limited impact studies which cover only the conifer belt exist (Gratzer et al. Citation2002; Roder et al. Citation2002; Gratzer & Rai Citation2004; Moktan et al. Citation2009a, Citation2009b; Wangchuk et al. Citation2014).
In our study we will focus on broad-leaved forests which are widely distributed in the Eastern Himalayas (Davidson Citation2000; Ohsawa Citation2002), including Southern and Eastern Bhutan (Norbu Citation2002), spreading along the major river basins (MoAF Citation2010). While previous studies focused on forest composition and regeneration in broad-leaved forests of dry valley slopes in Western Bhutan (Wangda & Ohsawa Citation2002, Citation2006a, Citation2006b), and the effects of grazing and logging on tree regeneration and diversity in mixed species forest areas (Van Ijssel Citation1990; Davidson Citation2000; Norbu Citation2002; Buffum et al. Citation2009; Covey et al. Citation2015), there are very limited or no studies focusing on species diversity and composition in relation to ‘natural’ and ‘disturbed forests’ due to anthropogenic utilization. The mission of this study is to investigate and assess management effects on forest biodiversity and timber species abundance in the broad-leaved forests within the watershed 144a in Dagana, Bhutan. We are specifically interested in:
species composition and species richness of different management zones,
differences in the biodiversity across the different management zones, and
the impact of selective cutting and/or other anthropogenic activities on timber and non-timber species.
2. Materials and methods
2.1. Study area
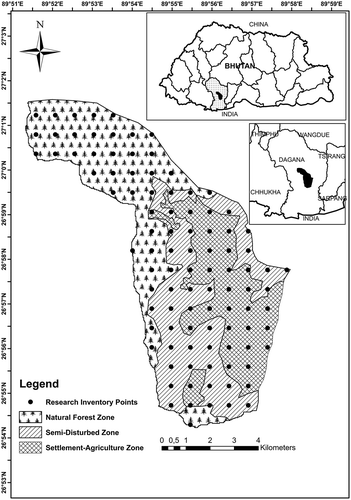
The study area is located within the geographical limit of 26°54ʹ16ʺ to 27°01ʹ36ʺ north and 89°51ʹ19ʺ to 89°57ʹ58ʺ east in the district of Dagana (). The area, which has a low socioeconomic development status (NSB Citation2014), is classified as degraded (WMD Citation2015), and is part of the Dagachhu sub-basin which belongs to the bigger Punatsangchhu river basin. The watershed has an area of 6422 ha and consists of the Buedulumchhu, Baleychhu, Zharingaychhu, and Lemichhu sub-watersheds. It comprises four geogs (group of villages) with 701 households and has an officially registered population of 6136.
Figure 1. Map of the study area showing the three different management zones along with the inventory plots.
The altitude of the watershed ranges from 500 to 2400 m.a.s.l (). The three climate stations located in the watershed at elevation of 1600, 1200, and 1000 m.a.s.l were established only in 2015. The 1 year climate data collected from these three stations corresponds with the data obtained from the two nearby stations. The mean annual precipitation is 2020 mm at Daga Dzong (1460 m.a.s.l.) and 1770 mm at Drujaygang station (1140 m.a.s.l.). The maximum precipitation occurs within the monsoon period from June to September. Mean monthly temperatures in Dagana range from 9.3°C in January to 20.7°C in July. Mean temperatures in Drujaygang range from 12.7°C in January to 24.2°C in July. Geologically, the area is located within the crystalline zone of the high Himalayas which was formed from migmatites and granite-gneisses (WMD Citation2015). The forest covers about 76% of the land area. According to the classification of Grierson and Long (Citation1983), three types of forests can be distinguished: (i) sub-tropical forests (up to 1000 m), (ii) warm, broad-leaved forests (1000–2000 m), and (iii) cool, broad-leaved forests (2000 m and above). The dominant species recorded within the watershed are given in Appendix 1.
Table 1. Mean, minimum (Min), maximum (Max), and standard deviation (Sd) of (i) site characteristics elevation and slope as wells as (ii) the stand characteristics density, basal area, volume, mean dbh, and mean height.
2.2. Study design
The forest area can be divided in to three different anthropogenic ‘disturbance zones’: (i) settlement-agriculture, (ii) semi-disturbed, and (iii) natural forest zones (). The settlement-agriculture zone with an area of 1666 ha consists of settlements and private agricultural fields with a few patches of private forests that are strongly protected. The settlement-agriculture zone is located within elevation range from 906 to 1778 m.a.s.l with mean elevation of 1229 m.a.s.l. The slope of the area is gentle to moderately steep with a mean slope of 22.5° (). The semi-disturbed zone with an area of 1834 ha is the buffer zone between settlement-agriculture and the natural forest zone. Timber and other resources for the rural households are supplied from these forests based on a single tree selection system without any forest management plans. It shows strong anthropogenic management impacts because it is located next to human settlements within the elevation range of 675–1848 m.a.s.l and thus easy to assess for extracting timber, fuel wood, non-timber forest products, and cattle grazing. The road network is concentrated in these two zones of settlement-agriculture and semi-disturbed zones (Waiba Citation2015). The slope of the area varies from 5° to 44° with a mean slope of 26.8°. The natural forest zone overlaps with the semi-disturbed zone with elevation ranging from 1392 to 2413 m.a.s.l and mean elevation of 1951 m.a.s.l (). In the natural forest zone, only minor anthropogenic disturbances due to harvesting and limited forest grazing are evident. The natural forest zone with an area of 2921 ha is difficult to access, and farther away from the human settlement areas. The slope of the natural forest zone varies from a very gentle slope of 3° to a steep slope of 60° and a mean slope of 27.5o. The steeper slopes are only found in the upper part of the forest zone with bamboo undergrowth. The mean elevation and mean slope of the three zones confirms the comparability of the results and therefore assumes that the differences in the diversity and species composition are mainly due to anthropogenic disturbances ().
In summer 2014 a forest inventory was conducted within the study area. The inventory design was adopted from the design of the national forest inventory of Bhutan (FRMD Citation2012). Within the watershed, we established 96 inventory points in a systematic grid of 800 m by 800 m. Thirty-six plots of the 96 plots are in the natural forest zone, 31 in the semi-disturbed zone, and 29 in the settlement-agriculture zone (). Circular plots with plot size of 500 m2 (r = 12.62 m) were established to collect sapling (dbh ≥5 to <10 cm) and tree data (dbh ≥10 cm). The regeneration data of the plots was collected on an inner plot area of 40 m2 (r = 3.57 m). No herbaceous data were collected. All plot radii were corrected for slope.
2.3. Forest stand data
Recorded tree data consists of the tree species, dbh, tree height, height to live crown base, azimuth, and horizontal distance from the plot center. For saplings, only the dbh and the height were recorded. Regeneration data were recorded by the number of (i) established seedlings (dbh <5 cm and height >2 m), (ii) un-established seedlings (dbh <5 cm and height <2 m), and (iii) recruits (current year seedlings) within the regeneration plot. In addition to the tree parameters, information on elevation, topography, slope, aspect, land ownership, forest management type, understory, and disturbances (grazing, forest fire, etc.) were recorded.
The number of trees, mean tree height, quadratic mean diameter, basal area, and stand volume were calculated for each plot (). We sorted the trees into timber and non-timber species to study the impact of selective cutting on the species composition. A taxonomist from the Department of Forests & Park Services (DoFPS) and a local timber harvesting contractor were part of the inventory team which helped with the identification of the species. The existing forest management plans of same forest types in the country, having information on the available timber species, their harvesting plan and management, also aided in sorting the species into timber and non-timber (FRDD Citation2005a, Citation2006, Citation2008). Additionally, we also interviewed representatives of the local people on their preferred tree species for timber, firewood, and fodder species. The interviews were conducted during the Watershed Management Planning workshop held by the office of the Watershed Management Division, DoFPS (WMD Citation2015). The timber species, as per the forest management plans, are shown with one asterisk (*) and results from the interview with the local people are shown with two asterisks (**) (Appendix 1). We calculated the stem number per hectare by dbh class for, ‘timber’ and ‘non-timber’ species, and management zone. Basal area and diameter distribution were used to study the abundance of timber species in the zones. The age distribution within the forest could not be assessed due to lack of information and when age is not available, dbh is often used as a substitute for age (Veblen Citation1992). Volume was calculated according to the volume equations of Laumans (Citation1994), FSI (Citation1996), FRDD (Citation2005c) – Appendix 2.
Next we calculated the diversity indices, species richness, and evenness for each of our inventory plots. Biodiversity was assessed with the species richness, Shannon index, Simpson’s index, and evenness. Species richness refers to the number of species recorded in each of the plot (Spellerberg & Fedor Citation2003). The Shannon index H’ (Shannon & Weaver Citation1949) is a measure of the number of species S and their even distribution according to the proportion of species pi and can be calculated as:
The Simpson index D is measure of diversity, which takes into account the number of species S and the relative abundance of each species with values starting from one. Higher values obtained with the Simpson index indicate greater diversity. It is calculated as (Simpson Citation1949):
Evenness indicates how even the species are distributed in the forest with values ranging from 0 to 1, where 1 means an equal distribution and values approaching 0 mean unequal distribution. It was calculated as:
where H’ is the Shannon index and S is the number of species (Pielou Citation1969).
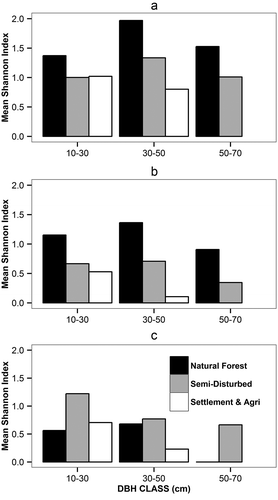
The plot results were assigned to one of our management zones (natural forest zone, semi-disturbed zone or settlement-agriculture zone) and the corresponding means and the standard deviations were calculated. In order to compare the diversity between different dbh classes, we grouped the diversity (Shannon index) of trees, saplings, and seedlings based on the quadratic mean dbh classes (10–30, 30–50 & 50–70) of trees for each of the zones. We then calculated the mean of the diversity for each of the dbh classes for trees, saplings, and established seedlings.
All data and statistical analysis were conducted in R environment (R Core Team Citation2015). We used the generalized linear model (glm) to assess any significant differences between the three different zones, while considering other available variables such as elevation, slope, and forest type (Equation 4).
The variables which were significant at 5% probability and with variance inflation factor (VIF) <5 were taken up in the final model for assessing the significant differences in the means between the zones. A 5% probability is required for significance and a VIF of <5 to avoid multicollinearity. The glm procedure with the Tukey’s multiple comparison of means test was used for post-hoc comparison of the means between the zones (Bretz et al. Citation2010). Regeneration of the different zones was compared using the Kruskal–Wallis test for all means. When the Kruskal–Wallis statistic was significant, we employed the post-hoc Kruskal Nemenyi test for multiple means comparison (Pohlert Citation2014).
3. Results
3.1. Species composition, richness, and diversity of the different zones
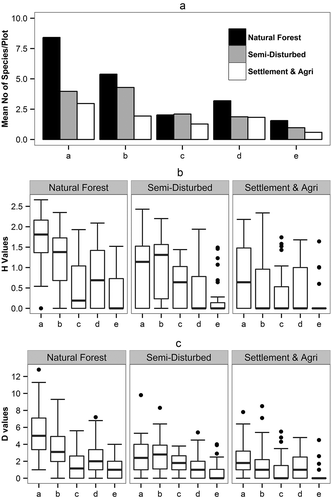
We started our analysis by comparing the stand characteristics and the biodiversity between the three different management zones of: (i) the settlement-agriculture zone where we expect the highest impact on biodiversity, (ii) the semi-disturbed zone with the cuttings of only highly valuable tree species and resource extraction, and (iii) a natural forest zone with only very minor or no anthropogenic activities (). This natural forest zone may be seen as undisturbed or old growth forests representing a ‘reference area’ to assess the ‘potential’ species richness and biodiversity in our study area. Our forest inventory within the watershed 144a recorded 124 tree species () and 16 shrub species (). These values indicate that the biodiversity of the forests within the area is rich. The highest number of species (83 tree and 12 shrub species) were recorded in the natural forest zone, 51 tree and 8 shrub species in the semi-disturbed zone and 41 tree and 3 shrub species in the settlement-agriculture zone ( and ). Maximum species richness for trees (8 species/plot) was recorded in the natural forest and least in the settlement-agriculture zone (3 species/plot) ()). The glm with Tukey’s multiple comparison of means indicated no significant differences for the density of trees (N ha−1) between natural forests and the semi-disturbed zones (). The basal area (m2 ha−1) and volume (m3 ha−1) of trees (dbh ≥10 cm) were significantly higher in the natural forest zone than the other two disturbed zones (). The diversity (Shannon and Simpson’s) indices were significantly higher in the natural forest zone than in the other two zones (; ,)). However, the diversity of established seedlings were highest in the semi-disturbed zone (,)). Oliver and Larson (Citation1996) and Buffum et al. (Citation2008) also confirmed the increase in seedling diversity after disturbances. As with the diversity, the evenness was highest in the natural forest zones, except for the established seedlings which had highest numbers in the semi-disturbed zone (). The differences in mean biodiversity values for the trees and saplings between the zones were higher for the quadratic dbh class of 30–50 and 50–70 indicating that cutting of trees was frequent in the higher dbh classes ().
Table 2. Density (N ha−1) and standard deviation of the tree species in the three zones.
Table 3. Density (N ha−1) and standard deviation of the shrub species in the three zones.
Table 4. Mean values ± standard deviations of density, basal area, and diversity indices for the different zones.
Figure 2. Species richness and diversity: (a) mean number of species per plot, (b) Shannon–Weaver index, and (c) Simpson’s index. Minima, median, maxima, and outliers are indicated. a = Trees (dbh ≥10 cm), b = Saplings (dbh ≥5 to <10 cm), c = Established Seedlings (dbh <5 cm and height >2 m), d = Unestablished seedlings (dbh <5 cm and height <2 m), e = Recruits (current year seedlings).
3.2. Impact of selective cutting in different zones
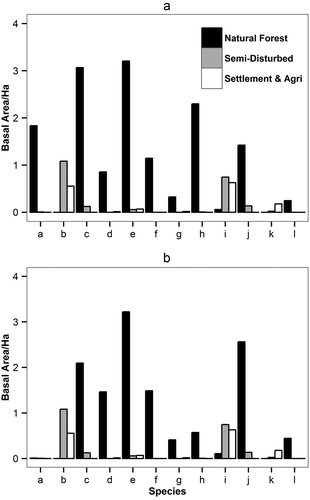
In the watershed 144a about 17 species of the 124 tree species are of commercial value (; Appendix 2). Thus we grouped our data by ‘timber’ and ‘non-timber’ species to analyze the potential differences in the tree species composition by management intensity (our forest zones). The diameter distribution of all the trees in the study area shows un-evenly aged forest (). In the natural forest zone, the density of timber species was 118 N ha−1, in the semi-disturbed zone it was 42 N ha−1, and in settlement-agriculture zone it was 45 N ha−1 ()). The density of timber species above the dbh of 40 cm in the natural forest zone was 47 N ha−1, while it was only 6 N ha−1 in the semi-disturbed zone followed by 4 N ha−1 in the settlement-agriculture zone ()). Bigger dbh trees of the timber species were found only in the natural forest zone ()), while bigger dbh trees of non-timber species were present in the semi-disturbed zone (,)). The basal area calculated showed maximum basal area for the preferred timber species in the natural forest zone rather than in the semi-disturbed and settlement-agriculture zones ()). The elevation gradient effect on the missing of certain commercial species from the semi-disturbed zone was studied by comparing the data from plots below 2000 m.a.s.l. The effect was only seen for the Acer spp. but not for other commercial species ()).
Figure 4. Diameter distribution of timber and non-timber species across the three zones. (a) Timber species distribution, (b) timber species above dbh class of 40 cm, (c) non-timber species distribution, and (d) non-timber species of dbh above 40 cm. * dbh of 40 cm and above are usually cut for timber.
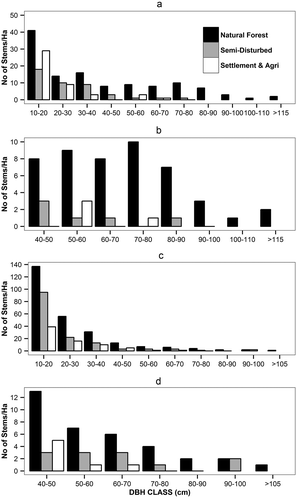
Figure 5. Basal area for the timber species across the three zones: (a) basal area/ha and (b) basal area/ha (plots below 2000 m.a.s.l). a = Acer spp., b = Alnus nepalensis, c = Castanopsis spp., d = Cinnamomum spp., e = Michelia spp., f = Persea spp., g = Phoebe spp., h = Quercus spp., i = Schima wallichi, j = Syzigium spp, k = Terminalia myriocarpa, l = Toona ciliata.
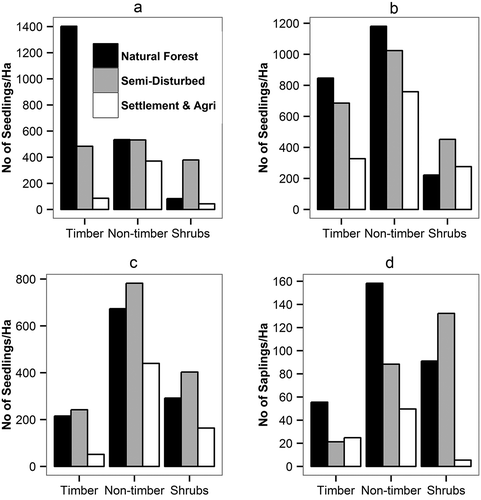
We studied the regeneration pattern in three zones by analyzing the seedlings and sapling density (N ha−1) of the ‘timber’, ‘non-timber’, and ‘shrub’ species in each of the management zones. The density (N ha−1) of unestablished seedlings (dbh <5 cm and height <2 m) and recruits (current year seedlings) of timber species were significantly higher in the natural forest zone than in the semi-disturbed and settlement-agriculture zones (; ). The Kruskal–Wallis test indicated no significant differences for the saplings (dbh ≥5 to <10 cm) and established seedlings of the timber species (dbh <5 cm and height >2 m) between the three zones. The presence of higher established seedling density of timber species (242 N ha−1) in the semi-disturbed zone, as compared to the natural forest zone, (215 N ha−1) was due to the presence of high Schima wallichii and Alnus nepalensis seedlings in the private forests, as well as in the landslide and erosion areas in the semi-disturbed zone ().
Table 5. Density (N ha−1) ± standard deviation of saplings and seedlings for the different zones.
Figure 6. Seedling and Sapling density (N ha−1) of the timber, non-timber and shrub species across the three zones. (a) Recruits (current year seedlings), (b) Unestablished Seedlings (dbh <5 cm and height <2 m), (c) Established Seedlings (dbh <5 cm and height >2 m), and (d) Saplings (dbh ≥5 to <10 cm).
4. Discussion
Species composition and species richness are important indicators for assessing the biodiversity (Husch et al. Citation2002) and may strongly depend and/or be influenced by the applied management practices. The listing of 140 plant species in the area shows the forest is rich in diversity ( and ) and can be considered to be among the highly diverse forests in the Himalayan region (Ohsawa Citation1987, Citation1991, Citation2002; Singh & Singh Citation1987). The record of 83 trees and 12 shrub species in the natural forest zone is comparable to other studies carried out in similar forest types of Bhutan, India, and Nepal. Wangda and Ohsawa (Citation2006b) listed 78 tree species in west central part of Bhutan and Buffum et al. (Citation2008) reported 39 tree species from the 260 ha forest in eastern part of Bhutan. Sundriyal and Sharma (Citation1996) recorded 81 tree species in the temperate forest in Sikkim, while Hussain et al. (Citation2008) counted 63 tree and 56 shrub species in Kumaon Himalaya, India. Carpenter (Citation2005) reported 159 tree species in eastern part of Nepal and Shrestha et al. (Citation2013) recorded 31 (19 tree and 12 shrub) and 37 (23 tree and 14 shrub) plant species in the two sites within the elevation range of 2650–2800 m.a.s.l in Nepal.
The lower number of species recorded in the semi-disturbed and settlement-agriculture zones ()) as compared to in the natural forest agree with the findings of earlier studies. Bhuyan et al. (Citation2003) reported only 16 species in highly disturbed site as compared to 47 species in the least disturbed site in the eastern part of India and Sunil et al. (Citation2011) observed 34 tree species in the low disturbed sites against tree species of 14 in the highly disturbed sites in the southern part of India. In Bangladesh, Rahman et al. (Citation2009) recorded 57 mature tree species in the low disturbed site, compared to 2 mature tree species in the highly disturbed sites. A tree species richness of 37 was the highest value found in natural forests, followed by the successional forest (31 species). The lowest was found in plantation forests, with only 9 species in Nepal (Webb & Sah Citation2003). All these studies attribute the differences in the results to the degree of disturbances caused by anthropogenic activities. Similarly, significant differences in diversity (Shannon and Simpsons) were seen between three zones, with the natural forest zone having highest diversity (; ,)) indicating the negative impact of the anthropogenic activities on the tree species diversity in the two disturbed zones. This corresponds with earlier studies (Bhat et al. Citation2000; Hall et al. Citation2003; Sagar et al. Citation2003; Onaindia et al. Citation2004; Kariuki et al. Citation2006; Rahman et al. Citation2009; Wangda et al. Citation2009; Semwal et al. Citation2010; Sunil et al. Citation2011; Joshi & Yadava Citation2015).
Harvesting or tree cutting influences the species distribution, especially if only a limited number of trees are removed. The remaining trees may get a competitive advantage and thus effect the regeneration of the forest stand. Additionally, the diameter distribution will change due to harvesting operations and this may be seen as a measure for assessing the disturbance effects within forests (Hitimana et al. Citation2004). The analysis of diameter distribution and basal area suggests that selective felling has a negative impact on the diameter distribution of preferred timber species ( and ). The differences in basal area (m2 ha−1) between the three zones were highest for the highly valuable and most preferred species, such as Michelia spp., Phoebe spp., Persea spp., and Toona ciliata ()). Our results also suggest that the lack of timber species or reduction in their density (N ha−1) is due to selective felling and not caused by changing site conditions due to elevation ()). Ohsawa (Citation2002) reported that species richness in mixed forest stands is independent from elevation gradient, however, the dominance and structure may change with elevation (Sharma et al. Citation2009; Kumar & Sharma Citation2014).
Forests which are rich in biodiversity, when accessible and unrestricted for resource extraction, can lead to forests with lower species diversity (Vetaas Citation1993; Sundriyal & Sharma Citation1996; Murali & Setty Citation2001; Kumar & Ram Citation2005). The natural forest zone which is not under any form of sustainable management lies farther away from the settlements and is not easily accessible by roads. In this zone, the disturbances are limited to minor harvesting of non-timber forest products and limited cattle grazing. However, the two disturbed zones of settlement-agriculture and semi-disturbed are in close proximity to human settlements and are accessible by roads. The road density of 10.6 m/ha (Waiba Citation2015) in the watershed is fairly high and concentrates in the semi-disturbed and settlement-agriculture zones. Road networks increase resource extraction and encroachment into the forest leading to a reduction in biodiversity (Sundriyal & Sharma Citation1996; Watkins et al. Citation2003; Hitimana et al. Citation2004). Timber and other resources for the rural households are supplied on standing tree basis (Moktan et al. Citation2009a) from the forests of the semi-disturbed zone because of the area being easily accessible. However, there is no management plan or a resource inventory for ensuring sustainable cutting of the trees leading to reduction or removal of the timber species from the semi-disturbed and settlement-agriculture zones. Sundriyal and Sharma (Citation1996) and Bhuyan et al. (Citation2003) reported indiscriminate cutting of trees and selective felling for timber as the major cause of forest destruction in the eastern part of India while Sunil et al. (Citation2011) described the lack of native tree species and bigger trees in the disturbed zones due to timber harvesting and fuel wood extraction in southern India. The density of shade intolerant species was higher due to removal of bigger commercial trees by selective logging in Australia (Kariuki et al. Citation2006). Rahman et al. (Citation2009) and Hitimana et al. (Citation2004) also observed standing large size stems and basal area only in the least disturbed sites in the forests of Bangladesh and Kenya, respectively.
Selection cutting is assumed to mimic natural forest development by leaving trees distributed in all age classes (Oliver & Larson Citation1996). However, our results indicate that the selective felling in combination with other anthropogenic disturbances leads to regeneration and establishment of non-timber and shrub species in the two disturbed zones. This may have significant impact on the forest structure in the coming years (; ). Peltzer et al. (Citation2000) in Western Kenya, Kumar and Ram (Citation2005) in Eastern India and Joshi and Yadava (Citation2015) in the central part of India also observed higher shrub density in disturbed sites. The lack of timber species regenerating in the forests could also mean less availability of the timber for people in the future. Although the local forest department office have initiated plantations of commercial species in the barren areas of the semi-disturbed zones, there seems to have been neither follow up nor monitoring on the natural regeneration after removal of trees, which explains for the less regeneration of timber species in the two disturbed zones. Gaps created by selective felling, if not treated, can be colonized by competing plants other than timber species (Fredericksen & Mostacedo Citation2000; Kariuki et al. Citation2006; Moktan et al. Citation2009a) and aggravated further by grazing (Davidson Citation2000; Tashi Citation2004).
Forest management interventions will be required to deal with the existing disturbances and expected disturbances under climate change (Dale et al. Citation2000). Therefore, we recommend some interventions but not limited to for improving the conditions of the two disturbed zones. Urgently, the forest areas without a management plan must immediately be treated under the management regime of either a forest management plan or a community based forest management plan (FRDD Citation2005b). The ‘natural forests’ which may be seen as a reserve, should focus on biodiversity and nature conservation issues. The management plans must be accompanied by regular (every 10 years) forest inventories to control the past management and update and adjust future sustainable management activities to avoid further loss in biodiversity. For instance, the establishment of community forests has led to the improvement of forest conditions by reducing deforestation (Nagendra Citation2007; Nagendra et al. Citation2008; Chhetri et al. Citation2009; Porter-Bolland et al. Citation2012) with a positive outcome in biodiversity conservation (Persha et al. Citation2010).
The forest field officials need to be trained in silviculture practices, reforestation, and afforestation activities (FRDS Citation1996, Citation1997; Beck Citation2011). Reforestation and afforestation activities should be carried out in the two disturbed zones through plantation of local and native species. Research on the use of non-commercial tree species to reduce the pressure on commercial species (see Beck (Citation2011) and enforcement of forest laws and regulations is needed. Additionally, strict monitoring on the amount of resource extraction and on the status of the regeneration/plantation should be carried out to improve the forest conditions (Nagendra Citation2007, Citation2009). This ensures that exploitation is avoided and the forest management activities are under control.
5. Conclusion
Forests with rich biodiversity provide various ecosystem services for sustaining rural livelihood and national development in Bhutan. The well-being of forest dependent communities rely on how well the forests are conserved and managed (Norbu Citation2002). However, the impacts of anthropogenic disturbances are now felt in these forests which are expected to increase further with the growing population and enhanced accessibility. The significantly lower biodiversity and stand characteristics in the disturbed zones confirm the increasing anthropogenic activities and its negative impact on diversity. Easy accesses to these forests have further contributed toward the decline in diversity.
Anthropogenic disturbances have also increased the composition of non-valuable species, which may not be useful for the local population. The single tree selection system seems to be failing in regenerating the timber species, probably due to lack of monitoring or silviculture stand improvement after felling of the trees. A single tree selection system, if carried out repeatedly without silvicultural stand improvement, might increase the proportion of non-timber species (Kariuki et al. Citation2006). It also leads to the removal of only bigger and more valuable timber species. This indicates that such forest areas, which are accessible and managed based on single tree selection system, warrant more attention from the concerned agencies to ensure their sustainability. Our findings may also be considered as a test case for providing insights into the change of the forest structure if no regular monitoring and management plan is in place.
Acknowledgments
This study was carried out under the project ‘Climate change adaptation potentials of forests in Bhutan – building human capacities and knowledge base (BC-CAP)’ with funding from the Austrian Government through the Austrian Federal Ministry of Agriculture, Forestry, Environment and Water Management, executed by the Department of Forests & Park Services, Ministry of Agriculture and Forests, Bhutan, and the University of Natural Resources and Life Sciences, Austria. We would like to thank: Georg Gratzer, Pema Wangda, Kinley Tenzin for the managerial support, Kezang Yangden for the technical inputs on GPS for data management, Karma Tenzin, Dorji Gyeltshen (Taxonomist), Karma Wangdra, Cheten Wangdra, Kinga Thinley, Janga B. Mongar, and Tendi Zangmo for helping with the field work, Rinchen Dorji for the GIS support, Younten Phuntsho for arranging relevant documents on forestry in Bhutan, Andras Darabant for providing helpful comments on an earlier version of the manuscript, and Adam Moreno for English editing. Finally we thank the editors and the three anonymous reviewers for their helpful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Beck M. 2011. Economic analysis of timber production from community forests and forest management units. Three case studies from central and eastern Bhutan [ MSc thesis]. Zurich: ETH Zurich.
- Bengtsson J, Nilsson SG, Franc A, Menozzi P. 2000. Biodiversity, disturbances, ecosystem function and management of European forests. For Ecol Manage. 132:39–50.
- Bhat D, Naik M, Patagar S, Hegde G, Kanade Y, Hegde G, Shastri C, Shetti D, Furtado R. 2000. Forest dynamics in tropical rain forests of Uttara Kannada district in Western Ghats, India. Curr Sci. 79:975–985.
- Bhuyan P, Khan M, Tripathi R. 2003. Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodivers Conserv. 12:1753–1773.
- Bretz F, Hothorn T, Westfall P. 2010. Multiple comparisons using R. Boca Raton (FL): Chapman & Hall/CRC,Taylor & Francis Group.
- Buffum B, Gratzer G, Tenzin Y. 2008. The sustainability of selection cutting in a late successional broadleaved community forest in Bhutan. For Ecol Manage. 256:2084–2091.
- Buffum B, Gratzer G, Tenzin Y. 2009. Forest grazing and natural regeneration in a late successional broadleaved community forest in Bhutan. Mt Res Dev. 29:30–35.
- Cannon CH, Peart DR, Leighton M. 1998. Tree species diversity in commercially logged Bornean rainforest. Science. 281:1366–1368.
- Carpenter C. 2005. The environmental control of plant species density on a Himalayan elevation gradient. J Biogeogr. 32:999–1018.
- Chazdon RL. 2003. Tropical forest recovery: legacies of human impact and natural disturbances. Perspect Plant Ecol Evol Syst. 6:51–71.
- Chhetri B, Schmidt K, Gilmour D. 2009. Community forestry in Bhutan-exploring opportunities and facing challenges. Community Forestry International Workshop; Pokhara, Nepal.
- Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science. 199:1302–1310.
- Covey K, Carroll CJW, Duguid MC, Dorji K, Dorji T, Tashi S, Wangdi T, Ashton M. 2015. Developmental dynamics following selective logging of an evergreen oak forest in the Eastern Himalaya, Bhutan: structure, composition, and spatial pattern. For Ecol Manage. 336:163–173.
- Dale VH, Joyce LA, McNulty S, Neilson RP. 2000. The interplay between climate change, forests, and disturbances. Sci Total Environ. 262:201–204.
- Davidson J. 2000. Ecology and management of broadleaved forests of eastern Bhutan. Khangma (Bhutan): Third Forestry Development Project, Department of Forestry Services, Ministry of Agriculture, Royal Government of Bhutan.
- Dhital DB. 2009. Bhutan forestry outlook study. Bangkok (Thailand): Food and Agriculture Orgazination of the United Nations Regional Office for Asia and the Pacific.
- DoFPS. 2013. Forestry: facts and figures 2013. Thimphu (Bhutan): Department of Forests & Park Services, Ministry of Agriculture & Forests, Royal Government of Bhutan.
- DoFPS. 2014. Forestry statistics 2014. Thimphu (Bhutan): Department of Forests & Park Services, Ministry of Agriculture & Forests, Royal Government of Bhutan.
- Drapeau P, Leduc A, Giroux J-F, Savard J-PL, Bergeron Y, Vickery WL. 2000. Landscape-scale disturbances and changes in bird communities of boreal mixed-wood forests. Ecol Monogr. 70:423–444.
- Franklin JF, Lindenmayer D, MacMahon JA, McKee A, Magnuson J, Perry DA, Waide R, Foster D. 2000. Threads of continuity. Conserv Biol Pract. 1:8–17.
- Franklin JF, Spies TA, Pelt R, Carey AB, Thornburgh DA, Berg DR, Lindenmayer DB, Harmon ME, Keeton WS, Shaw DC, et al. 2002. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For Ecol Manage. 155:399–423.
- FRDD. 2005a. Forest management plan for wangdigang forest management unit (2005-2015). Thimphu (Bhutan): Department of Forests, Ministry of Agriculture, Royal Government of Bhutan.
- FRDD. 2005b. Management of forest areas outside FMU systems-planning guidelines. Thimphu (Bhutan): Department of Forests, Ministry of Agriculture, Royal Government of Bhutan; p. 66.
- FRDD. 2005c. Volume table of Bhutan. Thimphu (Bhutan): Department of Forests, Ministry of Agriculture, Royal Government of Bhutan.
- FRDD. 2006. Management plan for Korila forest management unit (2006-2016). Thimphu (Bhutan): Department of Forests, Ministry of Agriculture, Royal Government of Bhutan.
- FRDD. 2008. Management plan for Lingmethang forest management unit (2008-2018). Thimphu: Deparment of Forests, Ministry of Agriculture, Royal Government of Bhutan.
- FRDS. 1996. Proceedings of the fifth workshop on implementation of forest management plans. Thimphu: Forest Resources Development Section, Forestry Services Division, Ministry of Agriculture, Royal Government of Bhutan.
- FRDS. 1997. Workshop on forest management planning and draft forest and nature conservation rules and regulations. Thimphu: Forest Resources Development Section, Forestry Services Division, Ministry of Agriculture, Royal Government of Bhutan.
- Fredericksen TS, Mostacedo B. 2000. Regeneration of timber species following selection logging in a Bolivian tropical dry forest. For Ecol Manage. 131:47–55.
- FRMD. 2012. National forest inventory field manual. Thimphu (Bhutan): Department of Forests & Park Services, Ministry of Agriculture & Forests, Royal Government of Bhutan.
- FRMD. 2013. Forest resources potential assessment of Bhutan 2013. Thimphu (Bhutan): Department of Forest & Park Services, Ministry of Agriculture & Forests, Royal Government of Bhutan; p. 23.
- FSI. 1996. Volume equations for forests of India, Nepal and Bhutan. Dehradun (India): Ministry of Environment and Forests, Govt. of India.
- Führer E. 2000. Forest functions, ecosystem stability and management. For Ecol Manage. 132:29–38.
- Gratzer G, Rai PB. 2004. Density-dependent mortality versus spatial segregation in early life stages of Abies densa and Rhododendron hodgsonii in Central Bhutan. For Ecol Manage. 192:143–159.
- Gratzer G, Rai PB, Schieler K. 2002. Structure and regeneration dynamics of Abies densa forests in central Bhutan. Aust J For Sci. 119:279–288.
- Grierson AJC, Long DG. 1983. Flora of Bhutan vol 1. Part 1. Edinburgh (UK): Royal Botanical Garden Edinburgh.
- Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, et al. 2015. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv. 1:e1500052.
- Hall JS, Harris DJ, Medjibe V, Ashton PMS. 2003. The effects of selective logging on forest structure and tree species composition in a Central African forest: implications for management of conservation areas. For Ecol Manage. 183:249–264.
- Hanewinkel M, Breidenbach J, Neeff T, Kublin E. 2008. Seventy-seven years of natural disturbances in a mountain forest area-the influence of storm, snow, and insect damage analysed with a long-term time series. Can J For Res. 38:2249–2261.
- Hasenauer H, Petritsch R, Zhao M, Boisvenue C, Running SW. 2012. Reconciling satellite with ground data to estimate forest productivity at national scales. For Ecol Manage. 276:196–208.
- Hillebrand H, Bennett DM, Cadotte MW. 2008. Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology. 89:1510–1520.
- Hitimana J, Kiyiapi JL, Njunge JT. 2004. Forest structure characteristics in disturbed and undisturbed sites of Mt. Elgon Moist Lower Montane Forest, western Kenya. For Ecol Manage. 194:269–291.
- Hiura T, Sano J, Konno Y. 1996. Age structure and response to fine-scale disturbances of Abies sachalinensis, Picea jezoensis, Picea glehnii, and Betula ermanii growing under the influence of a dwarf bamboo understory in northern Japan. Can J For Res. 26:289–297.
- Husch B, Beers TW, Kershaw Jr JA. 2002. Forest mensuration. New York (NY): John Wiley & Sons.
- Hussain MS, Sultana A, Khan JA, Khan A. 2008. Species composition and community structure of forest stands in Kumaon Himalaya, Uttarakhand, India. Trop Ecol. 49:167.
- Joshi A, Yadava AK. 2015. Effect of anthropogenic disturbances on plant diversity in Oak dominated forests of Nainital, Kumaun Himalaya, India. N Y Sci J. 8:22–27.
- Kariuki M, Kooyman RM, Smith RGB, Wardell-Johnson G, Vanclay JK. 2006. Regeneration changes in tree species abundance, diversity and structure in logged and unlogged subtropical rainforest over a 36-year period. For Ecol Manage. 236:162–176.
- Khan M, Rai J, Tripathi R. 1987. Population structure of some tree species in disturbed and protected subtropical forests of north-east India. Acta Oecol-Oec Appl. 8:247–255.
- Kumar A, Ram J. 2005. Anthropogenic disturbances and plant biodiversity in forests of Uttaranchal, central Himalaya. Biodivers Conserv. 14:309–331.
- Kumar S, Sharma S. 2014. Diversity, disturbance and regeneration status of forests along an altitudinal gradient in Paddar Valley, Northwest Himalayas. Indian For. 140:348–353.
- Laumans P. 1994. Height-diameter functions from PIS for country level site classification and local volume table selection. Working Document No.11(FO: DP/BHU/911002). Thimphu (Bhutan): UNDP/FAO Forest Resources Management and Institutional Development Project, Deparment of Forestry, Ministry of Agriculture, Royal Government of Bhutan.
- Lorimer CG, White AS. 2003. Scale and frequency of natural disturbances in the northeastern US: implications for early successional forest habitats and regional age distributions. For Ecol Manage. 185:41–64.
- Magnusson WE, de Lima OP, Reis FQ, Higuchi N, Ramos JF. 1999. Logging activity and tree regeneration in an Amazonian forest. For Ecol Manage. 113:67–74.
- Masaki T, Tanaka H, Tanouchi H, Sakai T, Nakashizuka T. 1999. Structure, dynamics and disturbance regime of temperate broad-leaved forests in Japan. J Veg Sci. 10:805–814.
- McKinney ML. 2002. Urbanization, biodiversity, and conservation the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience. 52:883–890.
- Mitchell RJ, Palik BJ, Hunter ML. 2002. Natural disturbance as a guide to silviculture. For Ecol Manage. 155:315–317.
- MoAF. 2010. Bhutan land cover assessment 2010 (LCMP 2010). Thimphu: Ministry of Agriculture & Forests, Royal Government of Bhutan; p. 35.
- Moktan MR, Gratzer G, Richards WH, Rai TB, Dukpa D. 2009a. Regeneration and structure of mixed conifer forests under single-tree harvest management in the western Bhutan Himalayas. For Ecol Manage. 258:243–255.
- Moktan MR, Gratzer G, Richards WH, Rai TB, Dukpa D, Tenzin K. 2009b. Regeneration of mixed conifer forests under group tree selection harvest management in western Bhutan Himalayas. For Ecol Manage. 257:2121–2132.
- Murali K, Setty SR. 2001. Effect of weeds Lantana camara and Chromelina odorata growth on the species diversity, regeneration and stem density of tree and shrub layer in BRT sanctuary. Curr Sci. 80:675–678.
- Nagendra H. 2007. Drivers of reforestation in human-dominated forests. Proc Natl Acad Sci. 104:15218–15223.
- Nagendra H. 2009. Drivers of regrowth in South Asia’s human impacted forests. Curr Sci. 97:1586–1592.
- Nagendra H, Pareeth S, Sharma B, Schweik CM, Adhikari KR. 2008. Forest fragmentation and regrowth in an institutional mosaic of community, government and private ownership in Nepal. Landscape Ecol. 23:41–54.
- Norbu L. 2002. Grazing management in Broadleaf Forests. J Bhutan Stud. 7:99–129.
- NSB. 2005. Population and housing census of Bhutan. Thimphu: National Statistical Bureau, Royal Government of Bhutan.
- NSB. 2014. Bhutan poverty assessment 2014. Thimphu: National Statistics Bureau, Royal Government of Bhutan; p. 130.
- Ohsawa M. 1987. Life zone ecology of the Bhutan Himalaya I. Chiba (Japan): Chiba University.
- Ohsawa M. 1991. Life zone ecology of Bhutan Himalaya II. Chiba (Japan): Chiba University.
- Ohsawa M. 2002. Life zone ecology of Bhutan Himalaya III. Chiba (Japan): Chiba University.
- Oliver CD, Larson BC. 1996. Forest stand dynamics. 1st ed. New York (NY): McGraw Hill.
- Onaindia M, Dominguez I, Albizu I, Garbisu C, Amezaga I. 2004. Vegetation diversity and vertical structure as indicators of forest disturbance. For Ecol Manage. 195:341–354.
- Paillet Y, Bergès L, Hjältén J, Ódor P, Avon C, Bernhardt‐Römermann M, Bijlsma RJ, De Bruyn L, Fuhr M, Grandin U, et al. 2010. Biodiversity differences between managed and unmanaged forests: meta‐analysis of species richness in Europe. Conserv Biol. 24:101–112.
- Pedro MS, Rammer W, Seidl R. 2015. Tree species diversity mitigates disturbance impacts on the forest carbon cycle. Oecologia. 177:619–630.
- Pelissier R, Pascal J-P, Houllier F, Laborde H. 1998. Impact of selective logging on the dynamics of a low elevation dense moist evergreen forest in the Western Ghats (South India). For Ecol Manage. 105:107–119.
- Peltzer DA, Bast ML, Wilson SD, Gerry AK. 2000. Plant diversity and tree responses following contrasting disturbances in boreal forest. For Ecol Manage. 127:191–203.
- Pereira HM, Leadley PW, Proença V, Alkemade R, Scharlemann JP, Fernandez-Manjarrés JF, Araújo MB, Balvanera P, Biggs R, Cheung WW, et al. 2010. Scenarios for global biodiversity in the 21st century. Science. 330:1496–1501.
- Persha L, Fischer H, Chhatre A, Agrawal A, Benson C. 2010. Biodiversity conservation and livelihoods in human-dominated landscapes: forest commons in South Asia. Biol Conserv. 143:2918–2925.
- Pielou EC. 1969. An introduction to mathematical ecology. New York: Wiley-Interscience; p. 286.
- Pohlert T. 2014. The pairwise multiple comparison of mean ranks package (PMCMR) [Internet]. R package. Available from: http://CRAN.R-project.org/package=PMCMR
- Porter-Bolland L, Ellis EA, Guariguata MR, Ruiz-Mallén I, Negrete-Yankelevich S, Reyes-García V. 2012. Community managed forests and forest protected areas: an assessment of their conservation effectiveness across the tropics. For Ecol Manage. 268:6–17.
- R Core Team. 2015. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Rahman M, Nishat A, Vacik H. 2009. Anthropogenic disturbances and plant diversity of the Madhupur Sal forests (Shorea robusta CF Gaertn) of Bangladesh. Int J Biodiv Sci Manage. 5:162–173.
- Rands MR, Adams WM, Bennun L, Butchart SH, Clements A, Coomes D, Entwistle A, Hodge I, Kapos V, Scharlemann JP, et al. 2010. Biodiversity conservation: challenges beyond 2010. Science. 329:1298–1303.
- Roder W, Gratzer G, Wangdi K. 2002. Cattle grazing in the Conifer forests of Bhutan. Mt Res Dev. 22:1–7.
- Sagar R, Raghubanshi A, Singh J. 2003. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For Ecol Manage. 186:61–71.
- Schweitzer CJ, Dey DC. 2011. Forest structure, composition, and tree diversity response to a gradient of regeneration harvests in the mid-Cumberland Plateau escarpment region, USA. For Ecol Manage. 262:1729–1741.
- Seidl R, Fernandes PM, Fonseca TF, Gillet F, Jönsson AM, Merganičová K, Netherer S, Arpaci A, Bontemps J-D, Bugmann H, et al. 2011. Modelling natural disturbances in forest ecosystems: a review. Ecol Model. 222:903–924.
- Semwal DP, Uniyal PL, Bhatt AB. 2010. Structure, composition and dominance–diversity relations in three forest types of a part of Kedarnath Wildlife Sanctuary, Central Himalaya, India. Not Sci Biol. 2:128–132.
- Shannon CE, Weaver W. 1949. The mathematical theory of communication. Urbana: University of Illinois Press; 117pp.
- Sharma C, Suyal S, Gairola S, Ghildiyal S. 2009. Species richness and diversity along an altitudinal gradient in moist temperate forest of Garhwal Himalaya. J Am Sci. 5:119–128.
- Shrestha KB, Måren IE, Arneberg E, Sah JP, Vetaas OR. 2013. Effect of anthropogenic disturbance on plant species diversity in oak forests in Nepal, Central Himalaya. Int J Biodivers Sci Ecosyst Serv Manage. 9:21–29.
- Simpson EH. 1949. Measurement of diversity. Nature. 163:688.
- Singh J, Singh S. 1987. Forest vegetation of the Himalaya. Bot Rev. 53:80–192.
- Spellerberg IF, Fedor PJ. 2003. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Global Ecol Biogeogr. 12:177–179.
- Sundriyal R, Sharma E. 1996. Anthropogenic pressure on tree structure and biomass in the temperate forest of Mamlay watershed in Sikkim. For Ecol Manage. 81:113–134.
- Sunil C, Somashekar R, Nagaraja B. 2011. Impact of anthropogenic disturbances on riparian forest ecology and ecosystem services in Southern India. Int J Biodivers Sci Ecosyst Serv Manage. 7:273–282.
- Tashi S. 2004. Regeneration of Quercus semecarpifolia Sm. in an old growth oak forest under Gidakom FMU - Bhutan. Department of Forestry. Wageningen (Netherlands): Wageningen University, p. 58.
- Thom D, Seidl R. 2015. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol Rev. doi:10.1111/brv.12193
- Ulanova NG. 2000. The effects of windthrow on forests at different spatial scales: a review. For Ecol Manage. 135:155–167.
- Van Ijssel W. 1990. The effect of grazing and seedbed type on forest regeneration under two silvicultural systems in a mixed broadleaf forest, Gedu. Tsenden. 3:32–44.
- Veblen TT. 1992. Regeneration dynamics. In: Glenn-Lewin DC, Peet RK, Veblen TT, editors. Plant succession: theory and prediction. London: Chapman & Hall; p. 152–187.
- Vetaas OR. 1993. Spatial and temporal vegetation changes along a moisture gradient in northeastern Sudan. Biotropica. 25:164–175.
- Waiba TN. 2015. Evaluation of old and recently built farm roads in the selected watershed of Bhutan-an evaluation from the prospective of assessing environmental impacts. Vienna (Austria): Department of Forest and Soil Sciences BOKU University.
- Wangchuk K, Darabant A, Rai PB, Wurzinger M, Zollitsch W, Gratzer G. 2014. Species richness, diversity and density of understory vegetation along disturbance gradients in the Himalayan conifer forest. J Mt Sci. 11:1182–1191.
- Wangda P, Ohsawa M. 2002. Transition of forest structure and environmental conditions along the altitudinal gradient in a dry valley, upper Lobesa, Bhutan. IUCN/WCPA-EA-4 Taipei Conference; Taipei, Taiwan; p. 8.
- Wangda P, Ohsawa M. 2006a. Structure and regeneration dynamics of dominant tree species along altitudinal gradient in a dry valley slopes of the Bhutan Himalaya. For Ecol Manage. 230:136–150.
- Wangda P, Ohsawa M. 2006b. Gradational forest change along the climatically dry valley slopes of Bhutan in the midst of humid eastern Himalaya. Plant Ecol. 186:109–128.
- Wangda P, Tashi S, Norbu L, Gyaltshen D. 2009. Change in forest structure and diversity after the human disturbances in the cool montane evergreen broad-leaved forest. J Renew Nat Resour Bhutan. 5:55–67.
- Watkins RZ, Chen J, Pickens J, Brosofske KD. 2003. Effects of forest roads on understory plants in a managed hardwood landscape. Conserv Biol. 17:411–419.
- Webb EL, Sah RN. 2003. Structure and diversity of natural and managed sal (Shorea robusta Gaertn. f.) forest in the Terai of Nepal. For Ecol Manage. 176:337–353.
- WMD. 2013. REDD+ readiness preparation proposal. Thimphu (Bhutan): Department of Forests & Park Services, Ministry of Agriculture & Forests, Royal Government of Bhutan; p. 159.
- WMD. 2015. Watershed Management Plan: Watershed 144a, Dagachhu sub-basin, Punatsangchhu basin (Draft Plan). Department of Forests&Park Services, MoAF, Royal Government of Bhutan, Unpublished.
Appendices
Appendix 1. Forest types and dominant species encountered in the watershed
Appendix 2. Equations used for calculating volume
Table