Abstract
Common carp (Cyprinus carpio) is considered to be a very important aquaculture species in many Asian and some European countries. It affects the aerobic decomposition of organic matter and nutrient availability in the water column via bioturbation of benthic sediment during feeding on benthic organisms. If the density of common carp is not excessive, an increase in nutrient availability may enhance photosynthesis and plankton production, whereas if it is excessive, it causes dramatic ecological disruption at both the community and ecosystem levels by changing the abiotic properties of the water. Therefore, the density of common carp is a very important factor that has a great effect on the aquatic ecosystem. The critical density of common carp largely depends on its habitat. In polyculture ponds, water quality, natural food resources and fish growth are strongly affected when the density of common carp approaches more than about 1000 kg ha−1. The critical density can be doubled if artificial feed is supplied to the carp. When its preferred food is not sufficient, the common carp switches to less preferred food and changes its behaviour and feeding niche. These factors make common carp a potential candidate not only for monoculture but also for polyculture ponds. This article reviews the role of common carp on the aquatic ecosystem, and the production and behaviour of fish in aquaculture production systems.
Introduction
Common carp (Cyprinus carpio) belongs to the order Cypriniformes and the family Cyprinidae, which is considered the largest family of freshwater fish. It generally inhabits freshwater environments, especially ponds, lakes and rivers, and also rarely inhabits brackish-water environments (Barus et al. Citation2001). It is widely distributed in almost all countries of the world but is very popular in Asia and some European countries (Weber & Brown Citation2011; Kloskowski Citation2011a; Parkos & Wahl Citation2014). Because of its high popularity, its distribution has been widely extended by human introduction. Common carp is the third most frequently introduced species worldwide. It is being considered as a potential candidate for commercial aquaculture in Asia and some European countries as it has a very high adaptive capability to both environment and food (Soltani et al. Citation2010; Manjappa et al. Citation2011; Rahman Citation2015). In some European countries, more than 80% of total fish production comes from common carp (Woynarovich et al. Citation2010; Anton-Pardo et al. Citation2014).
Common carp is frequently called an ‘ecological engineer' because it can modify ecological characteristics of aquatic systems (Matsuzaki et al. Citation2009; Bajer & Sorensen Citation2015; Rahman Citation2015). In some western countries, it is frequently reported as a nuisance fish as it causes dramatic ecological disruption to both the aquatic community and ecosystem. In the USA, common carp is considered as the greatest threat to the biodiversity of wetland and shallow lake ecosystems. Therefore, many studies have been conducted to identify appropriate methods to control common carp populations in wetlands and shallow lakes (Baldry Citation2000; Weber & Brown Citation2009, Citation2011).
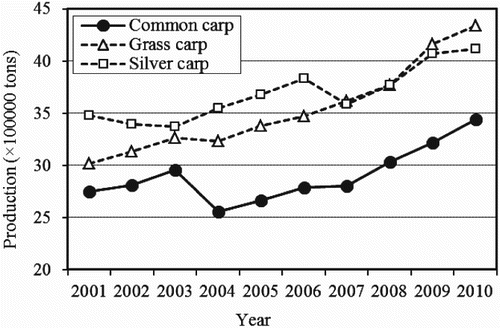
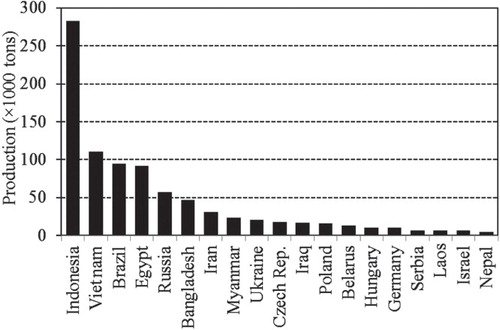
Common carp is the third most widely cultivated and commercially important freshwater fish species in the world (FAO Citation2013). In 2010, it ranked third (grass carp ranked first and silver carp second) in terms of worldwide finfish aquaculture production (), contributing 9% of the world's total finfish aquaculture production, and Asia accounted for more than 90% of common carp's aquaculture production (). China alone contributed 77% (2,462,346 tons) of the world's aquaculture production of common carp (3,216,203 tons) in 2009 (FAO Citation2012). In Asia, common carp is normally cultured in various aquaculture systems but the most common is the semi-intensive pond polyculture system (FAO 2012).
Figure 2. Major common carp-producing countries (except China) and their production in 2010 (FAO Citation2013).
Common carp is a very well-known benthivorous fish that has larger bottom–up effects than other benthivorous fish. The bottom–up effects of common carp mainly depend on the incorporation of benthos-derived nutrients and the release of nutrients from bottom sediment during grazing on benthos. The productivity of freshwater systems is often limited by a lack of soluble phosphorus (PO4-P), which acts as a limiting nutrient for phytoplankton growth. Common carp enhances phytoplankton productivity by releasing nutrients including soluble phosphorus from the sediment. This accelerates the nutrient fluxes to the next trophic levels (Rahman et al. Citation2008a; Rahman Citation2015). This positively influences the production of fish, which depend directly or indirectly on natural food. For example, rohu (Labeo rohita) is a planktivorous fish, which grows better in ponds with common carp than in a monoculture (Rahman et al. Citation2006). Therefore, common carp is commonly reared in polyculture ponds; however, in cold climate regions, especially in Europe, monoculture of common carp is more popular (Szucs et al. Citation2007).
The density of animals is a critical factor that strongly affects the aquatic ecosystem (Soundarapandian & Kannan Citation2008; Rahman et al. Citation2012; Khatune-Jannat et al. Citation2012; Amira et al. Citation2015). Excess densities of common carp have many negative effects on aquatic ecosystems (Rahman et al. Citation2006; Kloskowski Citation2011a; Rahman Citation2015). For example, after reaching a critical density, common carp changes the physical condition of an aquatic system from clear-water conditions dominated by macrophytes to a turbid state with a loss of macrophytes. In polyculture ponds, the optimum density of common carp can improve synergistic effects with other fish, which potentially increase nutrient retention efficiency in fish and decrease nutrient loss in the sediment (Rahman et al. Citation2008a).
This paper reviews the effects of common carp on the aquatic environment and fish production. When food resources are not sufficient, common carp changes its food habits, feeding niche and behaviour. Common carp also influences other fish species to change their food habits and behaviour, which affects their growth and behaviour either positively or negatively. This paper also reviews the food habits, feeding niche and behaviour of common carp in response to changing food resources, based on recently published information.
Effects of common carp on the aquatic environment
The nutrient turnover of the aquatic environment is strongly influenced by the fish population, especially planktivorous and benthivorous fish populations. The majority of nutrients in ponds are stored in bottom sediments, in both organic and inorganic forms. Sediment can store more than 100 times more nutrients than the water column (Rahman & Verdegem Citation2007). The transfer of nutrients back into the water column by the resuspension of sediment can have an important influence on the limnology of ponds. Fish species, size, density, food availability and foraging behaviour (benthic feeding) are important critical factors that greatly affect sediment resuspension. Several species often resuspend bottom sediment; the best known is the common carp, which has strong effects on the aquatic environment owing to its browsing activity for benthic macroinvertebrates in the sediment (Rahman et al. Citation2008a).
Common carp is specialized to browse for benthic macroinvertebrates in the sediment. By doing this, it affects water visibility by resuspending clay particles in the water, cycling of nutrients, and affecting the abundance of phytoplankton, zooplankton and benthic macroinvertebrates (Rahman et al. Citation2008a, Citation2009). Among various benthic macroinvertebrates, chironomid larvae are an important benthic food source for common carp. Depending on species, size and type of sediment, chironomid larvae live up to several centimetres deep in the sediment. Therefore, common carp requires a special technique to pick up chironomids from sediments (Rahman & Verdegem Citation2007). The carp separates benthic macroinvertebrates by digging and sieving of sediments. Its digging activity for browsing benthic macroinvertebrates is effective down to approximately 3 cm (Ritvo et al. Citation2004). During the digging and sieving of sediments, it causes bottom soil resuspension, which increases oxygen availability in the bottom soil. Generally, diffusion is an important mechanism to make oxygen available from the water column into the bottom soil, but this process is very slow and extremely insufficient, especially in natural feed-driven aquaculture systems. However, resuspension by common carp increases aerobic decomposition in the sediment by increasing oxygen availability in the bottom soil. This process accelerates the mineralization of organic matter in the bottom soil. The mineralization of organic matter happens more rapidly under aerobic than under anaerobic conditions (Beristain Citation2005; Rahman et al. Citation2008a, Citation2008b). Therefore, favouring aerobic decomposition of organic matter stimulates nutrient cycling in ponds, which in turn has a large impact on the abiotic and biotic properties of the overlying water column (Rahman et al. Citation2015; Rahman Citation2015). Common carp accelerates the decomposition of organic matter in the following two ways.
Decomposition in the bottom soil: In general, oxygen is mixed in to the bottom soil by diffusion. Wind and mechanically driven water currents mix the water column and bring some oxygen to the bottom layers. However, in most cases, this process is not sufficient (Ritvo et al. Citation2004). Common carp improves the oxidizing conditions in the bottom soil by disturbing the transition zone between the pond bottom soil and the overlying water (Rahman et al. Citation2008a; Yathavamoorthi et al. Citation2010). The transition zone between the pond bottom soil and the overlying water varies greatly under dynamic conditions. The transition between oxygenated overlying water and anaerobic soil takes place along a gradient of a few millimetres. However, disturbance of the bottom soil by common carp raises the depth and extent of oxygen penetration into the soil. The accelerated aerobic conditions due to carp bioturbation leads to increased decomposition and mineralization of organic matter in the bottom soil.
Decomposition in the water column: In general, the upper layer of the pond bottom soil is enriched with organic matter, which cannot be decomposed entirely owing to a lack of oxygen. Resuspension of bottom soil by common carp induces decomposition by exporting the organic matter to the water column, where the concentration of oxygen is generally high (Ritvo et al. Citation2004).
Dissolved oxygen is reduced during aerobic decomposition. Carbon dioxide is also released during decomposition, and contributes to lower pH and reduced alkalinity (Rahman et al. Citation2008c). Resuspension increases the mineralization rate, with increased nitrate nitrogen (NO3-N), total ammonia nitrogen (TAN), total nitrogen (TN) and phosphate phosphorus (PO4-P) and total phosphorus (TP) in the water (). Common carp also substantially accelerates nitrogen and phosphorus transport from the bottom sediment into the water column via excretion (Morgan & Hicks Citation2013). The increased nutrients stimulate photosynthesis, thus increasing phytoplankton biomass in the water column (). Phytoplankton production is limited by low PO4-P concentration in most freshwater ponds and lakes. Resuspension driven by common carp decreases this limitation by increasing P flux from the sediment to the water column (Rahman Citation2015). Zooplankton production rates increase owing to the enhancement of phytoplankton biomass in the presence of common carp.
Table 1. Water quality in systems with various densities of common carp.
Table 2. Total phytoplankton and zooplankton biomass in systems with various densities of common carp.
The density of common carp is a very critical factor for the aquatic environment. A substantial impact on aquatic ecology occurs after exceeding the critical common carp density, which largely depends on the habitat. For example, in shallow unfertile lakes, submerged macrophytes can be significantly affected by common carp when the common carp biomass approaches 200 kg ha−1 (Kloskowski Citation2011a), whereas a fertile lake can support up to 1125 kg ha−1, which is some five times higher than in shallow unfertile lakes (Baldry Citation2000). In the case of polyculture ponds, water quality, abundance of plankton and benthic macroinvertebrates, and fish growth can be strongly affected when the common carp density approaches more than about 1000 kg ha−1 (Rahman et al. Citation2006).
Artificial feeding is another very important factor that influences the critical density of fish (Rahman et al. Citation2008e; Rajkumar et al. Citation2013; Wu at al. Citation2015). The critical density can be doubled if pelleted artificial feed is supplied to common carp. In the presence of artificial feed, common carp shifts its preference from zooplankton and benthic macroinvertebrates to artificial feed (Rahman et al. Citation2006; Rahman & Meyer Citation2009). Common carp grows better in ponds supplied with pelleted artificial feed than with extruded feed and cereals. Pelleted artificial feed acts as a source of nutrients for common carp growth, but it also indirectly maintains ecological stability and controls cyanobacterial blooms in ponds (Ciric et al. Citation2013). Therefore, common carp can be stocked at high density in polyculture ponds supplied with artificial feed without any negative effects on water quality and natural food resources. Rahman et al. (Citation2006) observed 1628 kg ha−1 common carp biomass in ponds supplied with artificial feed without any negative effects on water quality and natural food resources. However, an excess of common carp has several negative effects on pond or lake ecology.
Decreased soluble phosphorus in the water: Excessive resuspension of sediment may increase the redox potential. An increased redox potential may have a positive effect on the precipitation of soluble phosphorus (PO4-P) through the formation of phosphate-rich inorganic particles, e.g. with iron as iron (III) phosphate (Rahman Citation2015). Sometimes, nutrients appear to be abundant in the water but the pond has a relatively low primary productivity of algae owing to a lack of bioavailable phosphorus (PO4-P). Although this pattern may be linked to the complex chemical interactions in water, excess common carp decreases the concentration of PO4-P in the water (Zambrano et al. Citation1999; Rahman et al. Citation2008b).
Increased turbidity of water: A high resuspension of bottom soil increases turbidity, which reduces light penetration (). The biomass of planktivorous fish decreases with increasing turbidity and their resulting inability to locate and feed on plankton. High turbidity also affects the growth of submerged vegetation in shallow lakes and ponds. Common carp may directly consume many types of macrophytes and indirectly uproot or break macrophytes while searching for benthic macroinvertebrates. However, the carp's indirect effects are more severe than its direct effects on aquatic macrophytes. Common carp density has a strong negative linear correlation with vegetation yields. Areas macrophytes exist provide shelter and food for invertebrates and larval fish. Therefore, many invertebrate groups may be reduced in abundance and the invertebrate community composition may be altered if macrophytes are reduced by common carp activity. Biodiversity in the aquatic ecosystem can be severely affected by a high density of common carp (Zambrano et al. Citation2001; Kloskowski Citation2011a).
Decreased system production: High turbidity and low phosphorus concentration reduce photosynthesis. Subsequently, primary, secondary and tertiary production in the aquatic ecosystem is reduced (Rahman et al. Citation2008e, Citation2009). At high density, common carp increase grazing pressure on natural food, especially zooplankton and benthic macroinvertebrates, to such an extent that recovery is not possible. Therefore, the abundance of zooplankton and benthic macroinvertebrates can be strongly suppressed at a high density of common carp. Depending on the environment, there is a threshold in the density of common carp above which zooplankton and benthic macroinvertebrate populations collapse (Rahman et al. Citation2008a; Zambrano et al. Citation2001).
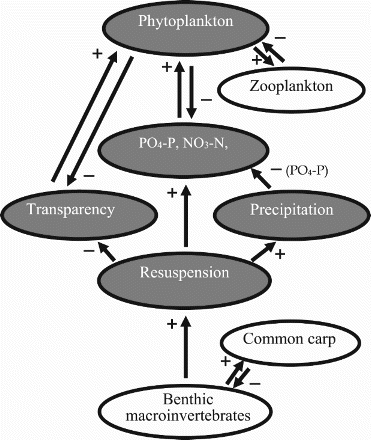
Figure 3. Schematic representation of the effects of common carp on nutrients (PO4-P, PO4-P, NO3-N, NO2-N, total ammonia nitrogen) and natural food availability. + and – indicate positive and negative effects, respectively. Filled ovals indicate processes and states that illustrate the influence of common carp on primary production.
The effects of common carp on environmental conditions are also size dependent. Aquatic environments are differently affected by different ontogenetic stages of common carp (Driver et al. Citation2005; Kloskowski Citation2011b). However, changes in environmental conditions are not linearly related to common carp size. The effect of common carp size on the aquatic environment almost disappears when the fish are more than 1 year old (Kloskowski Citation2011b).
Effects of common carp on fish production
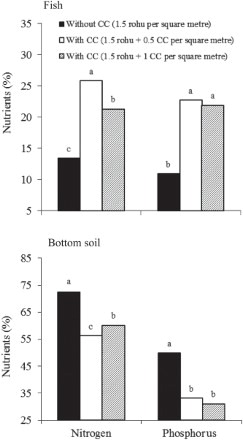
Resuspension of bottom soil by benthivorous fish affects not only the aquatic environment but also the level of fish production. Thus, increasing fish production by the addition of common carp is a common practice in many parts of the world, including Asia and Europe. This technique is very useful for semi-intensive polyculture systems where fish production depends almost entirely on natural food (Mohapatra et al. Citation2007; Rahman Citation2015). The interspecific interactions among fish species are important in the sustenance of any polyculture system (EL-Shebly et al. Citation2007; El-Sherif & Mervat Citation2009). Polyculture systems increase the utilization of the natural foods in fish ponds, by stocking a proper combination of two or more fish species, especially planktivorous and benthivorous fish, at proper densities (Rahman et al. Citation2008e). This strongly influences the nutrient turnover of the pond. For example, when planktivorous fish are kept together with common carp, the ponds generally require 20–40% less fertilizer to maintain adequate natural food levels than those with planktivorous fish in monoculture. Nitrogen and phosphorus retention efficiency in fish biomass increases in polyculture with planktivorous fish and common carp. This results in more nutrients passing through the pond's food web, and fewer nutrients in the bottom soil ().
Figure 4. Comparison of the effect of culture systems with common carp (CC) (polyculture of rohu and common carp) and without CC (monoculture of rohu) on nitrogen and phosphorus accumulation (%) in bottom soil and fish. Nitrogen and phosphorus retention efficiencies with no letters in common among three attached bars are significantly different (p < 0.05). Retention efficiency is calculated based on total input nutrients, higher total nutrient input and total fish (data from Rahman et al. Citation2008a).
Many benthivorous carp species can be used in polyculture to increase the production of planktivorous fish. These include common carp (Rahman et al. Citation2006, Citation2008a), mrigal (Cirrhinus cirrhosus) (Rahman & Verdegem Citation2010), calbasu (Labeo calbasu) (Rahman & Verdegem Citation2010), bream (Abramis brama) (Driver et al. Citation2005) and roach (Rutilus rutilus) (Driver et al. Citation2005). Among these, the best known and most widely used in semi-intensive polyculture is the common carp (Rahman et al. Citation2007; Ciric et al. Citation2013). The common carp has a greater effect than any other benthivorous fish on aquatic ecology and fish production (Wahab et al. Citation2002; Rahman et al. Citation2008c). Wahab et al. (Citation2002) observed 1.6 times higher yield of rohu in the presence common carp than in the presence of mrigal in semi-intensive polyculture ponds. In another study, Rahman et al. (Citation2008c) observed 1.5 times higher growth of rohu in the presence of common carp than in the presence of calbasu. Parkos et al. (Citation2003) observed greater effects on phytoplankton and total phosphorus availability in the presence of common carp than in the presence of channel catfish (Ictalurus punctatus). Common carp (under optimum density) has a strong synergistic effect in terms of producing natural food, which is used as food for other fish living in the water column. Therefore, total fish production is increased in the presence of common carp (). Common carp also influence aquaculture production indirectly by creating favourable conditions at the pond bottom, or by increasing the diffusion of toxic compounds (e.g. organic acids, manganese and sulphur compounds) from the bottom soil upwards to the water column (Ritvo et al. Citation2004; Rahman et al. Citation2008a).
Table 3. Growth and production of fish in systems with various densities of common carp.
Stocking density is important not only for common carp but also for planktivorous fish to obtain high production levels from a polyculture system. Pond productivity or fertility is a very important factor in calculating the optimum stocking density in a polyculture system. The stocking ratios vary widely in different areas, based on the management techniques used (Hossain et al. Citation2009; Khatune-Jannat et al. Citation2012). If the stocking ratio of benthivorous and planktivorous fish is not appropriate, then many negative effects on fish growth and production can be observed. For example, rohu growth can be lower in ponds with a higher density of common carp (1 common carp m−2) than in ponds with lower stocking density (0.5 common carp m−2) (Rahman et al. Citation2006). A similar effect of common carp density on the growth of rohu was also observed in tanks simulating the pond environment (Rahman et al. Citation2008d). In large lakes, common carp biomass is often negatively related to the abundance of other fish, especially bluegill (Lepomis macrochirus), black crappie (Pomoxis nigromaculatus), largemouth bass (Micropterus salmoides), smallmouth bass (Micropterus dolomieu), black bullhead (Ameiurus melas), walleye (Sander vitreus), yellow perch (Perca flavescens), northern pike (Esox lucius) and white bass (Morone chrysops) (Jackson et al. Citation2010; Weber & Brown Citation2011). The most important reason may be habitat degradation by common carp, which increases the turbidity of the water in the lake through its benthic foraging behaviour, switching lakes from the clear- to the turbid-water state (Stewart & Downing Citation2008).
Effects of common carp on food habits, feeding niche and behaviour
Food habits of fish are highly variable and depend on a wide variety of factors, including the species and age of the fish, the availability of preferred food and the combination of fish species (Rajkumar et al. Citation2013; Antony et al. Citation2014; Wu et al. Citation2015). Fish consume different food organisms in different amounts depending on the availability of the food items and the combinations and densities of species (Rahman et al. Citation2009). The proper combination of fish species may result in synergism. For example, strong synergistic effects in terms of food availability, food intake, growth and production of fish can be obtained in rohu ponds with 0.5 common carp per m2. These effects nearly disappear in rohu ponds with 1 common carp per m2 (Rahman et al. Citation2006; Rahman Citation2015). The correct stocking density influences individual food availability, and high densities cause preferred foods to become depleted. This leads common carp to shift its food habits. Common carp preferentially consumes benthic macroinvertebrates. A low abundance of benthic macroinvertebrates or a high stocking density of common carp could cause the carp to switch to its next preferred food items (zooplankton), leading to significant dietary competition with other fish which prefer zooplankton (Rahman et al. Citation2009). However, common carp has excellent adaptive capabilities in the presence of insufficient food. There is evidence that common carp eats the fry of other fish at high density, when there are insufficient other natural foods (Weber & Brown Citation2011). There is also evidence that the common carp predates on crayfish (Cambarellus montezumae) larvae when they live in the same habitat (Hinojosa-Garro & Zambrano Citation2004).
Common carp has mechanisms to maximize fitness not only by shifting food preference but also by modifying its feeding niche and behaviour. Common carp can modify its feeding niche and behaviour in the presence of superior fish, making it a valuable species not only for monoculture but also for polyculture (Rahman et al. Citation2009). When two species forage on the same limited food resources, interspecific competition for food will be intense. In this situation, common carp follows the classical optimal foraging theory, whereby it broadens its feeding niche to maximize its food intake (Rahman & Meyer Citation2009).
Common carp is an omnivorous fish that primarily feeds on benthic macroinvertebrates (chironomids, tubificids) and zooplankton, but the bulk of its diet consists of detritus (Garcia-Berthou Citation2001; Parkos et al. Citation2003; Rahman et al. Citation2009). Therefore, the feeding niche of common carp in natural systems is largely benthic (Rahman et al. Citation2010). Common carp generally ignores phytoplankton, strongly selects benthic macroinvertebrates and weakly selects zooplankton. These results suggest that common carp prefers benthic macroinvertebrates to zooplankton when plankton and benthic macroinvertebrates are provided together. This preference for benthic macroinvertebrates probably influences the behaviour of the common carp, as its spends the majority of its grazing and swimming time near the bottom in an aquatic system (Rahman et al. Citation2008f). When preferred food (benthic macroinvertebrates) becomes depleted, common carp increases its preference for zooplankton, which can be a very dominant food in the absence of benthic macroinvertebrates. The dependency on zooplankton shifts the common carp's feeding niche from the benthic zone to the water column. In this case, common carp spends the majority of its grazing and swimming time in the water column.
The presence of common carp can also alter the behaviour of other fish. Rohu is a planktivorous fish, which spends the majority of its grazing and swimming time in the water column. Rohu grazes more than three times longer in the water column than near the pond bottom. In the presence of common carp, rohu decreases its grazing time in the water column and increases its grazing time near the pond bottom. This effect is greater at higher than at lower densities of common carp. Rohu also increases its time spent actively swimming and searching for food in the presence of common carp (Rahman et al. Citation2008f). Common carp influences the behaviour not only of fish but also of crustaceans. Crayfish generally lives in a habitat where submerged macrophytes (e.g. Potamogeton pectinatus) and algae (e.g. Cladophora glomerata) are available. When submerged macrophytes and algae are affected by the presence of common carp, crayfish not only displaces its habitat but also increases its displacement speed (Hinojosa-Garro & Zambrano Citation2004).
Ontogenetic shifts in dietary preferences of common carp
Ontogenetic shifts in diet are very common in fish. Many factors are responsible for these changes in diet, and can be divided into two categories: external factors (e.g. habitat, food supply, predation risk) and internal factors (e.g. anatomical structures, behaviour, physiological demands). In many species, dietary changes are associated with habitat shifts. Changes in the size of the mouth and oral anatomy may also correspond to ontogenetic dietary shifts. Like many fish, common carp shows ontogenetic shifts in food habits.
Common carp is a benthivorous fish feeding mainly on benthic macroinvertebrates. Although this statement is commonly accepted, it is not true for all common carp. Small common carp are known to feed preferentially on zooplankton, whereas larger common carp avoid zooplankton and concentrate on benthic macroinvertebrates (Adamek et al. Citation2003; Rahman et al. Citation2009). According to Rahman et al. (Citation2009), common carp of up to 15.4 cm total length preferentially select zooplankton, but common carp larger than 18.9 cm total length avoid this food item. Ingestion of benthic macroinvertebrates by common carp increases significantly with its increasing size, but common carp size has no significant effect on phytoplankton consumption. All sizes of common carp avoid phytoplankton (Rahman et al. Citation2009). Overall, the proportion of zooplankton ingestion decreases with increasing common carp size. The diet of common carp is influenced by many factors, including the age of the fish, the availability of natural food and the season.
Effect of common carp on the diel feeding rhythm
Diel activities of most fish species are mainly synchronized with alterations in day and night. Fish can be classified as either diurnal feeders which rely on vision, or nocturnal feeders which rely more on tactile, chemical or electrical senses. However, diel activities are largely species specific. Some fish search for food during both dark and light periods, but are more active during daytime. The grazing behaviour of those fish depends on both light and the availability of food. Common carp is a very active fish, which grazes during both day and night but prefers to graze during daytime (Rahman et al. Citation2008d; Rahman & Meyer Citation2009). It shows the opposite pattern for non-feeding swimming behaviour (). Diel variations in the vertical swimming behaviour of common carp are related to available food types. In the absence of benthic macroinvertebrates, it spends most of its time in the water column, whereas when benthic macroinvertebrates are present, it spends more time near the pond bottom ().
Figure 5. Mean (bar: ± standard deviation) grazing and swimming activity of common carp (data calculated from Rahman et al. Citation2008d).
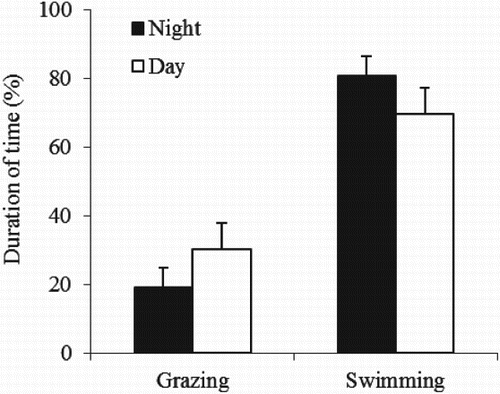
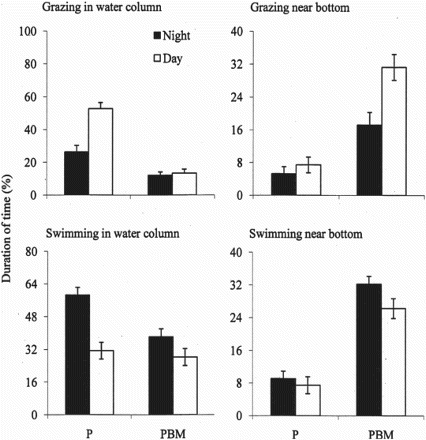
Figure 6. Mean (bar: ± standard deviation) grazing and swimming activity of common carp in simulated ponds with only plankton (P) and simulated ponds with plankton and benthic macroinvertebrates (PBM) (data calculated from Rahman & Meyer Citation2009).
Common carp affects the diel feeding rhythms of other fish (Rahman et al. Citation2008d). For example, the presence and density of common carp affect the diel rhythms of rohu in polyculture ponds. Rohu is a diurnal feeder, spending the majority of its grazing and swimming time in the water column during daytime. In the presence of common carp, the difference between day and night grazing and swimming in the water column by rohu decreases, and the difference between day and night grazing and swimming near the pond bottom increases. This effect is greater at higher than at lower densities of common carp.
Final remarks and future research
Aquaculture of high-value carnivorous fish is rapidly expanding to bridge the gap between supply and demand. Fish from natural food-driven aquaculture receive low priority because of their low demand and slow growth rate, and because they are difficult to culture. However, carnivorous fish consume considerably more fish protein than they produce. According to Naylor et al. (Citation2000), culturing of carnivorous fish uses up to five times more fish protein than is produced. The protein that is not retained by cultured fish causes various environmental problems including eutrophication and disease outbreaks. The major source of aquafeed protein is fishmeal produced from trash fish/low-value marine fish. Therefore, some marine fish stocks may be decreasing with the rapid expansion of carnivorous fish farming. According to the FAO (Citation2005), around 7% of marine fish stocks have been totally depleted, 17% are overexploited and 52% are completely exploited. The expansion of natural food-driven aquaculture with benthivorous and filter-feeding fish may minimize this problem. Filter-feeding fish species depend on natural productivity, which may be enhanced by stocking benthivorous fish such as common carp.
Common carp can be used as a management tool to manipulate the system ecology to achieve high growth and production of filter-feeding fish. However, more research is needed to understand the complex interactions between common carp and locally available filter-feeding fish. Although many studies have focused on the effects of common carp on pond and lake ecology, it is still difficult to separate the biotic and abiotic processes that are affected by common carp because biotic and abiotic processes are strongly interrelated. For example, depredation of phytoplankton grazers (filter-feeding fish) reinforces turbid conditions. Therefore, more research is needed to understand the effects of common carp on biotic and abiotic processes separately.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Adamek Z, Sukop I, Rendon PM, Kouril J. 2003. Food competition between 2+ tench (Tinca tinca L.), common carp (Cyprinus carpio L.) and bigmouth buffalo (Ictiobus cyprinellus Val.) in pond polyculture. J Appl Ichthyol. 19:165–169. doi: 10.1046/j.1439-0426.2003.00467.x
- Amira FS, Rahman MM, Kamaruzzaman Y, Jalal KCA, Hossain MY, Khan NS. 2015. Relative abundance and growth of male and female Nemipterus furcosus population. Sains Malays. (in press).
- Anton-Pardo M, Hlavac D, Masilko J, Hartman P, Adamek Z. 2014. Natural diet of mirror and scaly carp (Cyprinus carpio) phenotypes in earth ponds. Folia Zool. 63:229–237.
- Antony PJ, Rahman MM, Rajkumar M, Kamaruzzaman BY, Khan SA. 2014. Relative growth of Harpiosquilla raphidea (Fabricius, 1798) (Crustacea: Stomatopoda) male and female populations. Sains Malays. 43:1305–1310.
- Bajer PG, Sorensen PW. 2015. Effects of common carp on phosphorus concentrations, water clarity, and vegetation density: a whole system experiment in a thermally stratified lake. Hydrobiologia. 746:303–311. doi: 10.1007/s10750-014-1937-y
- Baldry I. 2000. Effect of Common Carp (Cyprinus carpio) on Aquatic Restorations. University of Minnesota, Department of Horticultural Science. Retrieved from the University of Minnesota Digital Conservancy, http://purl.umn.edu/60111.
- Barus V, Peaz M, Kohlmann K. 2001. Cyprinus carpio (Linnaeus, 1758). In Banarescu PM, Paepke HJ, editor. The freshwater fishes of Europe, v. 5/III; Cyprinidae 2/III, and Gasterosteidae. Germany: AULA-G GmbH Wiebelsheim; p. 85–179.
- Beristain BT. 2005. Organic matter decomposition in simulated aquaculture ponds. PhD thesis, Fish Culture and Fisheries Group. The Netherlands: Department of Animal Science, Wageningen University, 138 pp.
- Ciric M, Subakov-Simic G, Dulic Z, Bjelanovi K, Cicovacki S, Markovic Z. 2013. Effect of supplemental feed type on water quality, plankton and benthos availability and carp (Cyprinus carpio L.) growth in semi-intensive monoculture ponds. Aquacult Res 46:777–788. doi: 10.1111/are.12230
- Driver PD, Closs GP, Koen T. 2005. The effects of size and density of carp (Cyprinus carpio L.) on water quality in an experimental pond. Arch Hydrobiol. 163:117–131. doi: 10.1127/0003-9136/2005/0163-0117
- EL-Shebly AA, El-Kady MAH, Hussin AB, Hossain MY. 2007. Preliminary observations on the pond culture of meagre, Argyrosomus regius (Asso, 1801) (Sciaenidae) in Egypt. J Fish Aquat Sci. 2:345–352. doi: 10.3923/jfas.2007.345.352
- El-Sherif MS, Mervat AMA. 2009. Effect of rearing systems (mono-and poly-culture) on the performance of freshwater prawn (M. rosenbergii) juveniles. J Fish Aquat Sci. 4:117–128. doi: 10.3923/jfas.2009.117.128
- FAO. 2005. FAO Fisheries Report No. 779. Rome. 108 pp.
- FAO. 2012. Fishstate plus: Universal software for fishery statistical time series (available at: http://www.fao.org/fi/statist/fisoft/fishplus.aspwww.fao.org/fi/statist/fisoft/fishplus.asp).
- FAO. 2013. Fishstate plus: Universal software for fishery statistical time series (available at: www.fao.org/fi/statist/fisoft/fishplus.asp).
- Garcia-Berthou E. 2001. Size- and depth-dependent variation in habitat and diet of the common carp (Cyprinus carpio). Aquat Sci. 63:466–476. doi: 10.1007/s00027-001-8045-6
- Hinojosa-Garro D, Zambrano L. 2004. Interactions of common carp (Cyprinus carpio) with benthic crayfish decapods in shallow ponds. Hydrobiologia. 515:115–122. doi: 10.1023/B:HYDR.0000027323.77213.39
- Hossain MY, Rahman MM, Mollah MFA. 2009. Threatened fishes of the world: Pangasius pangasius Hamilton-Buchanan, 1822 (Pangasiidae). Environ Biol Fish. 84:315–316. doi: 10.1007/s10641-008-9422-y
- Jackson ZJ, Quist MC, Downing JA, Larscheid JG. 2010. Common carp (Cyprinus carpio), sport fishes, and water quality: ecological thresholds in agriculturally eutrophic lakes. Lake Reserv Manage. 26:14–22. doi: 10.1080/07438140903500586
- Khatune-Jannat M, Rahman MM, Bashar MA, Hasan MD, Ahamed F, Hossain MY. 2012. Effects of stocking density on survival, growth and production of Thai climbing perch (Anabas testudineus) under fed ponds. Sains Malays. 41:1205–1210.
- Kloskowski J. 2011a. Impact of common carp Cyprinus carpio on aquatic communities: direct trophic effects versus habitat deterioration. Fundam Appl Limnol. 178:245–255. doi: 10.1127/1863-9135/2011/0178-0245
- Kloskowski J. 2011b. Differential effects of age-structured common carp (Cyprinus carpio) stocks on pond invertebrate communities: implications for recreational and wildlife use of farm ponds. Aquacult Int. 19:1151–1164. doi: 10.1007/s10499-011-9435-y
- Lougheed LV, Crosbie B, Chow-Fraser P. 1998. Predictions on the effect of common carp (Cyprinus carpio) exclusion on water quality, zooplankton, and submergent macrophytes in a Great Lakes wetland. Can J Fish Aquat Sci. 55:1189–1197. doi: 10.1139/f97-315
- Manjappa K, Keshavanath P, Gangadhara B. 2011. Influence of sardine oil supplemented fish meal free diets on common carp (Cyprinus carpio) growth, carcass composition and digestive enzyme activity. J Fish Aquat Sci. 6:604–613. doi: 10.3923/jfas.2011.604.613
- Matsuzaki SS, Usio N, Takamura N, Washitani I. 2009. Contrasting impacts of invasive engineers on freshwater ecosystems: an experiment and meta-analysis. Oecologia. 158:673–686. doi: 10.1007/s00442-008-1180-1
- Mohapatra BC, Singh SK, Sarkar B, Majhi D, Sarangi N. 2007. Observation of carp polyculture with giant freshwater prawn in solar heated fish pond. J Fish Aquat Sci. 2:149–155. doi: 10.3923/jfas.2007.149.155
- Morgan DK, Hicks BJ. 2013. A metabolic theory of ecology applied to temperature and mass dependence of N and P excretion by common carp. Hydrobiologia. 705:135–145. doi: 10.1007/s10750-012-1388-2
- Naylor RL, Goldburg RJ, Primavera J, Kautsky N, Beveridge M, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. 2000. Effect of aquaculture on world fish supplies. Nature. 405:1017–1024. doi: 10.1038/35016500
- Parkos JIII, Wahl D. 2014. Effects of common carp (Cyprinus carpio), an exotic fish, on Aquatic Ecosystems. Ilinois Natural History Survey report of January/ February 2000. University of Illinois Board of Trustees, Center for Aquatic Ecology; Victor Santucci, Jr., Max McGraw Wildlife Foundation.
- Parkos JJ, Santucci VJJr., Wahl DH. 2003. Effects of adult common carp (Cyprinus carpio) on multiple trophic levels in shallow mesocosms. Can J Fish Aquat Sci. 60:182–192. doi: 10.1139/f03-011
- Rahman MM. 2015. Effects of co-cultured common carp on nutrients and food web dynamics in rohu aquaculture ponds. Aquacult Environ Interact. 6:223–232. doi: 10.3354/aei00127
- Rahman MM, Hossain MY, Jo Q, Kim SK, Ohtomi J, Meyer C. 2009. Ontogenetic shift in dietary preference and low dietary overlap in rohu (Labeo rohita) and common carp (Cyprinus carpio) in semi-intensive polyculture ponds. Ichthyol Res. 56:28–36. doi: 10.1007/s10228-008-0062-1
- Rahman MM, Jo Q, Gong YG, Miller SA, Hossain MY. 2008c. A comparative study of common carp (Cyprinus carpio L.) and calbasu (Labeo calbasu Hamilton) on bottom soil resuspension, water quality, nutrient accumulations, food intake and growth of fish in simulated rohu (Labeo rohita Hamilton) ponds. Aquaculture. 285:78–83. doi: 10.1016/j.aquaculture.2008.08.002
- Rahman MM, Kadowaki S, Balcombe SR, Wahab MA. 2010. Common carp (Cyprinus carpio L.) alter their feeding niche in response to changing food resources: direct observations in simulated ponds. Ecol Res. 25:303–309. doi: 10.1007/s11284-009-0657-7
- Rahman MM, Kadowaki S, Linn SM, Yohei Y. 2012. Effects of protein skimming on water quality, bacterial abundance and abalone growth in landbased recirculating aquaculture systems. J Fish Aquat Sci. 7:150–161. doi: 10.3923/jfas.2012.150.161
- Rahman MM, Meyer CG. 2009. Effects of food type on diel behaviours of common carp Cyprinus carpio L. in simulated aquaculture pond conditions. J Fish Biol. 74:2269–2278. doi: 10.1111/j.1095-8649.2009.02236.x
- Rahman MM, Normawaty MN, Shahbudin S, Kamaruzzaman Y. 2015. Coastal water quality of Tioman Island: effects of human activity and the distance from shoreline. Desalin Water Treat. (in press), doi: 10.1080/19443994.2015.1006820.
- Rahman MM, Verdegem M. 2010. Effects of intra- and interspecific competition on diet, growth and behaviour of Labeo calbasu (Hamilton) and Cirrhinus cirrhosus (Bloch). Appl Anim Behav Sci. 128:103–108. doi: 10.1016/j.applanim.2010.09.015
- Rahman MM, Verdegem MCJ. 2007. Multi-species fishpond and nutrients balance. In: ven der Zijpp AJ, Verreth AJA, Tri LQ, ven Mensvoort MEF, Bosma RH, Beveridge MCM, editor. Fishponds in farming systems. Wageningen: Wageningen Academic Publishers; p. 79–88.
- Rahman MM, Verdegem MCJ, Nagelkerke LAJ, Wahab MA, Milstein A, Verreth JAJ. 2008a. Effects of common carp Cyprinus carpio (L.) and feed addition in rohu Labeo rohita (Hamilton) ponds on nutrient partitioning among fish, plankton and benthos. Aquacult Res. 39:85–95. doi: 10.1111/j.1365-2109.2007.01877.x
- Rahman MM, Verdegem MCJ, Nagelkerke LAJ, Wahab MA, Verreth JAJ. 2008f. Swimming, grazing and social behaviour of rohu Labeo rohita (Hamilton) and common carp Cyprinus carpio (L.) in tanks under fed and non-fed conditions. Appl Anim Behav Sci. 113:255–264. doi: 10.1016/j.applanim.2007.09.008
- Rahman MM, Verdegem MCJ, Nagelkerke LAJ, Wahab MA, Verreth JAJ. 2008b. Relationships among water quality, food resources, fish diet and fish growth in polyculture ponds: a multivariate approach. Aquaculture. 275:108–115. doi: 10.1016/j.aquaculture.2008.01.027
- Rahman MM, Verdegem MCJ, Nagelkerke LAJ, Wahab MA, Milstein A, Verreth JAJ. 2006. Growth, production and food preference of rohu Labeo rohita (H.) in monoculture and in polyculture with common carp Cyprinus carpio (L.) under fed and non-fed ponds. Aquaculture. 257:359–372. doi: 10.1016/j.aquaculture.2006.03.020
- Rahman MM, Verdegem MCJ, Wahab MA. 2008e. Effects of tilapia (Oreochromis nilotica L.) addition and artificial feeding on water quality, and fish growth and production in rohu-common carp bi-culture ponds. Aquacult Res. 39:1579–1587. doi: 10.1111/j.1365-2109.2008.02007.x
- Rahman MM, Verdegem MCJ, Wahab MA, Hossain MY, Jo Q. 2008d. Effects of day and night on swimming, grazing and social behaviours of rohu Labeo rohita (Hamilton) and common carp Cyprinus carpio (L.) in simulated ponds. Aquacult Res. 39:1383–1392. doi: 10.1111/j.1365-2109.2008.02007.x
- Rahman MM, Wahab MA, Verdegem MCJ. 2007. Common carp increases rohu production in farmers ponds. Stream Journal. 6:14–15.
- Rajkumar M, Rahman MM, Reni Prabha A, Phukan B. 2013. Effect of cholymbi on growth, proximate composition, and digestive enzyme activity of fingerlings of long whiskered catfish, Mystus gulio (Actinopterygii: Siluriformes: Bagridae). Acta Ichthyol Piscat. 43:15–20. doi: 10.3750/AIP2013.43.1.03
- Ritvo G, Kochba M, Avnimelech Y. 2004. The effects of common carp bioturbation on fishpond bottom soil. Aquaculture. 242:345–356. doi: 10.1016/j.aquaculture.2004.09.013
- Sidorkewicj NS, Freije H, López Cazorla AC. 1999. Effects of young common carp (Cyprinus carpio L.) on seston dynamics in experimental culture. Ecol Environ Conserv. 5:35–39.
- Soltani M, Sheikhzadeh N, Ebrahimzadeh-Mousavi HA, Zargar A. 2010. Effects of Zataria multiflora essential oil on innate immune responses of common carp (Cyprinus carpio). J Fish Aquat Sci. 5:191–199. doi: 10.3923/jfas.2010.191.199
- Stewart TW, Downing JA. 2008. Macroinvertebrate communities and environmental conditions in recently constructed wetlands. Wetlands. 28:141–150. doi: 10.1672/06-130.1
- Soundarapandian P, Kannan A. 2008. South Indian technology of nursery farming for better survival and production of Macrobrachium rosenbergii (De Man). J Fish Aquat Sci. 3:137–144. doi: 10.3923/jfas.2008.137.144
- Szucs I, Stundi L, Varadi L. 2007. Carp farming in Central and Eastern Europe and a case study in multifunctional aquaculture. In: Leung PS, Lee CS, O'Bryan PJ, editor. Species and system selection for sustainable aquaculture. Ames: Blackwell Publishing; p. 389–413.
- Wahab MA, Rahman MM, Milstein A. 2002. The effect of common carp, Cyprinus carpio (L.) and mrigal, Cirrhinus mrigala (Hamilton) as bottom feeders in major Indian carp polycultures. Aquacult Res. 33:547–556. doi: 10.1046/j.1365-2109.2002.00654.x
- Weber MJ, Brown ML. 2009. Effects of common carp on aquatic ecosystems 80 years after ‘carp as a dominant’: ecological insights for fisheries management. Rev Fish Sci. 17:524–537. doi: 10.1080/10641260903189243
- Weber MJ, Brown ML. 2011. Relationships among invasive common carp, native fishes, and physicochemical characteristics in upper Midwest (USA) lakes. Ecol Freshw Fish. 20:270–278. doi: 10.1111/j.1600-0633.2011.00493.x
- Woynarovich A, Moth-Poulsen T, Peteri A. 2010. Carp polyculture in Central and Eastern Europe, the Caucasus and Central Asia: a manual. Rome: FAO.
- Wu B, Xia S, Rahman MM, Rajkumar M, Fu Z, Tan J, Yang A. 2015. Substituting seaweed with corn leaf in diet of sea cucumber (Apostichopus japonicus): effects on growth, feed conversion ratio and feed digestibility. Aquaculture. 444:88–92. doi: 10.1016/j.aquaculture.2015.03.026
- Yathavamoorthi R, Surendraraj A, Sabeena Farvin KH. 2010. Enteric bacteria and water quality of freshwater prawn Macrobrachium rosenbergii (De Man) in culture environment from Kerala, India. J Fish Aquat Sci. 5:282–292. doi: 10.3923/jfas.2010.282.292
- Zambrano L, Hinojosa D. 1999. Direct and indirect effects of carp (Cyprinus carpio L.) on macrophyte and benthic communities in experimental shallow ponds in central Mexico. Hydrobiologia. 408/409:131–138. doi: 10.1023/A:1017085129620
- Zambrano L, Perrow MR, Macias-Garcia C, Aguirre-Hidalgo V. 1999. Impact of introduced carp (Cyprinus carpio) in subtropical shallow ponds in Central Mexico. J Aquat Ecosyst Stress Recovery. 6:281–288. doi: 10.1023/A:1009958914016
- Zambrano L, Scheffer M, Martınez-Ramos M. 2001. Catastrophic response of lakes to benthivorous fish introduction. Oikos. 94:344–350. doi: 10.1034/j.1600-0706.2001.940215.x