Abstract
The antioxidant and antimycobacterial activities of Opuntia ficus-indica (OFI) fruit extracts were evaluated according to seasonal cultivation. The antioxidant activities of OFI extracts were assessed by different in vitro methods and the total phenolic and flavonoid contents were also examined. The antimycobacterial activity was measured against Mycobacterium tuberculosis strain H37Rv (ATCC 27294) by the microplate alamar blue assay method. In the antioxidant assay, methanol extracts of OFI in summer showed the highest antioxidant activity of all tested methods, and the lowest IC50 was observed in the 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay. Pearson's correlation coefficient was evaluated and the results from the extracts of summer and rainy season OFI were significantly correlated with the total phenolic and flavonoid contents. The methanol extracts of OFI in summer showed a high level of antimycobacterial activity against M. tuberculosis H37Rv, with a minimum inhibitory concentration of 50 µg ml−1. The results of this study suggest that OFI could be an important source of antioxidants as well as antimycobacterials against M. tuberculosis.
Introduction
The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), including free radical formation, plays a vital role in oxidation, which leads to degenerative and chronic diseases including cancer, diabetes and cardiovascular diseases (Lee et al. Citation2004). Low concentrations of ROS are compatible with normal physiological functions, whereas high concentrations of ROS are considered to be harmful to cells, leading to oxidative stress. A variety of enzymatic and non-enzymatic antioxidant systems has been developed to neutralize ROS (Fernando et al. Citation1992). To prevent the oxidation of proteins, lipids and DNA in tissues, antioxidants may be used to inhibit or delay the oxidation process. The use of currently available synthetic antioxidants in food is restricted, since they are suspected of being carcinogenic.
Tuberculosis (TB) is a communicable disease caused by the bacterium Mycobacterium tuberculosis, which most commonly affects the lungs and is transmitted from person to person via droplets from the throat and lungs of infected people. Worldwide, it is estimated that one-third of the total population is infected with the tubercle bacillus (Dye et al. Citation1999; WHO Citation2009), although only a small percentage of infected individuals will develop clinical TB. The World Health Organization (WHO) estimates that there are approximately 9.4 million new TB cases globally each year and around 1.7 million people died from TB in 2009 (WHO Citation2009).
Numerous antitubercular drugs were discovered between 1940 and 1960, and rifampicin was discovered last, in the late 1960s. These drugs are more efficacious when used in combination, preventing the development of drug resistance. The overuse or misuse of these drugs has led to an increasing prevalence of multidrug-resistant (MDR) strains (Cox et al. Citation2003). Therefore, there is an urgent need to develop new effective agents to reduce the prevalence of MDR-TB. Natural products play a vital role in the drug discovery and development process. Plant-derived compounds are a potential source of new antioxidants and antimycobacterials, among other pharmaceutical compounds (Newman et al. Citation2003).
Opuntia ficus-indica L. (OFI) is a tropical or subtropical plant belonging to the family Cactaceae. The plant grows wild in arid or semi-arid countries and is widely cultivated all over the world. It is native to Mexico, and widely distributed in Central and South America, Australia, South Africa and some parts of Asia (Leo et al. Citation2010). The plant is commonly known as the prickly pear or cactus pear, and less commonly as the Barbary fig or Indian fig. It is mainly used for its fruits, which are eaten after peeling. They are sweet and juicy, and rich in nutritionally valuable compounds, such as ascorbic acid and polyphenols. These fruits have shown antiulcerogenic (Galati et al. Citation2003), antioxidant (Kuti Citation2004), anticancer (Zou et al. Citation2005), neuroprotective (Dok et al. Citation2003), hepatoprotective (Galati et al. Citation2005) and antiproliferative activity (Sreekanth et al. Citation2007). Moreover, the fruits may be used in the treatment of gastritis, hyperglycaemia, arteriosclerosis, diabetes and prostate hypertrophy. In traditional Chinese medicine cactus pear is used against inflammation, pain and snake bite (Zou et al. Citation2005). Different parts of this plant are included in the traditional medicine of several countries: the cladodes are used to reduce serum cholesterol level and blood pressure, and in the treatment of ulcers, rheumatic pain, wounds, fatigue, capillary fragility and liver conditions (Agozzino et al. Citation2005). In addition, Zorgui et al. (Citation2009) showed the potential antigenotoxic activities of cactus cladodes against the mycotoxin zearalenone, a potent estrogenic metabolite.
Although OFI is used to treat various disorders, no study has yet been conducted to examine the in vitro antioxidant activity using multiple assays and antimycobacterial activities. Therefore, the present study investigates the antioxidant and antimycobacterial effects of OFI extracts collected in the rainy and summer seasons. The results should provide a better understanding of this plant for further investigation.
Materials and methods
Chemicals and reagents
Aluminium chloride (AlCl3.6H2O) and gallic acid were purchased from Acros Organics, New Jersey, USA. 2,4,6-Tripyridyl-s-triazine (TPTZ), 1,1-diphenyl,2-picryl hydrazyl (DPPH), ascorbic acid, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), Folin–Ciocalteu phenol reagent, quercetin, methanol, nitroblue tetrazolium (NBT), ferrous sulphate, sodium hydroxide (NaOH), dimethyl sulphoxide (DMSO), salicylic acid, hydrogen peroxide (H2O2), sodium nitroprusside, sulphanilamide, phosphoric acid, N-(1-naphthyl)ethylenediamine dihydrochloride, ethylenediaminetetraacetic acid (EDTA), ferrous sulphate (FeSO4), sodium carbonate (Na2CO3) and sodium nitrite (NaNO2) were purchased from Sigma Chemical Company (Aldrich, St. Louis, MO, USA). All other chemicals and reagents were of analytical grade.
Plant material and extraction
The plant material was collected from the tropical Western Ghat regions of Erode, Tamilnadu, India, in summer (April–May) and rainy (July–August) seasons. It was shade-dried at room temperature, authenticated and deposited (SC 23/559) in the Herbarium of the Laboratory of Botany, Coimbatore, Tamilnadu, India. The OFI collected in the rainy and summer seasons (each 100 g) was mixed in 1 l of methanol [rainy methanol extract (RM), summer methanol extract (SM)] and water [rainy water extract (RW), summer water extract (SW)] separately for 1 day in an automatic shaker. The suspension was filtered using a 300-mesh filter paper (50 mm) (Advantec; Toyo Roshi Kaisah, Tokyo, Japan), and the filtrate was concentrated in a rotary evaporator and lyophilized.
Total phenolic content
The total phenolic content (TPC) of extracts was determined using 400 µl of Folin–Ciocalteu reagent mixed with 100 µl of different solvent extracts in a volumetric flask (Kim et al. Citation2003). The solution was left at 25 °C for 5–10 min, mixed with 0.5 ml of Na2CO3 (1 M) solution, and finally diluted to 10 ml in a volumetric flask with deionized distilled water. Before reading the absorbance at 765 nm, the mixture was held at 25 °C for 2 h. A calibration curve was plotted for gallic acid and TPC was calculated as milligrams of gallic acid equivalents per 100 grams of the dry weight fraction (mg 100 g–1 DW).
Total flavonoid content
A calorimetric assay was used to estimate the total flavonoid content (Park et al. Citation2008). In a 10 ml volumetric flask, an aliquot of 0.1 ml of extracts, distilled water (4 ml), 5% NaNO2 (0.3 ml) and 10% AlCl3.6H2O (0.3 ml) was mixed and left at room temperature for 6 min. After addition of 2 ml of NaOH (1 M), the solution was diluted with 2.4 ml of distilled water. The absorbance of the coloured solution was recorded at 510 nm against a blank (containing all reagents except for the sample) in an ultraviolet spectrophotometer (Agile microplate reader, Piscataway, New Jersey, USA). Quercetin was used for plotting the calibration curve. The total flavonoid content was calculated as milligrams of quercetin equivalents per 100 grams of dried extract (mg 100 g−1 DW).
Antioxidant assays
DPPH assay
The scavenging activity of DPPH was assessed by the scavenging of 2,2-diphenyl-1-picrylhydrazyl radicals (Brand Williams et al. Citation1995). A stock solution of 0.3 mM DPPH was made by dissolving the DPPH in methanol. Then, 100 µl of DPPH solution was added to 100 µl of extracts at varying concentrations (3.125–100 µg ml−1) and shaken vigorously. Absorbance was recorded at 517 nm after incubation for 15 min at room temperature. Water and DPPH were used as controls. The experiment was conducted three times and the results were averaged. The DPPH scavenging activity was calculated by the following equation:
where Abs is absorbance.
Superoxide scavenging assay
The alkaline DMSO method was used to determine the superoxide radical scavenging assay (Elizabeth & Rao Citation1990). In the wells of a microtitre plate, 10 µl of nitroblue tetrazolium (NBT) (1 mg ml−1 prepared in DMSO) was added to 30 µl of different concentrations of extracts or standard. Then, 100 µl of alkaline DMSO (90 µl of DMSO + 10 µl of 5 mM NaOH) was added. The absorbance was measured at 560 nm in a spectrophotometer (Agile microplate reader, USA). DMSO was used as a control instead of sample. The percentage scavenging activity was calculated by the same equation as given for the DPPH assay (see above).
Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity was analysed by the method described previously (Smirnoff & Cumbes Citation1989), with some minor modifications. To 1 ml of different extract concentrations, 300 µl of FeSO4 (8 mM) solution and 250 µl of H2O2 (20 mM) were added and mixed. To initiate the reaction, 250 µl of salicylic acid in ethanol (3 mM) was added. The reaction mixture was allowed to stand for 30 min in a water bath at 37 °C, after which 450 µl of distilled water was added and the mixture was centrifuged at 10,000 rpm for 10 min. The absorbance of the supernatant was measured at 510 nm. Extracting solvent was used as a control instead of sample. The percentage scavenging activity was calculated by the same equation as given for the DPPH assay (see above).
Nitric oxide scavenging assay
The nitric oxide scavenging capacity was measured by the previous method (Sreejayan & Rao Citation1997), with some minor modifications. First, 500 µl of sodium nitroprusside (10 mM) was incubated with 500 µl of phosphate buffer (1 mM) and 30 µl of extracts at 25 °C for 90 min. After incubation, an aliquot of 1 ml solution (1:1) was prepared by mixing the incubated solution with freshly prepared Griess reagent [2% of sulphanilamide in 4% phosphoric acid and 0.2% of N-(1-naphthyl)ethylenediamine dihydrochloride] and the absorbance was measured at 546 nm after 3–5 min. The percentage scavenging activity was calculated by the same equation as given for the DPPH assay (see above).
Hydrogen peroxide scavenging assay
The H2O2 scavenging assay was carried out according to the previous method (Muller Citation1985), with some modifications. First, 80 µl of extracts was mixed with 20 µl of H2O2 (10 mM) in a microtitre plate. Then, 100 µl of phosphate buffer pH 5 (0.1 M) was introduced to the wells. The plate was incubated at 37 °C for 5 min. Finally, 60 µl of ABTS (1.25 mM) prepared with 1 IU ml−1 of peroxidase was mixed and the plates were incubated at 37 °C for 10 min. The absorbance was measured at 405 nm. The percentage scavenging activity was calculated by the same equation as given for the DPPH assay (see above).
Iron chelating assay
The ability of extracts to chelate Fe2+ was determined as described previously (Dinis et al. Citation1994), with some minor modifications. In brief, 0.1 ml of FeSO4 (0.1 mM) was mixed with 0.1 ml of extracts and 0.1 ml of ferrozinc (0.25 mM) in microtitre plates. The mixture was allowed to stand at room temperature for 10 min. The absorbance was measured at 562 nm. EDTA was used as a standard. The percentage chelating activity was calculated by the same equation as given for the DPPH assay (see above).
Ferric reducing antioxidant power assay
To evaluate the reducing power of extracts, the ferric ion reducing antioxidant power (FRAP) assay (Benzie & Strain Citation1996), modified for 96-well microplates, was undertaken. FRAP reagent was prepared by mixing 10 mM of TPTZ in 40 mM HCl, 0.02 M FeCl3 and acetate buffer pH 3.6 at a ratio of 1:1:10, respectively. Following the addition of different concentrations of extracts or standard (10 µl) to 290 µl of FRAP reagent (substituted with distilled water in the blank probe), the absorbance at 593 nm was measured after 6 min. All samples were made in triplicate and the mean values of reducing power were expressed as micrograms of ferrous sulphate equivalents per gram of dry weight, calculated according to the standard calibration curve.
Total antioxidant capacity
The total antioxidant capacity (TAC) was measured using the method described previously (Kambayashi et al. Citation2009). First, 90 µl of phosphate buffer (1 M, pH 7.2) was mixed with 50 µl of myoglobin (0.018 mM), 20 µl of ABTS (3 mM) and 20 µl of different concentrations of sample or standard. After incubation for 3 min at room temperature, 20 µl of H2O2 (0.250 mM) was added and the mixture was again incubated at room temperature for 5 min. The absorbance was measured at 600 nm. To obtain good results, the whole experiment was carried out in cold conditions. Gallic acid was used as a standard and the results were expressed as micrograms of gallic acid equivalents per gram of dried extract (µg g−1 DW).
Antimycobacterial activity
The antimycobacterial activity of all extracts against M. tuberculosis H37Rv (ATCC 27294) strains was tested using the microplate alamar blue assay (MABA) as described previously, with slight modifications (Collins & Franzblau Citation1997; Jimenez Arellanes et al. Citation2003). Before the bioassay, the stock solutions were prepared in DMSO and the working stock solutions were prepared in sterile 7H9 broth. The test was performed in 96-well sterile microplates. The minimal inhibitory concentration (MIC) of four different extracts was tested in duplicate by a two-fold serial dilution method. All wells received 100 µl of supplemented Middlebrook 7H9 broth, then 100 µl of a working solution of OFI (100 µg ml−1) was added to the wells in rows A–H in column 1. Using a multichannel pipette, 100 µl was serially transferred from column 1 to column 2, then further to columns 3 and 4, and the contents of the wells were mixed thoroughly. Finally, 100 µl of excess medium was discarded from the wells in column 4. Next, 100 µl of M. tuberculosis inoculum was added to the wells in rows A–H in columns 1–10. Thus, the wells in columns 5 and 6 served as drug-free controls. Rifampicin (0.09–0.72 µg ml−1, four wells) and isoniazid (0.68–5.44 µg ml−1, four wells) were included as standard controls and tested in columns 7–10. Column 11 received only media to assess background fluorescence.
Final testing concentration ranges were 12.5–100 µg ml−1 and each microplate was incubated for 5 days at 37 °C. Any suspicious growth of other bacteria was checked using blood agar for each test. After the incubation time, one control growth (well A6) was developed with 20 µl of alamar blue solution (Trek Diagnostics, Westlake, OH, USA). The plates were reincubated at 37 °C for 24 h. After this incubation, if the well remained blue, the reagent was added to another control well and the result was read on the following day. If the well turned pink, all the wells in the microplate received alamar blue solution in the same way and were incubated for an additional 24 h, and the colours of all wells were recorded. A blue colour in the well was interpreted as no growth, whereas wells with a well-defined pink colour were scored as positive for growth. The fluorescence units of media-only wells were subtracted from all other wells. The MIC was defined as the lowest concentration of sample that prevented a colour change to pink.
Statistical analysis
All of the experiments were carried out in triplicate. Results were expressed as mean ± standard deviation (SD). Significant differences were assessed by one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test using SPSS software (version 13.0 for Windows; SPSS, Chicago, IL, USA). Pearson's correlation coefficients were calculated between total phenolics and flavonoids and antioxidant activity. In all methods, p < 0.05 was regarded as significant.
Results
Total phenolic and flavonoid contents
The total contents of phenolics and flavonoids were determined for fruit extracts of OFI collected in rainy and summer seasons. For total phenolics, the calibration curve prepared with gallic acid showed a correlation coefficient (r) of 0.998, while for total flavonoids, the calibration curve prepared from quercetin showed an r value of 0.995. The results were expressed as milligrams of gallic acid or quercetin equivalent per 100 g of dried extract and are presented in . Significant amounts of total phenolics and flavonoids were detected in all tested extracts. The total phenolic values of the different extracts ranged from 189.64 ± 1.06 for RW to 300.88 ± 2.2 for SM. As described above, a similar order of total flavonoids was obtained, ranging from 11.81 ± 1.09 for RW to 21.24 ± 1.73 for SM. Overall, the total phenolics and flavonoids were in the order SM > RM > SW > RW.
Table 1. Total phenolics, flavonoids and antioxidant capacity of Opuntia ficus-indica (OFI) fruit extract.
Antioxidant assays
Radical scavenging activities
In the DPPH assay, the scavenging activity of extracts against the stable radical was evaluated at a concentration of 100 µg ml−1 and the results are illustrated in (A). The SM showed the highest percentage inhibition (75%) with the lowest half-maximal inhibitory concentration (IC50) value of 28.71 ± 1.46 µg ml−1, followed by RM (72%; IC50 = 40.41 ± 3.72 µg ml−1), SW (71%; IC50 = 45.1 ± 0.99 µg ml−1) and RW (69%; IC50 = 47.95 ± 1.77 µg ml−1). The standards ascorbic acid (92%) and gallic acid (83%) were used and showed IC50 values of 10.2 ± 0.8 µg ml−1 and 15.9 ± 0.20 µg ml−1, respectively.
Figure 1. Free radical scavenging activity of Opuntia ficus-indica fruit extracts: (A) 2,2-diphenyl-1-picrylhydrazyl (DPPH); (B) superoxide, where 1 = water extract, 2 = methanol extract, 3 = ascorbic acid and 4 = gallic acid at 100 µg ml−1.
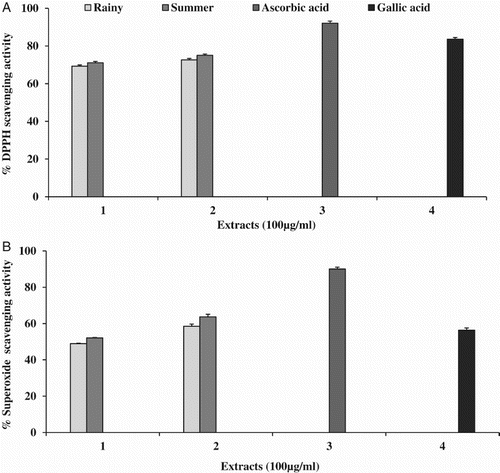
Different extracts of OFI were used for measuring the scavenging activity of superoxide radicals. The highest inhibitory activity was observed in SM (63%), with an IC50 value of 44.17 ± 2.54, followed by RM (58%; IC50 = 69.57 ± 3.70 µg ml−1) and SW (52%; IC50 = 85.44 ± 3.62 µg ml−1) (B). No IC50 value was found in RW, with a maximum scavenging activity of 49%. Ascorbic acid and gallic acid were used as standards and, when investigated under the same conditions, showed percentage inhibition of 90% (IC50 = 8.26 ± 0.70 µg ml−1) and 56% (IC50 = 56.44 ± 1.63 µg ml−1), respectively.
The hydroxyl ion scavenging activity of various extracts (100 µg ml−1) is presented in (A). The best scavenging activity was obtained in SM (60%; IC50 = 65.60 ± 1.91 µg ml−1), followed by SW (55%; IC50 = 78.90 ± 3.08 µg ml−1), RM (54%; IC50 =78.19 ± 1.76 µg −1) and RW (51%; IC50 = 85.89 ± 1.61 µg ml−1). No IC50 was observed in the ascorbic acid standard, with a maximum scavenging activity of 26%, while for gallic acid the maximum inhibition was 82% (IC50 = 60.28 ± 1.09 µg ml−1).
Figure 2. Free radical scavenging activity of Opuntia ficus-indica fruit extracts: (A) hydroxyl; (B) nitric oxide, where 1 = water extract, 2 = methanol extract, 3 = ascorbic acid and 4 = gallic acid at 100 µg ml−1.
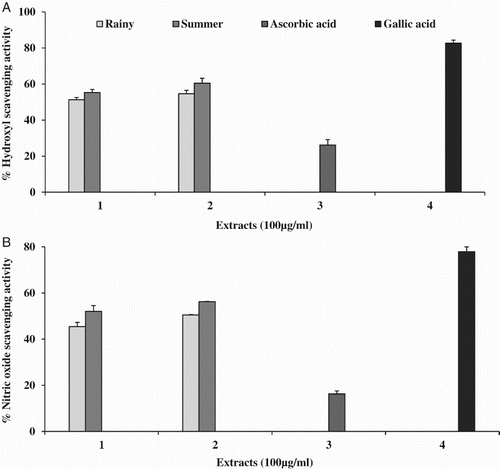
In the present study, SM of the fruits showed the highest nitric oxide scavenging activity (56%; IC50 = 68.28 ± 1.27 µg ml−1) of all the tested extracts, followed by SW (52%; IC50 = 82.04 ± 0.09 µg ml−1) and RM (50%; IC50 = 90 ± 1.60 µg ml−1). RW showed the maximum inhibition of 45% and no IC50 was observed. The standards ascorbic acid (13%) and gallic acid (78%; IC50 = 42.4 ± 2.51 µg ml−1) were used and clearly demonstrated that all extracts used in the study achieved higher percentage inhibition than ascorbic acid. The percentage inhibition results (100 µg ml−1) are shown in (B).
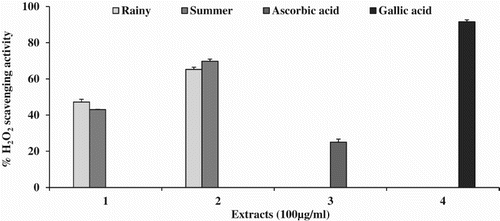
The highest H2O2 scavenging activity was observed in SM (70%; IC50 = 55.37 ± 2.24 µg ml−1), followed by RM (65%; IC50 = 66.56 ± 0.87 µg ml−1). The extracts of RW and SW showed the maximum inhibition of 47% and 43%, respectively, and no IC50 was observed. Gallic acid (91%; IC50 = 2.1 ± 0.93 µg ml−1) and ascorbic acid (25%) were used as standards. The highest percentage inhibition is presented in .
Iron chelating assay
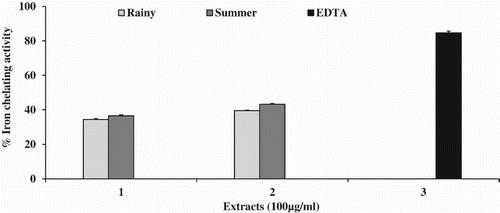
No IC50 was observed in any of the tested extracts. The chelation effect was in the order SM (43%) > RM (39%) > SW (36%) > RW (34%). EDTA was used as a standard and the maximum inhibition of 84% was obtained, with an IC50 value of 61.2 ± 0.79 µg ml−1. The results for iron chelating effects are shown in .
Ferric reducing antioxidant power assay
To determine the antioxidant capacity of the samples, the absorbance values were compared with those obtained from the linear standard curves of FeSO4 (r2 = 0.9980). The antioxidant capacity values were expressed as micrograms of FeSO4 equivalent per gram of extract (µg FeSO4 eq g−1 extract). SM displayed the highest FRAP value (2855.96 ± 32.63), followed by SW (1979.43 ± 29.33), RM (1441.37 ± 29.43) and RW (1268.08 ± 102.77). The results are presented in .
Total antioxidant capacity
The calibration curve was plotted using gallic acid (r2 = 0.995) and the results were expressed as micrograms of gallic acid per gram. The highest antioxidant capacity was found in SM (42.46 ± 0.88), followed by RM (34.05 ± 0.07), SW (32.94 ± 0.62) and RW (29.10 ± 0.86). The results are presented in .
Antimycobacterial activity
The MIC values of OFI extracts used to treat TB are presented in . Out of the four OFI extracts, RM and SM showed good antimycobacterial activity of 100 and 50 µg ml−1, respectively. The activity observed from the extracts of RW and SW shows that these extracts may have antimycobacterial activity higher than the concentration of 100 µg ml−1. Therefore, further studies aimed at isolating the bioactive compounds of OFI are necessary, as the findings indicate that they may be suitable future candidates for TB treatment.
Table 2. Minimum inhibitory concentration (MIC) values of Opuntia ficus-indica (OFI) fruit extracts against Mycobacterium tuberculosis H37Rv.
Correlation between total phenolic/flavonoid contents and antioxidant activities
Pearson's correlations between antioxidant activities determined by different methods and total phenolic and flavonoid contents were evaluated. The results indicate that all the methods had positive correlations, except for TAC. For all extracts, the correlation between total phenolics and total flavonoids was higher with FRAP than with the other methods. Sulaiman et al. (Citation2011) reported the highest correlations between total phenolics and FRAP with extracts of Coriandrum sativum. The highest correlation was found between FRAP and total phenolics and flavonoids (r = 0.999, p < 0.01) with the RW extract. The lowest correlation was found between total phenolics and flavonoids and TAC (r = 0.757, 0.756, respectively) () with the RM extract. These findings suggest that the activity of extracts in these methods may be attributed to the presence of non-phenolic compounds.
Table 3. Pearson's correlation coefficients of total phenolics, total flavonoids and antioxidant methods.
Discussion
Phenolic compounds in plants play a major role in their antioxidant activities. The major secondary metabolites from plants are polyphenols, which are reported to possess antioxidant and free radical scavenging activity. Several studies have shown that polyphenols act as antioxidants by inhibiting free radicals (Silva et al. Citation2004; Obed et al. Citation2011). In this study, the total phenolic and flavonoid contents were higher in OFI extracts collected in summer than in the rainy season. These results are in full agreement with previous reports (Kuti Citation2004; Chaalal et al. Citation2013; Yeddes et al. Citation2013).
DPPH is a free radical that is stable at room temperature and produces a violet solution in ethanol. In this assay, picrylhydrazyl radical (purple colour) is reduced to picrylhydrazine (pale yellow colour) by plant extracts or antioxidant compounds (Blois Citation1958). The colour change or discoloration indicates free radical scavenging activity of the tested sample. The capability of DPPH reduction was determined by the decrease in its absorbance at 517 nm, which is increased by antioxidants. Scavenging of superoxide radical is important because it is one of the precursors of the singlet oxygen and hydroxyl radicals. During oxidation reactions at a cellular level, superoxide radicals are normally formed first, and their effects can be magnified because they produce other kinds of cell-damaging free radicals and oxidizing agents (Marklund & Marklund Citation1974). The hydroxyl radical is an extremely reactive free radical formed in biological systems and has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule found in living cells. This radical has the capacity to join nucleotides in DNA and can cause strand breakage, which contributes to carcinogenesis, mutagenesis and cytotoxicity (Manian et al. Citation2008). The Fenton reaction generates hydroxyl radicals which degrade DNA deoxyribose, using Fe2+ salts as an important catalytic component. Oxygen radicals may attack DNA either at the sugar or at the base, giving rise to a large number of products (Rajeshwar et al. Citation2005). Nitric oxide is a gaseous free radical and is considered an important pleiotropic mediator of physiological processes such as smooth muscle relaxation, immune response, control of vasodilatation and blood pressure, neuronal signalling, inhibition of platelet aggregation and regulation of cell-mediated toxicity (Jagetia et al. Citation2002). H2O2 is a non-free radical and it can inactivate enzymes by the oxidation of thiol groups. It can cross the cell membrane quickly and slowly oxidize the various cell compounds. Hydroxyl radicals are formed by the reaction between H2O2 and the metal ions Fe2+ and Cu2+, and this may be the beginning of all its toxic effects (Bozin et al. Citation2008).
Food is often contaminated by transition metal ions, which may be introduced during processing. Bivalent ferrous ions play an important role as catalysts for oxidative processes, leading to the formation of superoxide radicals and hydroxyl radicals via Fenton reactions. It is well reported that the free radicals generated will cause the production of oxy radicals, lipid peroxidation and DNA damage (Luo et al. Citation2011a). The FRAP assay is commonly used in routine analysis for the evaluation of antioxidant capacity, since it is a rapid, simple, sensitive and reproducible method. The assay is based on the ability of antioxidant compounds to reduce complex ferric (Fe3+) to ferrous (Fe2+) iron. The Fe2+–TPTZ complex gives a blue colour with an absorbance maximum at 593 nm (Benzie & Strain Citation1996). Three TAC tests have been developed, namely TAC I (ABTS.+ generated enzymatically with metmyoglobin and H2O2), TAC II (radical generation with filtration over the MnO2 oxidant) and TAC III [with potassium persulphate (K2S2O8) oxidant]. These tests are totally different from each other and applicable to different solvent media, and their findings for a given antioxidant can vary significantly (Schleiser et al. Citation2002). The advantages of ABTS/TAC are reported to be operational simplicity, reproducibility, diversity and flexible usage in multiple media, since the reagent is soluble in both aqueous and organic solvent media (Awika et al. Citation2003).
In the present study, the antioxidant activities were estimated using five rapid and stable methods. The percentage antioxidant activity of OFI extracts was increased in a concentration-dependent manner, comparing favourably with standards (ascorbic acid and gallic acid) of 100 µg ml−1 in all the tested methods. Moreover, the highest percentage activity was measured in the DPPH scavenging assay, with the lowest IC50 value compared with other radical scavenging assays. In the superoxide scavenging assay, the highest percentage inhibition was obtained compared with standard gallic acid at 100 µg ml−1. These results are in good agreement with previously reported methods in which the DPPH assay had higher percentage inhibition than other assays (Kuti Citation2004; Chaalal et al. Citation2013; Yeddes et al. Citation2013).
Until now, no specific cut-off value has been established for a reference compound to compare with the results of plant extracts in different anti-TB testing methods. In the present study, all the extracts exhibited antimycobacterial activity, but to different extents. The methanolic extract of OFI collected in the summer season showed higher activity, with an MIC value of only 50 µg ml−1. All the other extracts had MIC values of 100 µg ml−1 or higher. In previous reports, plant extracts were considered active against M. tuberculosis if the MIC was ≤ 100 µg ml−1 (Green et al. Citation2010), ≤ 125 µg ml−1 (Luo et al. Citation2011b), ≤ 200 µg ml−1 (Jimenez Arellanes et al. Citation2003), ≤ 500 µg ml−1 (Newton et al. Citation2002), ≤ 1600 µg ml−1 (Mohamed et al. Citation2011), ≤ 2048 µg ml−1 (Tekwu et al. Citation2012) and ≤ 2500 µg ml−1 (Madikizela et al. Citation2014). In summary, the extracts of OFI exhibited good antimycobacterial activity compared with previous reports.
Conclusion
In the present investigation, aqueous and methanol extracts from Opuntia ficus-indica fruits, collected in rainy and summer seasons, displayed antioxidant as well as antimycobacterial activity, thus supporting the traditional use of this plant in the treatment of oxidative disorders and TB. Consequently, the promising activity of the extracts may guide future isolation and antimycobacterial evaluation of the active principles. Further phytochemical and pharmacological studies of this plant are evidently worthwhile and this group is already focusing its efforts in this area.
Acknowledgement
We thank Professor Scott G. Franzblau, Director, Institute for Tuberculosis Research, College of Pharmacy, University of Illinois, Chicago, USA, for providing antimycobacterial activities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This work was supported by the Korea CCS R&D Center, funded by the Ministry of Education, Science and Technology of the Korean Government.
References
- Agozzino P, Avellone G, Caraulo L, Ferrugia M, Flizzola F. 2005. Volatile profile of Sicilian prickly pear (Opuntia ficus-indica) by SPME-GC/MS analysis. Ital J Food Sci. 17:341–348.
- Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. 2003. Screening methods to measure antioxidant activity of Sorghum (Sorghum bicolor) and Sorghum products. J Agric Food Chem. 51:6657–6662. doi: 10.1021/jf034790i
- Benzie IFF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 239:70–76. doi: 10.1006/abio.1996.0292
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200. doi: 10.1038/1811199a0
- Bozin B, Mimica-Duki N, Samojlik I, Goran A, Igic R. 2008. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 111(4):925–929. doi: 10.1016/j.foodchem.2008.04.071
- Brand-Williams W, Cuvelier ME, Berset C. 1995. Use of free radical method to evaluate antioxidant activity. LWT – Food Sci Tech. 28:25–30. doi: 10.1016/S0023-6438(95)80008-5
- Chaalal M, Louaileche H, Touati N, Bey MB. 2013. Phytochemicals, in vitro antioxidant capacity and antiradical potential of whole and ground seeds of three prickly pear varieties: a comparative study. Ind Crops Prod. 49:386–391. doi: 10.1016/j.indcrop.2013.05.010
- Collins L, Franzblau SG. 1997. Microplate alamar blue assay versus BACTEC 460 system for high throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 41:1004–1009.
- Cox H, Hargreaves S, Ismailov G. 2003. Effect of multidrug resistance on global tuberculosis control. Lancet. 362:1858–1859. doi: 10.1016/S0140-6736(03)14918-5
- Dinis TCP, Madeira VMC, Almeida LM. 1994. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 315:161–169. doi: 10.1006/abbi.1994.1485
- Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J. 2003. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus indica var. saboten. Brain Res. 965:130–136. doi: 10.1016/S0006-8993(02)04150-1
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 282:677–686. doi: 10.1001/jama.282.7.677
- Elizabeth K, Rao MNA. 1990. Oxygen radical scavenging activity of curcumin. Int J Pharm. 58:237–240. doi: 10.1016/0378-5173(90)90201-E
- Fernando MR, Nanri H, Yoshitake S, Nagata-Kuno K, Minakami S. 1992. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 209:917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x
- Galati EM, Mondello MR, Giuffrida D, Dugo G, Miceli N, Pergolizzi S, Taviano MF. 2003. Chemical characterization and biological effects of Sicilian Opuntia ficus indica (L.) Mill. fruit juice: antioxidant and antiulcerogenic activity. J Agric Food Chem. 51:4903–4908. doi: 10.1021/jf030123d
- Galati EM, Mondello MR, Lauriano ER, Traviano MF, Galluzzo M, Miceli N. 2005. Opuntia ficus-indica (L.) Miller fruit juice protects liver from carbon tetrachloride induced injury. Phytother Res. 19:796–800. doi: 10.1002/ptr.1741
- Green E, Samie A, Obi CL, Bessong PO, Ndip RN. 2010. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. J Ethnopharmacol. 130:151–157. doi: 10.1016/j.jep.2010.04.033
- Jagetia GC, Baliga MS, Malagi KJ, Kamath MS. 2002. The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to γ-radiation. Phytomedicine. 9:99–108. doi: 10.1078/0944-7113-00095
- Jimenez-Arellanes A, Meckes M, Ramirez R, Torres J, Luna Herrera J. 2003. Activity against multidrug-resistant Mycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytother Res. 17:903–908. doi: 10.1002/ptr.1377
- Kambayashi Y, Binh NT,Asakura HW, Hibino Y, Hitomi Y, Nakamura H, Ogino K. 2009. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J Clin Biochem Nutr. 44:46–51. doi: 10.3164/jcbn.08-162
- Kim DO, Jeong SW, Lee CY. 2003. Antioxidant capacity of phenolic phytochemicals from various cultivars of plum. Food Chem. 81:321–326. doi: 10.1016/S0308-8146(02)00423-5
- Kuti JO. 2004. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 85:527–533. doi: 10.1016/S0308-8146(03)00184-5
- Lee J, Koo N, Min DB. 2004. Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp Rev Food Sci Food Saf. 3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x
- Leo MD, Abreu MBD, Pawlowska AM, Cioni PL, Braca A. 2010. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC-PDA-ESI-MS and GC/EIMS analyses. Phytochem Lett. 3:48–52. doi: 10.1016/j.phytol.2009.11.004
- Luo X, Pires D, Ainsa JA, Gracia B, Mulhovo S, Duarte A, Anes E, Ferreira MJU. 2011a. Antimycobacterial evaluation and preliminary phytochemical investigation of selected medicinal plants traditionally used in Mozambique. J Ethnopharmacol. 137:114–120. doi: 10.1016/j.jep.2011.04.062
- Luo W, Zhao M, Yang B, Ren J, Shen G, Rao G. 2011b. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 126:277–282. doi: 10.1016/j.foodchem.2010.11.018
- Madikizela B, Ndhlala AR, Finnie JF, Van Staden J. 2014. Antimycobacterial, anti-inflammatory and genotoxicity evaluation of plants used for the treatment of tuberculosis and related symptoms in South Africa. J Ethnopharmacol. 153:386–391. doi: 10.1016/j.jep.2014.02.034
- Manian R, Anusuya N, Siddhuraju P, Manian S. 2008. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 107:1000–1007. doi: 10.1016/j.foodchem.2007.09.008
- Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
- Mohamad S, Zin NM, Wahab HA, Ibrahim P, Sulaiman SF, Zahariluddin ASM, Noor SSM. 2011. Antituberculosis potential of some ethnobotanically selected Malaysian plants. J Ethnopharmacol. 133:1021–1026. doi: 10.1016/j.jep.2010.11.037
- Muller HE. 1985. Detection of hydrogen peroxide produced by microorganism on ABTS-peroxidase medium. Zentrabl Bakteriol Mikrobiol Hyg A. 259:151–154.
- Newman DJ, Cragg GM, Snader KM. 2003. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 66:1022–1037. doi: 10.1021/np030096l
- Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. 2002. The evaluation of forty-three plant species for invitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol. 79:57–67. doi: 10.1016/S0378-8741(01)00350-6
- Osorio-Esquivel O, Moreno A-O, Álvarez VB, Dorantes-Álvarez L,Mónica Giusti M. 2011. Phenolics betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res Int. 44:2160–2168. doi: 10.1016/j.foodres.2011.02.011
- Park Y-S, Jung S-T, Kang S-G, Heo BG, Arancibia-Avila P, Toledo F, et al. 2008. Antioxidants and proteins in ethylene treated kiwifruits. Food Chem. 107:640–648. doi: 10.1016/j.foodchem.2007.08.070
- Rajeshwar Y, Kumar GP, Gupta M, Mazumder UK. 2005. Studies on in vitro antioxidant activities of methanol extract of Mucuna pruriens (Fabaceae) seeds. Eur Bull Drug Res. 13:31–39.
- Schleiser K, Harwat M, Bohm V, Bitsh R. 2002. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 36:177–187. doi: 10.1080/10715760290006411
- Silva BM, Andrade PB, Valentao P. 2004. Quince (Cydonia oblonga Miller) fruit (pulp peel and seed) and jam: antioxidant activity. J Agric Food Chem. 52:4705–4712. doi: 10.1021/jf040057v
- Smirnoff N, Cumbes QJ. 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 28:1057–1060. doi: 10.1016/0031-9422(89)80182-7
- Sreejayan N, Rao MNA. 1997. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x
- Sreekanth D, Arunasree MK, Roy KR, Reddy TC, Reddy GV, Reddanna P. 2007. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia cell line-K562. Phytomedicine. 14:739–746. doi: 10.1016/j.phymed.2007.03.017
- Sulaiman SF, Bakar Sajak AA, Ooi KL, Supriatno, Seowa EM. 2011. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J Food Comp Anal. 24:506–515. doi: 10.1016/j.jfca.2011.01.020
- Tekwu EM, Askun T, Kuete V, Nkengfack AE, Nyasse BL, Etoa FX, Beng VP. 2012. Antibacterial activity of selected Cameroonian dietary spices ethno-medically used against trains of Mycobacterium tuberculosis. J Ethnopharmacol. 142:374–382. doi: 10.1016/j.jep.2012.05.003
- WHO. 2009. Global epidemiology control: epidemiology, strategy, financing [M]. Publication no. WHO/HTM/TB/2009, 6–33.
- Yeddes N, Cherif JK, Guyot S, Sotin H, Ayadi M. 2013. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants. 2(1):37–51. doi: 10.3390/antiox2020037
- Zorgui L, Ayed-Boussema I, Ayed Y, Bacha H, Hassen W. 2009. The antigenotoxic activities of cactus (Opuntia ficus-indica) cladodes against the mycotoxin zearalenone in Balb/c mice: prevention of micronuclei, chromosome aberrations and DNA fragmentation. Food Chem Toxicol. 47:662–667. doi: 10.1016/j.fct.2008.12.031
- Zou DM, Brewer M, Garcia F, Feugang JM, Wang J, Zang R, Liu H, Zou C. 2005. Cactus pear: a natural product in cancer chemoprevention. Nutr J. 4:25–36. doi: 10.1186/1475-2891-4-25