Abstract
Background: This study aims to tackle the impact of histological subtype on the presentation and treatment trends of renal cell carcinoma (RCC).
Methods: RCC cases, diagnosed from 2001 to 2013, were assembled from the Surveillance, Epidemiology and End Results (SEER) database. The incidence of different RCC histological subtypes (clear cell, papillary, chromophobe and collecting duct) was assessed and Join-Point analysis was conducted. Relevant clinicopathological characteristics were assessed and correlated with subtypes through chi-square testing. Survival analysis was then evaluated using Kaplan–Meier analysis and multivariate analyses for factors affecting overall and cancer-specific survivals were assessed.
Results: A total of 89,968 RCC patients were evaluated. The incidence of different RCC subtypes has increased during the study period. Chromophobe subtype seems to have the best overall and cancer-specific survivals, whilst collecting duct subtype has the worst outcomes (p < .0001 for all endpoints). Multivariate analysis for factors affecting overall survival among non-metastatic RCC patients showed that the following factors are associated with worse overall survival (collecting duct carcinoma histology, older age, T4 disease, N2 disease, no receipt of local treatment, black race, male gender and unmarried status) (p < .0001 for all parameters).
Conclusion: This analysis suggests that the prognosis of RCC varies widely according to the histological subtype. Biology-oriented preclinical and clinical assessments of novel therapeutics are needed particularly for non-clear cell RCC.
Introduction
Kidney cancer is an important global health problem affecting both men and women; according to GLOBOCAN 2012 data, it ranks as the 13th most common cancer in terms of incidence [Citation1]. Kidney cancer may arise from the renal parenchyma (mostly renal cell carcinoma (RCC)) or renal pelvis. Renal pelvic carcinomas are urothelial in origin and share common characteristics with other tumors arising from the urothelium [Citation2].
On the other hand, RCCs can be classified broadly into clear cell and non-clear cell carcinomas [Citation3]; non-clear cell histologies include papillary carcinoma, chromophobe carcinoma and collecting duct carcinoma in addition to some other rarer variants [Citation4,Citation5]. Clear cell carcinoma comprises the majority of RCC cases and account for approximately 70–75% of all cases [Citation6–8].
Treatment algorithms for RCC have been based on the studies incorporating mainly patients with clear cell histology with little representation for the rarer non-clear cell histologies [Citation9,Citation10]. This is particularly apparent in the systemic therapy arena and also in the epidemiology and local treatment arena. Taken into consideration the biological diversity of different subtypes, it is appropriate to study each subtype independently [Citation11].
Given the relative rarity of some RCC subtypes, population-based analyses (e.g. SEER analysis) are very useful to provide an overview about the epidemiological and therapeutic trends for these subtypes [Citation12]. The aim of this study is thus to evaluate the epidemiology, treatment trends and outcomes of different histological subtypes of RCC.
Methods
After obtaining the necessary approvals from the National Cancer Institute – Surveillance, Epidemiology and End Results (SEER) program, the SEER database from 2001 to 2013 was queried using SEER*Stat software version 8.3.2 [Citation13]. Patients with kidney cancer primary were identified using the variable (primary site-labeled: C64.9 kidney) and different RCC histological subtypes were identified using the variable (ICD-O-3 Hist/Behav, malignant) and using codes for the four most common subtypes (codes 8262/3: papillary, 8310/3: clear cell, 8317/3 and 8270/3: chromophobe, 8319/3: collecting duct). Patients with RCC, not otherwise specified (i.e. without definite histological subtype), were not included in this analysis. Sub-classification of papillary histology into types I and II is not available in the SEER database. Sarcomatoid variant was not considered in the current analysis as a separate subtype and was not analysed separately in the current study.
The query was further limited to patients diagnosed 2001–2013 to avoid potential diagnostic confusion among different subtypes before that date.
The incidence of different RCC subtypes with respect to time was assessed using rate session in the SEER*stat program and then trend was assessed using Join-Point program. In this analysis, incidence was calculated as the total count of cases per 100,000.
The baseline characteristics of different histological subtypes were evaluated through case listing session and the following variables have been evaluated: age at diagnosis, gender, marital status, race, RCC histological subtype and AJCC stage at presentation. Moreover, for metastatic patients diagnosed starting from 2010, the site(s) of metastases (brain, bone, liver or lung) were available in the SEER database and was reported. Local treatment modality of the primary tumor was reported including local tumor ablation, partial nephrectomy and total nephrectomy. This has been extracted from the variable “RX Summ Surg primary site”. Additionally, localized radiotherapy was also reported although it was not clear from the SEER database the site of radiotherapy.
Among the study cohort, overall and cancer-specific survivals were evaluated based on the histological subtype as well as local treatment modality using Kaplan–Meier analysis. Through a log-rank test, the survival differences were assessed. Multivariate analyses for factors affecting overall and cancer-specific survival among non-metastatic cases were then assessed using Cox proportional hazards model. p value < .05 was considered as the threshold for statistical significance. The above statistical analyses were conducted using the SPSS version 20.0 (IBM, NY) program.
Moreover among metastatic cases, one and three years relative survivals were assessed across different time periods according to the histological subtypes (using survival session in SEER*stat).
Results
Patients’ characteristics
A total of 89,968 of patients with RCC diagnosed 2001–2013 were identified among this cohort, 68,318 (75.9%) of patients have clear cell RCC, 14,791 (16.4%) of patients have papillary RCC, 6458 (7.1%) of patients have chromophobe RCC and 401 (0.6%) of patients have collecting duct RCC. summarizes baseline characteristics of different RCC histological subtypes. The majority of RCC patients among all histological subtypes are males, white race, married and diagnosed in the age group of 40–69. Most patients present at an earlier stage (stage I/II) except those with collecting duct carcinoma which present more at a more advanced stage. The predominant local treatment modality is total (radical) nephrectomy and radiotherapy has not been used in the majority of cases.
Table 1. Baseline characteristics of renal cell carcinoma (RCC) patients included in the analysis according to the histology (N = 89,968).
Incidence analyses
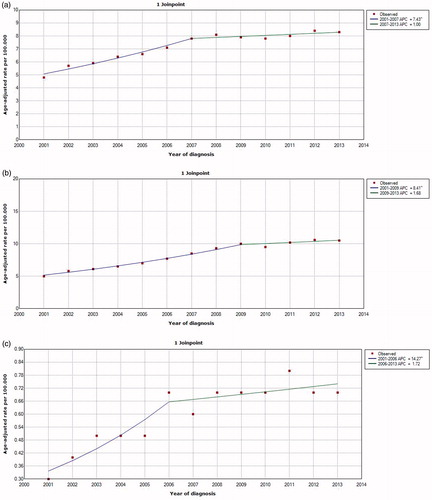
Looking at the Join-Point analyses curves ()), the incidence of the three major histological subtypes (clear cell, papillary and chromophobe) increased during the study period (from 2001–2013). This increase was more marked during the first half of this period compared to the second half. Because of the very small total number of collecting duct carcinoma enrolled, it was not feasible to establish similar Join-Point analysis curve for this subtype too.
Survival outcomes
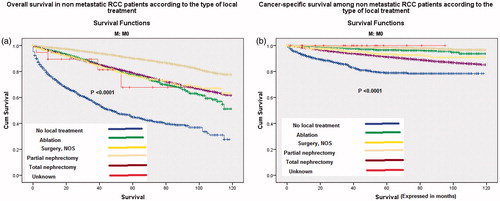
Kaplan–Meier curves of cancer-specific survival and overall survival according to the histological subtype for both non-metastatic (M0) and metastatic (M1) patients are represented in . For both non-metastatic and metastatic cohorts, chromophobe carcinoma has the best overall and cancer-specific survivals while collecting duct carcinoma has the worst overall and cancer-specific survivals (p < .0001 for all scenarios). Moreover, Kaplan–Meier assessment of cancer-specific survival and overall survival among non-metastatic patients according to the type of local treatment showed that partial nephrectomy had the best overall and cancer-specific survival ().
Figure 2. Kaplan–Meier curve according to histological subtype of: (a) overall survival of non-metastatic cases; (b) cancer-specific survival of non-metastatic cases; (c) overall survival of metastatic cases; (d) cancer-specific survival of metastatic cases.
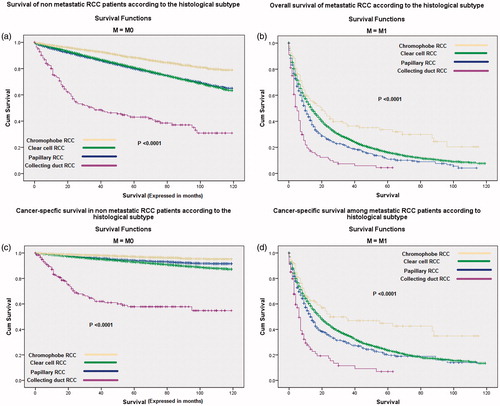
Figure 3. Kaplan–Meier curve of: (a) overall survival and (b) cancer-specific survival according to the type of local treatment of non-metastatic patients.
An additional multivariate analysis for factors affecting overall and cancer-specific survival among M0 RCC patients showed that the following factors are associated with worse overall survival (collecting duct carcinoma histology, older age, T4 disease, N2 disease, no receipt of local treatment, black race, male gender and unmarried status) (p < .0001 for all parameters); while the following factors were associated with worse cancer-specific survival (collecting duct carcinoma histology, older age, T4 disease, N positive disease, no receipt of local treatment and unmarried status) (p < .0001 for all parameters) ().
Table 2. Multivariate analysis of factors affecting overall and cancer-specific survival among non-metastatic RCC patients.
Moreover, an analysis of one and three year relative survival according among metastatic cases according to histological subtype and across different time periods (2001–2006/2007–2013) has been conducted (). The later time period has been correlated to the onset of the use of targeted agents for metastatic RCC. Modest improvement in relative survival has been noted only for clear cell RCC in the second time period; while the three non-clear cell histologies did not show similar improvement.
Table 3. Relative survival of metastatic RCC patients according to histological subtypes.
Discussion
This analysis aims to evaluate the incidence and treatment trends of different histological subtypes of RCC. The results of this analysis suggest that all the four major histological subtypes of RCC (clear cell, papillary, chromophobe and collecting duct) have increased over the past decade. Moreover, chromophobe subtype has the best prognosis while collecting duct subtype has the worst prognosis. The introduction of targeted treatment (starting from 2007) probably contributed to the modest improvement in relative survival of metastatic clear cell carcinomas while it had no effect on metastatic non-clear cell carcinomas.
Incidence analysis revealed an increasing incidence for the major subtypes of RCC across the past decade. This is in line with previous reports and it signifies the importance of further understanding of the pathogenesis of this disease as well as potentially preventable risk factors of each subtype [Citation14,Citation15]. Looking more closely at Join-Point analyses of major histological subtypes (), it seems that the major part of the increase in incidence occurred during the first half of the decade (2001–2007); while the slope almost flattened or increased minimally in the second half (beyond 2007). This point needs further analysis for its potential causes.
In the baseline characteristics of the current analysis, African-American race seems to be more represented in papillary subtype compared to clear cell subtype. Moreover, younger age (<40 years) and women seem to be more represented in chromophobe subtype compared to clear cell subtype. These results are in line with previously published data [Citation16–18].
The majority of patients present with early stage disease with the exception of collecting duct carcinomas which more commonly present with advanced stage. This may be related to the aggressive biological behavior of this histological subtype.
In the multivariate analysis, African-American race was predictive of poorer overall survival, but not cancer-specific survival. This is in line with previously published SEER analyses and it has been explained by racial differences in some co-morbidities such as cardiovascular and chronic renal disease [Citation19,Citation20]. Additionally, women have better overall, but not cancer-specific survival compared to men. This may also be explained by differences in prevalence and/or severity of medical co-morbidities between men and women [Citation21]. Moreover, being married is associated with better overall and cancer-specific survivals. This may be explained by better social support and early access to health care among married patients [Citation22].
The importance of histological subtype in predicting survival has been alluded to in a previous study; and this has suggested – similar to the current analysis – a favorable prognosis for chromophobe histology and worse prognosis for collecting duct histology [Citation23]. However, this previous study was only in the postoperative setting and it was restricted to the duration from 2000–2005. It is to be noted also that the majority of patients present with early stage disease with the exception of collecting duct carcinomas which more commonly present with advanced stage. This may be related to the aggressive biological behavior of this histological subtype.
In the current analysis, partial nephrectomy seems to perform better than total nephrectomy and ablation as a local treatment for localized disease. This is in line with previously published population-based analyses and it has been partially explained by the preservation of renal function with partial nephrectomy [Citation5,Citation24–26]. However, this apparent superiority of partial nephrectomy has been challenged by the possibility of observational selection bias in these retrospective studies [Citation27]; given the fact that patients referred for partial nephrectomy are more likely to be of earlier Tumor Node Metastasis (TNM) stage and/or more geographically favorable location compared to those referred for total nephrectomy and also given the fact that patients referred to ablative treatment are usually medically inoperable because of co-morbidities. Thus, plenty of confounding factors may hinder definite conclusions based solely on retrospective studies.
In the relative survival analysis, targeted therapy seems to modestly contribute to survival improvement among metastatic clear cell RCC, but not among metastatic non-clear cell RCC. The difference between histological types is expected given the fact that the vast majority of randomized studies evaluating targeted therapy have focused on clear cell RCC [Citation28]. Moreover, the non-striking survival improvement is also expected given the fact that most of the newly approved targeted agents have shown an improvement in progression free survival rather than overall survival in their registration trials [Citation29]. Some previous SEER analyses have evaluated the survival for pre-targeted vs. targeted therapy eras of the whole RCC population [Citation30–32]. However, none of them dissected the survival changes according to histology.
An important caveat to be noted is that some cases of clear cell RCC and papillary RCC were likely misclassified in the time frame of the study as clear cell tubulopapillary RCC, mucinous tubular and spindle cell carcinoma, and Xp11.2 RCC, and these misclassified subtypes could have an effect on outcome. Additionally, high grade chromophobe RCCs are not well recognized, also affecting prognosis. Likewise, the study duration crosses changes in WHO histological classifications as well as AJCC staging versions.
Sarcomatoid variants used to be considered previously as a separate histological entity. However, with the updates in histopathological classification of RCC, it has been considered as a variant that may occur with any other RCC subtype [Citation33]. Thus, it was not included in the current analysis as a separate subtype.
The output of this analysis should be interpreted with caution given some weaknesses; most notably the lack of information about performance status and co-morbidities of included patients. Moreover, there is a lack of information about systemic therapy used in metastatic cases. Additionally, the general shortcomings of administrative data (like SEER analyses) are applicable to this study. These include the retrospective nature of data collection and the heterogeneity of histological expertise within different healthcare settings from which these data were extracted.
Given the apparent differences in natural history and prognosis of different histological subtypes of RCC, further research into the distinct biological drivers of different subtypes is mandatory and grouping of all non-clear cell subtypes into a single category should be discouraged. This is particularly relevant with regard to the choice of systemic therapy for advanced disease.
In conclusion, this analysis suggests that the prognosis of RCC varies widely according to the histological subtype. Biology-oriented preclinical and clinical assessments of novel therapeutics are needed particularly for non-clear cell RCC.
Transparency
Declaration of funding
This manuscript was not funded.
Declaration of financial/other relationships
The authors have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article. Journal of Drug Assessment peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- GLOBOCAN. Available from: globocan.iarc.fr [cited 2016 Nov 27 ].
- Abdel-Rahman O. Squamous cell carcinoma of the bladder: a SEER database analysis. Clin Genitourin Cancer. 2017;15(3):e463-e468.
- Delahunt B, Bethwaite PB, Nacey JN. Outcome prediction for renal cell carcinoma: evaluation of prognostic factors for tumours divided according to histological subtype. Pathology. 2007;39:459–465.
- Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48:166–174.
- Abdel-Rahman O. Impact of tumor size on the outcome of patients with small renal cell carcinoma. Expert Rev Anticancer Ther. 2017;17:769–773.
- Su D, Singer EA, Srinivasan R. Molecular pathways in renal cell carcinoma: recent advances in genetics and molecular biology. Curr Opin Oncol. 2015;27:217–223.
- Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in metastatic renal cell carcinoma. Future Oncol. 2017.
- Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37:1469–1489.
- Kokabi N, Xing M, Duszak R, Jr, et al. Sociodemographic disparities in treatment and survival of small localized renal cell carcinoma: surgical resection versus thermal ablation. J Comp Eff Res. 2016;5:441–452.
- Abdel-Rahman O. Risk of subsequent primary kidney cancer after another malignancy: a population-based study. Clin Genitourin Cancer. 2017;15:e747–e754.
- Simone G, Tuderti G, Ferriero M, et al. Papillary type 2 versus clear cell renal cell carcinoma: Survival outcomes. Eur J Surg Oncol. 2016;42:1744–1750.
- Smaldone MC, Egleston B, Hollingsworth JM, et al. Understanding treatment disconnect and mortality trends in renal cell carcinoma using tumor registry data. Med Care. 2016;55:398–404.
- Surveillance, Epidemiology and End Results Program. About the SEER Program. Available from: http://seer.cancer.gov/about [cited 2016 June 25].
- Tyson MD, Humphreys MR, Parker AS, et al. Age-period-cohort analysis of renal cell carcinoma in United States adults. Urology. 2013;82:43–47.
- King SC, Pollack LA, Li J, et al. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol. 2014;191:1665–1670.
- Olshan AF, Kuo TM, Meyer AM, et al. Racial difference in histologic subtype of renal cell carcinoma. Cancer Med. 2013;2:744–749.
- Lee S, Jeon HG, Kwak C, et al. Gender-specific clinicopathological features and survival in patients with renal cell carcinoma (RCC). BJU Int. 2012;110:E28–E33.
- Daugherty M, Blakely S, Shapiro O, et al. Chromophobe renal cell carcinoma is the most common nonclear renal cell carcinoma in young women: results from the SEER database. J Urol. 2016;195:847–851.
- Schwartz K, Ruterbusch JJ, Colt JS, et al. Racial disparities in overall survival among renal cell carcinoma patients with young age and small tumors. Cancer Med.. 2016;5:200–208.
- Patel HD, Kates M, Pierorazio PM, et al. Race and sex disparities in the treatment of older patients with T1a renal cell carcinoma: a comorbidity controlled competing risks model. Urol Oncol. 2014;32:576–583.
- Aron M, Nguyen MM, Stein RJ, et al. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133–140.
- Lai H, Lai S, Krongrad A, et al. The effect of marital status on survival in late-stage cancer patients: an analysis based on surveillance, epidemiology, and end results (SEER) data, in the United States. Int J Behav Med. 1999;6:150–176.
- Keegan KA, Schupp CW, Chamie K, et al. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol. 2012;188:391–397.
- Roos FC, Steffens S, Junker K, et al. Survival advantage of partial over radical nephrectomy in patients presenting with localized renal cell carcinoma. BMC Cancer. 2014;14:372.
- Daugherty M, Bratslavsky G. Compared with radical nephrectomy, nephron-sparing surgery offers a long-term survival advantage in patients between the ages of 20 and 44 years with renal cell carcinomas (≤4 cm): an analysis of the SEER database. Urol Oncol. 2014;32:549–554.
- Hellenthal NJ, Mansour AM, Hayn MH, et al. Renal cell carcinoma in octogenarians: nephron sparing surgery should remain the standard of care. J Urol. 2011;185:415–420.
- Shuch B, Hanley J, Lai J, et al. Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer. 2013;119:2981–2989.
- Abdel-Rahman O, Fouad M. Efficacy and toxicity of sunitinib for non clear cell renal cell carcinoma (RCC): a systematic review of the literature. Crit Rev Oncol Hemat. 2015;94:238–250.
- Keizman D, Ish-Shalom M, Taksey JD, et al. Influence of risk factors for renal cell carcinoma (RCC) on outcome of patients (pts) with metastatic disease treated with sunitinib. J Clin Oncol. 2012;30(5 Suppl):437.
- Li P, Wong YN, Armstrong K, et al. Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer Med. 2016;5:169–181.
- Shah BK, Ghimire KB. Survival trends among patients with advanced renal cell carcinoma in the United States. Urol Int. 2015;94:133–136.
- Vaishampayan U, Vankayala H, Vigneau FD, et al. The effect of targeted therapy on overall survival in advanced renal cancer: a study of the national surveillance epidemiology and end results registry database. Clin Genitourin Cancer. 2014;12:124–129.
- Malouf GG, Ali SM, Wang K, et al. Genomic characterization of renal cell carcinoma with sarcomatoid dedifferentiation pinpoints recurrent genomic alterations. Eur Urol. 2016;70:348–357.