Abstract
Objective: To assess the effectiveness and safety of biosimilar infliximab (CT-P13) in a real-life setting in adults with moderate-to-severe active Crohn’s disease (CD) or ulcerative colitis (UC).
Methods: This multi-centre, observational cohort study was conducted at medical centres in Romania, Czech Republic, and Bulgaria. Effectiveness was measured using the Crohn’s Disease Activity Index (CDAI) for CD or partial Clinical Activity Index (pCAI) for UC. Quality-of-life (QoL) was measured using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ). Safety was assessed according to treatment withdrawals and adverse events (AEs) monitoring. Analyses were performed in the safety population and were reported based on the observed case (OC) or last observation carried forward (LOCF) method.
Results: Altogether, 85 patients with CD (n = 38) or UC (n = 47) received biosimilar infliximab for up to 30 weeks. Most patients (n = 68; 80.0%) had no prior exposure to infliximab. At the end of treatment, 65.8% (95% CI = 49.8–78.9) of CD patients and 55.3% (95% CI = 41.2–68.6) of UC patients showed a clinical response, and 47.4% (95% CI = 32.5–62.7) and 48.9% (95% CI = 35.3–62.8), respectively, were in remission. Statistically significant (p < 0.0001) improvements from baseline were observed in CDAI and pCAI scores (both LOCF). In the combined CD and UC population, SIBDQ was significantly improved (p < 0.0001) from baseline to end of treatment (OC). Two AEs (moderately severe infusion reactions) were judged by investigators to be definitely related to treatment, one of which led to treatment withdrawal.
Conclusion: Results align with those of previous studies demonstrating the effectiveness and safety of biosimilar infliximab in CD and UC.
Introduction
Over the past decade, biologics have significantly changed the management of inflammatory Crohn’s disease (CD)Citation1 and ulcerative colitis (UC)Citation2. However, access to treatment with biologics is frequently limited by their high costCitation3. The introduction of less costly biosimilars may increase patient access to well-established therapies while improving healthcare affordability, which is particularly important in lower GDP countries.
Biosimilar infliximab (CT-P13) was the first monoclonal antibody biosimilar approved in the European Union, and has also received regulatory approval in Australia, Canada, Japan, and the USCitation4. Early clinical trials to demonstrate the therapeutic equivalence of biosimilar infliximab to reference infliximab were conducted in patients with ankylosing spondylitis and rheumatoid arthritisCitation5–8. The results of these studies supported regulatory approval of biosimilar infliximab across all indications for which the parent biologic was approved, including inflammatory bowel disease (IBD)Citation4. Additional observational data are, thus, required to confirm the risk:benefit profile of biosimilar infliximab in patients with IBD under everyday use in clinical practice.
The aim of this study was to evaluate the effectiveness and safety of biosimilar infliximab administered in a real-life setting in adults with active CD or UC in three selected Eastern European countries.
Methods
Study design and inclusion criteria
This multi-centre, observational, non-interventional study was conducted at sites in Romania, the Czech Republic, and Bulgaria. Participating sites were medical centres experienced in the biological treatment of patients with CD and UC. Investigators were selected for participation if they had the available patient population (representative of the target patient population), and were able to conduct the observational study according to applicable legal and regulatory requirements of their respective country.
Eligible patients were adults greater than 18 years of age with moderate-to-severe active CD (not responding to corticosteroids and/or immunosuppressive agents) or moderate-to-severe active UC (inadequate response to conventional therapy) who were eligible for treatment with infliximab and were prescribed biosimilar infliximab.
Exclusion criteria were: known hypersensitivity to infliximab, other murine proteins, or any component of the formulation; moderate-to-severe heart failure (New York Heart Association Class III/IV); presence of fever >38 °C on the day of administration; received a live or live-attenuated vaccine within 8 weeks of screening or were scheduled to receive a live or live-attenuated vaccine before study end (killed vaccines were allowed); received an investigational agent for CD or UC within 5 half-lives or 90 days of screening, whichever was greater; currently receiving biologic therapy indicated for treatment of CD or UC (patients experiencing an inadequate response to previous biologic therapy and switching to biosimilar infliximab were allowed to participate); current history of chronic or active infection with hepatitis B (HBsAg), hepatitis C, or infection with human immunodeficiency virus 1 or 2; current diagnosis of tuberculosis or other severe chronic infection (e.g. sepsis, abscess or opportunistic infection, or invasive fungal infection such as histoplasmosis), or a past diagnosis without sufficient documentation of complete resolution following treatment; and patients who, in the opinion of their general practitioner or investigator, should not participate in the study.
Patients were treated with biosimilar infliximab as per the approved product labelCitation9,Citation10. Patients received biosimilar infliximab at baseline, and at Weeks 2, 6, 12, 14, 22, and 30 (end of treatment). Evaluations were conducted at enrolment (baseline) and at Week 30. In the event of early withdrawal from treatment, the patient’s next routine follow-up visit was the study termination visit and included efficacy evaluations.
The study was approved by the respective national Ethics Committees, and all patients provided written informed consent prior to participation.
Assessment criteria
The primary efficacy endpoint was the percentage of patients with a response to biosimilar infliximab at Week 30. The Crohn’s Disease Activity Index (CDAI) was used to evaluate effectiveness in CD patients. Clinical response was defined as a decrease of >70 points from the baseline CDAI score; clinical remission was defined as a CDAI score of 150 or lessCitation11. The partial version (without endoscopy) of the Clinical Activity Index (pCAI) was used to evaluate effectiveness in UC patients. Clinical response was defined as a decrease of at least 3 points from the baseline pCAI score and an absolute sub-score for bleeding not greater than 1 point; clinical remission was defined as a total pCAI score of 2 points or lowerCitation12,Citation13.
Secondary efficacy endpoints were changes from baseline to Week 30 in mean CDAI and pCAI scores, and change from baseline to Week 30 in quality-of-life (QoL) scores assessed using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ)Citation14.
Safety was assessed according to early withdrawals and adverse events (AEs) monitoring. Adverse events were categorized according to their severity (mild, moderate, or severe) and likely relationship to treatment (unrelated, possibly related, probably related, or definitely related). Treatment-related events were categorized as adverse drug reactions (ADRs).
Statistical analysis
Descriptive statistics were used. Continuous parameters were summarized using mean and standard deviation (SD). Qualitative data were reported as absolute (n) and relative (%) frequency distributions. Paired t-tests were used to compare mean change in disease activity from baseline to end of treatment. A p-value <0.05 was considered statistically significant. Analyses were conducted in the safety population, which included all patients who received at least one dose of biosimilar infliximab. For analyses of disease activity, the last available observation carried forward (LOCF) method was used for patients with no effectiveness measurements at Week 30 due to early termination. For analyses of response and remission, patients who discontinued treatment early were considered to be non-responders/non-remitters. The observed case (OC) method was used for analysis of QoL.
Based on patient enrolment (n = 85), the precision estimate of the 95% confidence interval (CI) for the primary outcome measure (clinical response rate) was ±10.6%. CIs for binomial proportions were calculated using the Agresti-Coull method.
Results
Patient characteristics
Demographics and clinical characteristics of patients are summarized in . A total of 85 patients were enrolled in the study, 38 (44.7%) with CD and 47 (55.3%) with UC. The mean age of the cohort was 40.7 ± 15.0 years and 58.8% of patients were male. The majority of patients with CD (n = 24; 63.2%) or UC (n = 44; 93.6%) were new to infliximab treatment.
Table 1. Baseline demographic and clinical characteristics (safety population).
The median follow-up was 210 (range = 43–282) days. The mean dose of biosimilar infliximab at Week 30 was 5.0 ± 0.6 mg/kg. Most patients (n = 67; 78.8%) received the six scheduled infusions of biosimilar infliximab.
Patient disposition
A total of 67 patients (CD: 27; UC: 40) completed the study as per protocol. Eighteen patients withdrew from biosimilar infliximab treatment prematurely. Of these, three patients discontinued treatment because of AEs (including one patient with a life-threatening infusion reaction), eight patients were withdrawn due to therapeutic failure, four patients were withdrawn due to low baseline CDAI or pCAI scores, two patients were withdrawn at their request, and one patient was lost to follow-up.
Effectiveness
Treatment response is summarized in . At Week 30, the response rate was 65.8% (95% CI = 49.8–78.9) in patients with CD and 55.3% (95% CI = 41.2–68.6) in patients with UC. Corresponding remission rates were 47.4% (95% CI = 32.5–62.7) and 48.9% (95% CI = 35.3–62.8), respectively.
Table 2. Response and remission rates at the end of treatment in patients with Crohn’s disease or ulcerative colitis.
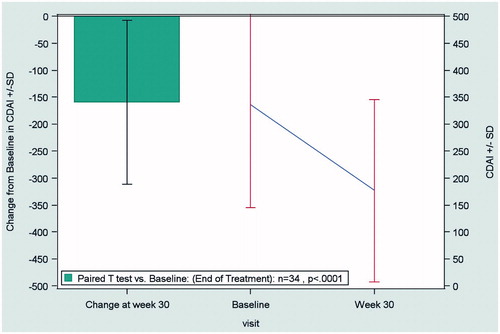
In patients with CD, the mean CDAI score decreased from 359 ± 175 at baseline to 190 ± 170 at the end of treatment (LOCF), indicating a significant improvement (p < 0.0001) in disease activity with biosimilar infliximab ().
Figure 1. Change from baseline to end of treatment (Week 30) in the CDAI score in patients with Crohn’s disease (n = 34), according to the LOCF method. Abbreviations. CDAI, Crohn’s Disease Activity Index; LOCF, last observation carried forward; SD, standard deviation.
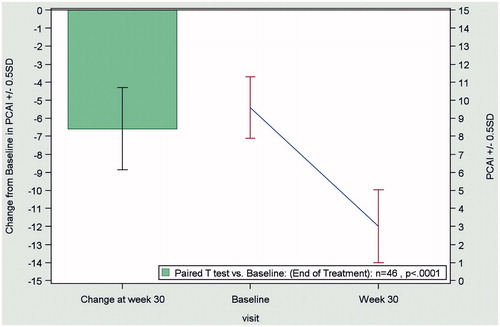
In patients with UC, the mean pCAI score decreased from 9.8 ± 3 at baseline to 3.1 ± 4.0 at the end of treatment (LOCF), indicating a significant improvement (p < 0.0001) in disease activity with biosimilar infliximab ().
Figure 2. Change from baseline to end of treatment (Week 30) in the pCAI score in patients with ulcerative colitis (n = 46), according to the LOCF method. Abbreviations. pCAI, partial version Clinical Activity Index; LOCF, last observation carried forward; SD, standard deviation.
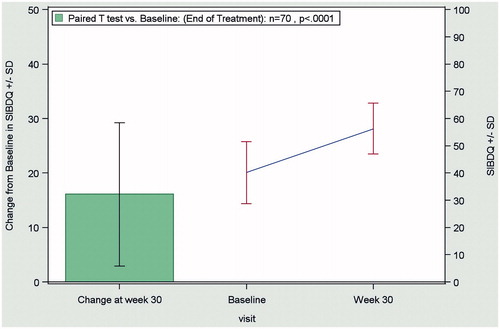
In the combined CD and UC patient population, QoL improved significantly (p < 0.0001) during the observation period. The mean SIBDQ score increased from 40.0 ± 11.3 at baseline to 56.2 ± 9.3 at the end of treatment (OC), as shown in .
Figure 3. Change from baseline to end of treatment (Week 30) in the SIBDQ score in the combined evaluable patient population (CD + UC patients; n = 70), according to the OC method. Abbreviations. CD, Crohn’s disease; OC, observed case; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; UC:,ulcerative colitis.
Safety
A total of 473 infusions of biosimilar infliximab were administered and 31 AEs (6.6%) were recorded. Two events (0.4%) were considered by investigators to be definitely related to treatment. Both events were infusion reactions of moderate severity, one of which led to treatment discontinuation. One AE was considered possibly related to treatment, and the remaining 28 AEs were considered unrelated to treatment with biosimilar infliximab. Of three treatment-related ADRs, two were of moderate and one was of mild intensity.
Discussion
The results of this study show that, in a real-life clinical setting in three selected Eastern European countries, biosimilar infliximab was effective and safe in patients with CD or UC. After 30 weeks’ treatment, 66% of the overall population had a clinical response and 48% were in remission. Highly significant improvements from baseline to Week 30 were observed in disease activity and QoL. Biosimilar infliximab was well tolerated. ADRs with a definite causal relationship with treatment as judged by investigators were limited to two events of moderately severe infusion reactions.
Subsequent to regulatory approval of biosimilar infliximab across all indications, numerous observational studies reported on its effectiveness and safety during use in everyday practice. Komaki et al.Citation15 reviewed 11 observational studies involving 829 patients with CD or UC who were either induced with or switched to biosimilar infliximab. Consistent with our findings, pooled clinical response rates at 24–30 weeks were 77% (CD) and 77% (UC), and pooled clinical remission rates were 60% and 52%, respectively. Gisbert and ChaparroCitation16 reviewed 24 studies in 1326 patients with IBD who had been switched from originator infliximab to biosimilar infliximab. These authors reported a weighted mean disease control rate of 88%, with no increased risks of immunogenicity or other safety concerns. The therapeutic equivalence of biosimilar infliximab observed after switching is not unexpected, as serum concentrations were shown to be non-inferior to those with originator infliximab in patients with CD or UC in clinical remissionCitation17. In the past few years, several other groups have added to the weight of evidence suggesting that biosimilar infliximab is effective and safe as induction or switch therapy in patients with IBDCitation18–22.
Although the number of patients (n = 17) in the current study with previous exposure to anti-TNF agents was too low to conduct a meaningful sub-group analysis, we were interested to compare outcomes in this sub-group with those in the overall population. Among 14 evaluable patients, nine (53%) responded to treatment, of which six (35%) were in remission. Although no data were collected about withdrawal of the initial anti-TNF in these patients, it has been reported that response to a second anti-TNF is better if the reason for switching is intolerance rather than primary or secondary failureCitation23.
Immunogenicity is a recognized risk associated with use of biologic agents in the management of chronic inflammatory disease. The risk has been shown to vary with different biologic/biosimilar agents, including infliximabCitation24. In one study of the use of biosimilar infliximab in 384 patients with IBD in Hungary and the Czech Republic, there were 28 reports of infusion reactions (7.3%). Patients with prior anti-TNF exposure or antidrug antibody positivity were significantly more likely to develop such reactionsCitation25. A similar incidence of infusion reactions was reported in the PROSIT cohort (8.8%, 71 of 810 IBD patients)Citation18. The incidence of infusion reactions in the current study (two of 84 patients, 2.4%) was less than that in these earlier reports, perhaps reflecting the lack of previous exposure to anti-TNFα treatment in the majority (80.0%) of the population.
Potential exists for biosimilar infliximab to generate costs savings sufficient to meet the needs of many more patients with IBD. A budget impact analysis in six European countries, including Romania and the Czech Republic, showed that interchange of medication from infliximab to biosimilar infliximab in patients with CD may result in budgetary savings adequate to treat an estimated 700–1500 additional patientsCitation26. Severs et al.Citation27 designed a stochastic economic model to simulate the introduction of biosimilars for IBD in the Netherlands over a 5-year time horizon. These authors concluded that, while the magnitude of the economic impact would ultimately depend on factors such as local pricing, procurement policies, and the willingness of physicians to switch patients to biosimilars, the potential cost savings would be in the vicinity of 30%. In 2015, annual direct drug cost savings in five European countries through the introduction of biosimilar infliximab were projected to range from 10–30%Citation28.
The study is limited by its observational design, which can introduce selection bias, and by the relatively modest sample size and short observation period. On the other hand, the study provided insight into the real-world management of patients with IBD in a naturalistic setting. Another limitation is the lack of objective efficacy measures such as biomarkers and endoscopy; however, as these evaluations are rarely performed in everyday practice, the amount of available patient data was insufficient to conduct meaningful analyses. Overall, we consider that the close alignment between our findings and those of large pooled analyses strengthens the perception that biosimilar infliximab is an effective and safe alternative to reference infliximab in patients with IBD, and has the potential to fulfil the unmet needs of more patients.
Conclusions
In the real-life setting, biosimilar infliximab administered for 30 weeks improved disease activity and was well tolerated in patients with CD or UC. In addition, biosimilar infliximab had a strong positive impact on patients’ QoL. Together with other studies reporting on the effectiveness and tolerability of biosimilar infliximab in patients with chronic inflammatory conditions including IBD, the weight of evidence suggests that biosimilar infliximab is an effective and cost-effective replacement for originator infliximab.
Transparency
Declaration of funding
This paper was not funded.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
This article was presented as a poster at the 13th Congress of the European Crohn’s and Colitis Organization (ECCO), 14–17 February 2018, in Vienna, Austria.
Acknowledgements
Statistical analysis was provided by Gábor Csukly of BioData Kft, Budapest, Hungary. Medical writing assistance was provided by Kerry Dechant of Content Ed Net (Budapest, Hungary), with funding from Egis Pharmaceuticals PLC (Budapest, Hungary).
References
- Randall CW, Vizuete JA, Martinez N, et al. From historical perspectives to modern therapy: a review of current and future biological treatments for Crohn’s disease. Therap Adv Gastroenterol. 2015;8:143–159.
- Park SC, Jeen YT. Current and emerging biologics for ulcerative colitis. Gut Liver. 2015;9:18–27.
- Stawowczyk E, Kawalec P. Cost-effectiveness of biological treatment of ulcerative colitis – a systematic review. PG. 2017;12:90–97.
- Bonovas S, Peyrin-Biroulet L, Danese S. Clinical development of biologicals and biosimilars - safety concerns. Exp Rev Clinical Pharmacol. 2017;10:567–569.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–1612.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016;18:82.
- Inflectra, INN-infliximab. Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002778/WC500151489.pdf [Accessed 29 August 2018].
- Remsima (infliximab). European Public Assessment Report (EPAR). http://www.ema.europa.eu:80/ema/index.jsp?curl=pages/medicines/human/medicines/002576/human_med_001682.jsp&mid=WC0b01ac058001d124 [Accessed 29 August 2018].
- Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–530.
- Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86.
- Cortot A, Maetz D, Degoutte E, et al. Mesalamine foam enema versus mesalamine liquid enema in active left-sided ulcerative colitis. Am J Gastroenterol. 2008;103:3106–3114.
- Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578.
- Komaki Y, Yamada A, Komaki F, et al. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:1043–1057.
- Gisbert JP, Chaparro M. Switching from an originator anti-TNF to a biosimilar in patients with inflammatory bowel disease: Can it be recommended? A systematic review. Gastroenterol Hepatol. 2018;41:389–405.
- Strik AS, van de Vrie W, Bloemsaat-Minekus JPJ, et al. Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 non-inferiority trial. Lancet Gastroenterol Hepatol. 2018;3:404–412.
- Armuzzi A, Fiorino G, Variola A, et al. The PROSIT cohort of infliximab biosimilar in IBD: a prolonged follow-up on the effectiveness and safety across Italy. Inflamm Bowel Dis. 2019;25:568–579.
- Guerra Veloz MF, Vázquez Morón JM, Belvis Jiménez M, et al. Switching from reference infliximab to CT-P13 in patients with inflammatory bowel disease: results of a multicenter study after 12 months. Rev Esp Enferm Dig. 2018;110:564–570.
- Høivik ML, Buer LCT, Cvancarova M, et al. Switching from originator to biosimilar infliximab – real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol. 2018;53:692–699.
- Kaniewska M, Moniuszko A, Rydzewska G. The efficacy and safety of the biosimilar product (Inflectra(®)) compared to the reference drug (Remicade(®)) in rescue therapy in adult patients with ulcerative colitis. Pg. 2017;12:169–174.
- Ratnakumaran R, To N, Gracie DJ, et al. Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience. Scand J Gastroenterol. 2018;53:700–707.
- Gisbert JP, Marín AC, McNicholl AG, et al. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613–623.
- Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31:299–316.
- Bálint A, Rutka M, Végh Z, et al. Frequency and characteristics of infusion reactions during biosimilar infliximab treatment in inflammatory bowel diseases: results from Central European nationwide cohort. Expert Opin Drug Saf. 2017;16:885–890.
- Brodszky V, Rencz F, Péntek M, et al. A budget impact model for biosimilar infliximab in Crohn's disease in Bulgaria, the Czech Republic, Hungary, Poland, Romania, and Slovakia. Expert Rev Pharmacoecon Outcomes Res. 2016;16:119–125.
- Severs M, Oldenburg B, van Bodegraven AA, et al. The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis. 2017;11:289–296.
- Jha A, Upton A, Dunlop WC, et al. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32:742–756.
