Abstract
Purpose
To describe a standardized protocol of the dexamethasone intravitreal (DEX) implant Ozurdex (Allergan, Dublin, Ireland) performed in a controlled environment surgical cabin (CESC).
Methods
Retrospective and observational study conducted on patients who underwent a DEX implant between May 2011 and June 2019, in a third level University Hospital. The controlled environment surgical cabin (ArcSterile, Imex, Valencia, Spain) used in this study was the MB 20 (2 m width, 1.60 m depth, and 2 m height) with an uninterrupted power system (ARSSAI1) to keep the cabin working for 20 min. The cabin was used in the open mode. A standardized protocol of intravitreal injections in controlled environment surgical cabin was designed.
Results
From May 2011 to February 2015, a total of 454 DEX implants were performed in the operating room, whereas from March 2015 to June 2019, 1054 DEX devices were implanted using the CESC. The mean number of DEX implants/per week was significantly lower in the operating room than in the CESC [2.3 (2.1 to 2.5) versus 3.8 (3.6 to 4.1), mean difference 1.5 (1.2 to 1.8), p < 0.0001]. The incidence of endophthalmitis was similar in the two populations, 0/454 (0.0%; 95% CI 0.0 to 0.81%) and 0/1054 (0.0%; 95% CI 0.0 to 0.35%) in the operating room and in the CESC, respectively.
Conclusions
The CESC may be a good alternative to the conventional operating room for the administration of the intravitreal DEX implant.
Introduction
Diabetic retinopathy (DR) and retinal vein occlusion (RVO) are two leading causes of visual impairment and blindnessCitation1,Citation2. Macular edema (ME) has been identified as the most common cause of vision loss in patients affected by DRCitation2,Citation3 and RVOCitation4,Citation5.
Among currently available treatment options, intravitreal injections, either anti-vascular endothelial growth factor (VEGF) or intravitreal corticosteroids, have become first-choice therapy for DME over the past several yearsCitation6,Citation7.
Despite the good functional and anatomical outcomes obtained with the anti-VEGF therapies, many patients do not adequately respondCitation8–11. The question of whether patients who do not adequately respond to anti-VEGF could benefit from an early change to another therapy has not been fully elucidated. Nevertheless, there is new evidence supporting an early switch to DEX in those patients who did not adequately respond to anti-VEGFCitation12–14.
Dexamethasone intravitreal (DEX) implant has shown to be an effective treatment for DME in clinical and real-life studiesCitation15–20.
The question of whether the DEX implant (Ozurdex®; Allergan, Irvine, CA) may be safely administered outside the operating room remainsCitation21. While in many countries, intravitreal injections are performed at the operating roomCitation22, in the USA and Canada are mainly performed in the officeCitation23. The Vitreo-Retina Spanish Society (SERV) guidelines did not establish any recommendation about the best place for performing the procedure, i.e. office setting, procedure room, operating room, etc.Citation24.
DEX implants should be administered in a space that present enough comfort, both for the patient and for the ophthalmologist, and likewise allow the realization of a sterile techniqueCitation21–26.
The results of a retrospective and observational study, carried out in Spain, which compared the profitability of the controlled environment surgical cabin (CESC) versus the operating room in ophthalmic minor surgical procedures, found that the use of the CESC was associated with an increase of 14% in the number of surgeriesCitation27. Additionally, the cost per hour of the CESC was 30.75€, while the cost per hour of the conventional operating room was 142.78€, which meant a reduction of the 78.5% in the cost per hourCitation27.
This paper aimed to describe a standardized protocol of the DEX implant performed in a controlled environment surgical cabin “The ArcSterile®” and its safety. Additionally, this study also compared the number of DEX implants performed in the operating room with those performed in the CESC.
Methods
Retrospective analysis of a register database of patients who underwent a DEX implant between May 2011 and June 2019, in a third level University Hospital. All the data were collected from the Hospital register database which included patient identification number, age, disease, type of procedure, and serious adverse events.
The study protocol was approved by the ethics committee of La Paz University Hospital (Protocol number HULP: PI-3797), that waived the need of informed consent for this study. All procedures were carried out in accordance with the tenets of the Declaration of Helsinki.
The ArcSterile
The controlled environment surgical cabin (ArcSterile®, Imex, Valencia, Spain) used in this study was the MB 20 (2 m width, 1.60 m depth, and 2 m height) with an uninterrupted power system (ARSSAI1) to keep the cabin working during 20 minCitation28. The ArcSterile® is an aluminum structure cabin, with sliding screens panel and folding front opening polyvinyl door for entry of patients. It recreates the conditions of asepsis and electrical safety of a conventional operating room, allowing the realization of invasive procedures in a safe way, in any hospital room (as long as it has an air-conditioned system)Citation28.
This device was designed for those processes in which it is crucial to have a low level of particles and microorganisms suspended in the air. The ArcSterile® can act as a chamber of controlled indoor air quality for both, surgical procedures and those that require aseptically special conditions, as isolation roomsCitation28.
Regarding its technical design, it includes two columns of impulsion and air filtration to generate the sterile horizontal laminar flow. This device ensures ISO 5 air quality in the operative fieldCitation29, throughout the duration of the surgical process. The level of suspended particles in the air is kept low due to: (1) the ultrafiltration of the air; (2) the laminated characteristics of the air flow; (3) the air renewal; and (4) the positive pressure generated, which prevents outside air penetrate inside the cabinCitation28,Citation29.
Mode of operation
Although the ArcSterile® can be placed in any Hospital room, the room should be previously equipped with an air-conditioned system. The laminar air flow tunnel can be switched to be generated from left to right or vice versa, to fit the work organization of the surgical team and the instrumental location.
The cabin could be used in the “open mode” or in the “close mode”. In this protocol, the cabin was used in the open mode.
Protocol of intravitreal injection pathway
A standardized protocol of intravitreal injections in CESC has been designed. This protocol defines step by step how to optimize the path from the medical retina office, when the doctor decides to set up a new intravitreal injection, until the patient leaves the CESC after the treatmentCitation30.
The work protocol is shown in .
Table 1. Work protocol for intravitreal injections in controlled environment surgical cabin (ArcSterile®).
The protocol of intravitreal injection pathway in the conventional operating room is similar to that of the CESC. The main difference falls to the intervention staff. In the CESC protocol were involved one ophthalmologist, one nurse, and one nurse assistant (recommended), whereas in the conventional operating room were involved the staff of the operating room (one ophthalmologist, one nurse, one nurse assistant, and one orderly) and those of the ophthalmology outpatient care (one nurse and one nurse assistant) ().
Statistical analysis
A standard statistical analysis was performed using MedCalc Statistical Software version 19.0.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019).
Data were evaluated in a masked fashion. The groups were coded and the statistician did not know where the procedure was performed.
Descriptive statistics mean [standard deviation (SD)], mean [95% confidence interval (95% CI)], median (25–75 quartile range), and percentages were used, as needed.
Data were tested for normal distribution using a D’Agostino-Pearson test.
The two-way independent sample t Student test was used to compare the number of DEX implant/per week between the operating room and the CESC.
Categorical variables were compared using a Chi-square test and a Fisher's exact test, as needed.
Results
From 10 May 2011 to 19 February 2015, a total of 454 DEX implants were performed in the operating room, whereas from 10 March 2015 to 25 June 2019, 1054 DEX devices were implanted using the CESC (). In other words, over the course of the study, in the operating room were performed, on average (95% confidence interval, 95% CI), 2.3 (2.1 to 2.5) DEX implants per week, while in the CESC were performed 3.8 (3.6 to 4.1) DEX implants per week, mean difference 1.5 (1.2 to 1.8), p < .0001.
Figure 1. Number of eyes treated with the intravitreal dexamethasone implant Ozurdex. Abbreviations. CESC: controlled environment surgical cabin; OR: operating room. First: Years 2011 and 2015 for the OR and CESC, respectively. Second: Years 2012 and 2016 for the OR and CESC, respectively. Third: Years 2013 and 2017 for the OR and CESC, respectively. Fourth: Years 2014 and 2018 for the OR and CESC, respectively. Fifth: Years 2015 and 2019 for the OR and CESC, respectively. *From 10 May 2011 in the OR and from 4 March 2015 in the CESC. **Until 19 February 2015 in the OR and till 25 June 2019 in the CESC.
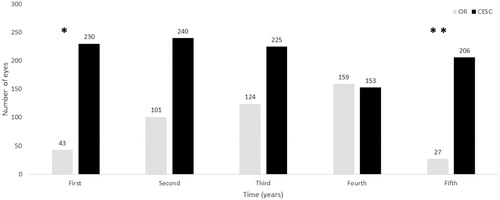
Mean (95% CI) age was 69.4 (68.2 to 70.5) years and 75.1 (74.0 to 76.2 years) for patients underwent DEX implant in the conventional operating room and in the CESC, respectively, p < 0.0001.
The incidence of endophthalmitis was similar in the two populations, 0/454 (0.0%; 95% CI 0.0 to 0.81%) and 0/1054 (0.0%; 95% CI 0.0 to 0.35%) in the operating room and in the CESC, respectively.
Accepting an alfa of 0.05 (bilateral hypothesis contrast), with 454 patients in the operating room group and 1054 patients in the controlled environment surgical cabin group, this analysis had a statistical power of the 75% to detect, as statistically significant, an endophthalmitis incidence of the 0.04% in the operating room group and the 0.075% in the CESC group.
With the exception of one eye, performed in the operating room, that received a DEX implant into the crystalline lens, there were not register serious adverse events. The incidence of minor adverse events was not collected.
Discussion
The CESC ArcSterile® is a tool that speeds up the entire process and allows to handle large volumes of patients with the necessary sterility guaranteesCitation27.
A CESC has been available in our Department since February 2015. As the results of the current study clearly suggested, its introduction in the clinical management of DEX implant administration has entailed a significant increase in the number of procedures, without a loss of the safety conditions. However, it cannot be ruled out that the increase in the number of DEX implants after the introduction of the CESC device was not related to the approval of DEX implant in the EEUU and most of the European countries for the treatment of DME in 2014Citation32. The Spanish National Health System (SNHS) is public, universal, and mostly free of charge for the patients except for the share of out-of-pocket expenditureCitation33. All the different treatment options currently available for DME are covered by the SNHSCitation33. Since there was not any change in the SNHS regulation, reimbursement of the DEX implant was not the reason of the increase in the number of procedures.
As a consequence of economic, social, and demographic changes, with the resulting implications for health care costs, increasing the efficiency and efficacy of health services became relevant to enable their greater profitabilityCitation34,Citation35.
Intravitreal injection is a daily practice in Retina SubspecialtyCitation22,Citation36. Moreover, the introduction of new intravitreal therapies predicts a future with a growing volume in the number of processes, which will suppose that the Health Systems will have to face the challenge of handling increased patient volumeCitation22. This fact forces us to create protocols and clinical pathways that allow us to manage a huge number of patients.
In this aspect, the CESC has shown to be effective for treating a greater number of patients and, additionally, to treat them earlier than with the conventional operating roomCitation27. Our protocol did not significantly differ from that of the European GuidelinesCitation22.
It was previously suggested that the CESC has a positive economic impact of the health systemCitation27. However, that variable was not analyzed in our study. Operating room time is a limited commodity, which should be optimized for those procedures that really need it. Administering intravitreal injections at the operating room would increase time per patient treated, running costs, and overall inconvenience to the patientCitation8,Citation27.
Patients underwent DEX implant in the CESC were significantly older than those performed in the operating room. In the one hand, this finding indicated that the DEX implant are administered to an older population. On the other hand, it also suggested that DEX implant administration can be safely performed in elderly people in a CESC.
Regarding the safety profile, the incidence of endophthalmitis did not significantly differ from those reported in the literatureCitation37–41. Based on the currently available scientific evidence, intravitreal injections performed at the operating room did not have lower rates of endophthalmitis than those performed in the officeCitation21–23,Citation37–41.
The incidence of endophthalmitis after intravitreal injections of anti-VEGF or corticosteroids is lowCitation37–41. In a Retrospective, nationwide multicenter case series conducted in France, which evaluated 316,576 intravitreal injections, the overall incidence of endophthalmitis was 0.021% (2.1 in 10,000 injections) (95% CI, 0.016%−0.026%)Citation37. Interestingly, VanderBeek et al. reported a higher risk of endophthalmitis after intravitreal corticosteroids (rate = 0.13% or 1/778 steroid injections) than after anti-VEGF (rate = 0.019% or 1/5283 anti-VEGF injections)Citation40. However, the incidence of endophthalmitis in the corticosteroid group was much higher than that observed in other studiesCitation37–39,Citation41. Although the number of injections in our study was much lower than that analyzed by other authorsCitation37–41, the incidence of endophthalmitis observed (95% CI 0.0 to 0.35%) was in line with those previously reportedCitation37–41.
Last but not least, some comments should be made about DME diagnosis. Although DME can be detected by stereoscopic slit-lamp examination using a fundus lens, the importance of optical coherence tomography (OCT) in diagnosing and managing DME needs to be emphasizedCitation42.
OCT was originally developed using time-domain acquisition of imagesCitation43. New OCT devices using spectral-domain acquisition of images (SD-OCT) and swept-source OCT (SS-OCT) have been developed. SD-OCT and SS-OCT provide faster acquisition of images, denser sampling of the macula, and better imaging of the choroid and outer retinaCitation44–47.
However, due to they use different segmentation algorithms for defining retinal layers, normal value for SD-OCT and SS-OCT differ, and measurements are not interconvertible across instruments made by different companiesCitation45,Citation46,Citation48.
This study has some limitations that should be taken into consideration when interpreting its results. First, its retrospective design. Bias and potential pitfalls are inherent to retrospective studies. Second, the results were obtained from a register database with limited information, which supposed that nonserious adverse events could not be analyzed. Third, it was a single-center study that adopted a new protocol and clinical pathway, so our results may be not exportable to other Hospitals. The fourth limitation is the lack of sample size calculation before to start the study. Although it was not originally planned, we have calculated the statistical power for the endophthalmitis rate comparison. We are aware that this was post-hoc analysis; nevertheless, it may provide a valuable information.
Despite these limitations, the controlled environment surgical cabin ArcSterile® may be a good alternative to the conventional operating room for the administration of the intravitreal dexamethasone implant Ozurdex®. The CESC is a tool that dynamizes the entire process and can handle large volumes of patients with the necessary guarantees of sterility. Finally, the CESC might allow to reserve the conventional operating room for patients who need a more complex surgery, optimizing the use of operating room
Further studies are needed, especially multicenter prospective trials, that evaluate the role of the CESC on clinical outcomes and health economics.
Transparency
Declaration of funding
Medical writing and editorial assistant services have been provided by Ciencia y Deporte S.L. and covered by a Grant from Allergan. Support for this assistance was funded by Allergan S.A. Medical writing services have been provided by Allergan. Allergan did not participate in either data analysis or redaction of the manuscript.
Declaration of financial/other relationships
MdPCB has received a grant from Allergan during the conduct of the study. None of the coauthors or peer reviewers of this manuscript have any competing interests to declare.
Author contributions
MdPCB: Conception and design of the work, interpretation of the data, and revision of the work; FAM: Conception and design of the work, interpretation of the data, and revision of the work; GAS: Acquisition and interpretation of data for the work; JCR: Acquisition and interpretation of data for the work; ODAM: Acquisition and interpretation of data for the work; IdlRP: Acquisition and interpretation of data for the work; BMM: Acquisition and interpretation of data for the work; JGM: Acquisition and interpretation of data for the work; MAD: Acquisition and interpretation of data for the work; GCA: Acquisition and interpretation of data for the work.
Acknowledgements
The authors want to thank Guillermo Soria-Tristán for his collaboration with the management of the Register Database.
References
- Klein R, Moss SE, Meuer SM, et al. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513–518.
- Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4):346–354.
- Bandello F, Battaglia Parodi M, Lanzetta P, et al. Diabetic macular edema. Dev Ophthalmol. 2017;58:102–138.
- Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56(4):281–299.
- Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8(9):1886–1894.
- Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222.
- Coscas G, Loewenstein A, Augustin A, et al. Management of retinal vein occlusion–consensus document. Ophthalmologica. 2011;226(1):4–28.
- Bressler SB, Ayala AR, Bressler NM, et al.; for the Diabetic Retinopathy Clinical Research Network. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134(3):278–285.
- Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema:analysis of protocol i data. Am J Ophthalmol. 2016;172:72–79.
- Bressler NM, Beaulieu WT, Glassman AR, et al. Diabetic Retinopathy Clinical Research Network. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257–269.
- Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to anti vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of protocol i study data. Retina. 2019;39(1):88–97.
- Busch C, Zur D, Fraser-Bell S, et al.; for the International Retina Group. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55(8):789–796.
- Hernández Martínez A, Pereira Delgado E, Silva Silva G, et al. Early versus late switch: how long should we extend the anti-vascular endothelial growth factor therapy in unresponsive diabetic macular edema patients? Eur J Ophthalmol. 2019. doi:10.1159/000506312 [Epub ahead of print].
- Demir G, Ozkaya A, Yuksel E, et al. Early and late switch from ranibizumab to an intravitreal dexamethasone implant in patients with diabetic macular edema in the event of a poor anatomical response. Clin Drug Investig. 2020;40(2):119–128.
- Iglicki M, Zur D, Busch C, et al. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR-Pro-DEX study’. Acta Diabetol. 2018;55(6):541–547.
- Iglicki M, Busch C, Zur D, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: the International Retina Group real-life 24-month multicenter study. The IRGREL-DEX study. Retina. 2019;39(1):44–51.
- Mello Filho P, Andrade G, Maia A, et al. Effectiveness and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: a real-world experience. Ophthalmologica. 2019;241(1):9–16.
- Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236.
- Iglicki M, Zur D, Fung A, et al.; for the International Retina Group (IRG). TRActional DIabetic reTInal detachment surgery with co-adjuvant intravitreal dexamethasONe implant: the TRADITION STUDY. Acta Diabetol. 2019;56(10):1141–1147.
- Zur D, Iglicki M, Sala-Puigdollers A, et al.; International Retina Group (IRG). Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2019;
- Meyers L, Almeida D, Abràmoff MD. Intravitreal injection technique. A primer for ophthalmology residents and fellows; [cited 2019 Nov 25]. Available from: https://webeye.ophth.uiowa.edu/eyeforum/tutorials/intravitreal-injection/index.htm
- Grzybowski A, Told R, Sacu S, et al. 2018 update on intravitreal injections: Euretina Expert Consensus Recommendations. Ophthalmologica. 2018;239(4):181–193.
- Xing L, Dorrepaal SJ, Gale J. Survey of intravitreal injection techniques and treatment protocols among retina specialists in Canada. Can J Ophthalmol. 2014;49(3):261–266.
- Arias-Barquet L, Basauri-Rementeria E, Gómez-Ulla d I F. Serv clinical practice guidelines: management of intravitreal injections; [cited 2019 Nov 26]. Available from: https://serv.es/wp-content/descargasWP/documentacionMedica/Guia_SERV_02_primeraRevision.pdf
- Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24(Suppl):S3–S19.
- Pilli S, Kotsolis A, Spaide RF, et al. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol. 2008;145(5):879–882.
- Fenoll MA. [Arcsterile: a cost-effective luxury]. Rev Enferm. 2015;38(5):8–12.
- Dossier of ArcSterile; [cited 2019 Nov 26]. Available from: https://www.imex.es/wp-content/uploads/2015/12/DOSSIER-Arc-Sterile-eng_1.pdf
- Authors not listed. International Standard. Cleanrooms and associated controlled environments; [cited 2019 Nov 26]. Available from: http://zoser.com.co/wp-content/uploads/2015/10/ISO%2014644-1%20Version%202015.pdf
- Cidad-Betegón P, Armadá-Maresca F, De la Rosa-Pérez I, et al. Arcsterile: intravitreal injections protocol. 10th Annual Congress on Controversies in Ophthalmology: Europe (COPHy EU); [cited 2020 Feb 7]. Available from: https://www.comtecmed.com/cophy/2019/Uploads/Editor/Docs/15.pdf
- Guigou S, Pommier S, Meyer F, et al. Efficacy and safety of intravitreal dexamethasone implant in patients with diabetic macular edema. Ophthalmologica. 2015;233(3–4):169–175.
- Boyer DS, Yoon YH, Belfort R, Jr, et al., Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914.
- Spanish national Health System; [cited 2020 Feb 7]. Available from: https://www.mscbs.gob.es/organizacion/sns/libroSNS.htm
- Barkun JS. Waiting lists for surgery. Can J Surg. 2002;45(5):328–329.
- Waddell JP. Wait times and health care resources. Can J Surg. 2004;47(6):405–407.
- Lai TY, Liu S, Das S, et al. Intravitreal injection – technique and safety. Asia Pac J Ophthalmol (Phila). 2015;4(6):321–328.
- Dossarps D, Bron AM, Koehrer P, et al.; FRCR net (FRenCh Retina specialists net). Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am J Ophthalmol. 2015;160(1):17–25.e1.
- Stem MS, Todorich B, Yonekawa Y, et al. Incidence and visual outcomes of culture-proven endophthalmitis following dexamethasone intravitreal implant. JAMA Ophthalmol. 2017;135(4):379–382.
- Rajesh B, Zarranz-Ventura J, Fung AT, et al.; International Ozurdex Study Group. Safety of 6000 intravitreal dexamethasone implants. Br J Ophthalmol. 2020;104(1):39–46.
- VanderBeek BL, Bonaffini SG, Ma L. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology. 2015;122(11):2311–2315.e1.
- Freiberg FJ, Brynskov T, Munk MR, et al. Low endophthalmitis rates after intravitreal anti-vascular endothelial growth factor injections in an operation room: a retrospective multicenter study. Retina. 2017;37(12):2341–2346.
- Bandello F, Cicinelli MV. 19th EURETINA congress keynote lecture: diabetic retinopathy today. Ophthalmologica. 2020. doi:10.1159/000506312 [Epub ahead of print].
- Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113(8):1019–1029.
- Forooghian F, Cukras C, Meyerle CB, et al. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Investig Ophthalmol Vis Sci. 2008;49(10):4290–4296.
- Kakinoki M, Sawada O, Sawada T, et al. Comparison of macular thickness between Cirrus HD-OCT and Stratus OCT. Ophthalmic Surg Lasers Imaging. 2009;40(2):135–140.
- Legarreta JE, Gregori G, Punjabi OS, et al. Macular thickness measurements in normal eyes using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2008;39(4 Suppl):S43–S9.
- Wang JC, Laíns I, Providência J, et al. Diabetic choroidopathy: choroidal vascular density and volume in diabetic retinopathy with swept-source optical coherence tomography. Am J Ophthalmol. 2017;184:75–83.
- Willoughby AS, Chiu SJ, Silverman RK, et al. Platform-independent cirrus and spectralis thickness measurements in eyes with diabetic macular edema using fully automated software. Transl Vis Sci Technol. 2017;6(1):9.
