Abstract
Objective
Hyperhidrosis (excessive sweating) is associated with significant quality-of-life burden yet is often undertreated. With limited FDA-approved treatments, health care providers must determine optimal treatment among approved and off-label options. Key objectives of this review were to reassess, update, and expand a previous systematic review of commonly used treatment options for primary hyperhidrosis, including consideration of aluminum and zirconium compounds.
Methods
We performed a qualitative systematic review of efficacy, health-related quality of life, satisfaction, and safety of interventions, replicating and expanding the strategy outlined in a previous systematic review, with the addition of studies utilizing a within-patient design. We performed a critical appraisal of identified studies to determine risk of bias (RoB) and strength of evidence (SOE).
Results
A total of 32 studies were eligible for critical appraisal. Only three studies – two clinical trials of glycopyrronium cloth (2.4%) and one trial of botulinum toxin A injections in axillary hyperhidrosis were rated as “low” RoB; both had SOE ratings of “moderate” for use in axillary hyperhidrosis – the highest rating included in this review.
Conclusions
Optimal treatment choice depends on several factors, including understanding the quality of evidence regarding each treatment’s efficacy and safety (considerations of convenience and cost are beyond the scope of this review). In hyperhidrosis, as in other clinical conditions, treatment decisions should be patient centered. At this time, because of the quality of evidence, only imprecise estimates of effect are possible for hyperhidrosis treatments included in this review, and statements about comparative effectiveness are not possible.
Introduction
Primary hyperhidrosis is characterized by excessive sweating beyond what is necessary for thermal homeostasisCitation1. It is an idiopathic condition (in contrast to secondary hyperhidrosis) that is estimated to occur in 4.8% of the U.S. population (∼15.3 million people) and most commonly affects the axillary, palmar, and plantar regions of the bodyCitation1,Citation2.
The negative impact of primary hyperhidrosis on quality of life is well established; sufferers experience embarrassment and negative effects on social and emotional health, with a disease impact similar to or greater than that of psoriasis or eczemaCitation1–4. Even so, many hyperhidrosis patients go years without seeking the help that could be afforded from prescription treatments. Many rely on over-the-counter products and employ coping strategies such as frequent showering or carrying around towels and sets of extra clothingCitation5. In a survey conducted by the International Hyperhidrosis Society, nearly half (48.9%) of patients waited a decade or longer before seeking medical help for their excessive sweatingCitation2.
The lack of patient engagement in seeking medical help is not due to a lack of available treatment options. A diverse array of interventions has been used for the treatment of primary hyperhidrosis, including topical, oral and injectable prescription treatments as well as medical device therapiesCitation6. Specific treatment choices may be impacted by the focal area affected and intrinsic patient factors; however, given the limited number of FDA-approved treatment options for hyperhidrosis, health care providers are left to determine for each patient what might be the optimal treatment course among the approved and off-label choices available to them. Aside from prescription-strength antiperspirants, only two pharmacological agents have been approved by the FDA for the treatment of hyperhidrosisCitation7–9.
This underscores how instructive a critical evaluation of the hyperhidrosis treatment evidence base becomes for practicing physicians trying to narrow down the best treatment decisions within a broad range of therapeutic options. Most patients and many clinicians are either unaware of the important connection between the quality of scientific studies and the reliability of the reported results, or they focus on the reported results and fail to consider the strength of the evidence (SOE) before making treatment decisionsCitation10. The reason for emphasizing evidence quality is that benefits and harms that patients may experience with various treatment choices can be more accurately predicted when evidence is at the lowest risk of bias (RoB).
A systematic review first reported by Wade et al.Citation11 and subsequently in Wade et al.Citation12 examined relevant evidence available in the scientific literature through July 2016 for primary hyperhidrosis treatments. However, several aspects of that analysis, including the lack of special consideration for those studies utilizing within-patient designs or with large response rates, the exclusion of aluminum/zirconium compounds commonly used for hyperhidrosis treatment, and the interim FDA approval of a new hyperhidrosis drug, warrant further exploration.
Here, we performed a qualitative systematic review of efficacy, health-related quality of life, satisfaction, and safety of commonly used interventions in the treatment of primary hyperhidrosis based on the strategy outlined by Wade et al.Citation12. The key objectives of this review are to provide a comprehensive literature appraisal that includes the most recently published studies as well as a critical reassessment of studies evaluating hyperhidrosis treatments via within-patient designs and those with large response rates. Evidence summaries are presented to assist decision-makers in choosing optimal treatment strategies for patients seeking treatment for primary hyperhidrosis across a range of focal areas.
Methods
Eligibility criteria
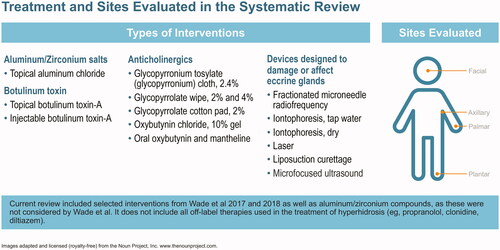
The protocol for this systematic review was registered in PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp identifier CRD42018104063). Clinical trials were eligible for inclusion. Eligibility was based on the Cochrane PICO annotation systemCitation13: population (patients with primary hyperhidrosis involving axillae, palms, soles or head without age restriction), intervention (four interventions used in the treatment of primary hyperhidrosis of the head, axilla, palms and soles in children and adults – see ), comparator (placebo, no treatment or any of the commonly used treatments listed above), outcome (efficacy, safety and quality of life outcomes using a variety of tools reported in the literature). Non-English language studies were excluded. Treatments evaluated include aluminum and zirconium compounds, anticholinergics (topical and systemic), botulinum toxin, and medical device therapies designed to alter the function of sweat production or damage eccrine glands (iontophoresis, curettage, laser therapy, microwave, fractional needle radiotherapy and ultrasound; ).
Search strategy and study selection
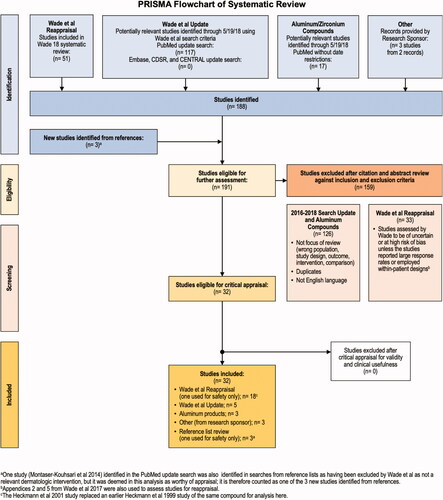
The following databases were searched: MEDLINE, Embase, the Cochrane Database of Systematic Reviews (CDSR) and Cochrane Central of Controlled Trials (CENTRAL). Studies were identified (1) by reviewing and assessing those included in two related publications by Wade et al. of the same search strategy and resultsCitation11,Citation12, (2) by performing an updated database search for studies published after the search date of 12 January 2016 used in the Wade et al. analysis, and (3) via PubMed and Embase searches for studies on aluminum/zirconium compounds. Search dates, terms and PubMed translations can be found in Supplementary Materials (p. 79).
Two investigators developed inclusion and exclusion criteria for study eligibility and agreed upon study inclusion. Studies considered were those studies from the Wade et al. analysis that met our inclusion criteria for critical appraisal, relevant studies obtained through database searches using the Wade et al. search terms (but published after the Wade et al. search date of 12 January 2016 and meeting our criteria), relevant studies of aluminum/zirconium salts (which were outside the scope of the Wade et al. analysis), and relevant studies identified via hand searches of retrieved study reference lists.
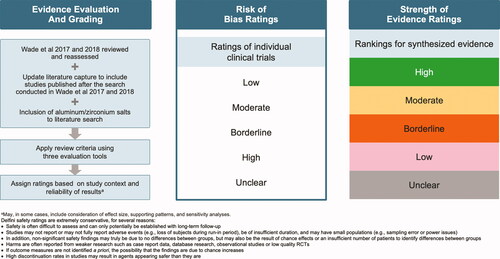
Trial quality was assessed in Wade et al.Citation12 using a modified version of the Cochrane Risk of Bias tool. The Cochrane Risk of Bias tool requires that a finding of high or uncertain RoB in any review domains renders the overall study as at high or uncertain RoBCitation14. Our assessment is that this standard is quite stringent and insufficiently contextual, which may result in the exclusion of results that are possibly reliable. For example, though studies using the “within-patient” design were included in the overall conclusions in Wade et al.Citation12 (despite being considered to be at high RoB), the Cochrane tool cannot account for the fact that this study design has unique strengths due to protections against confounding effects and has merit for both subjective and objective outcomes. Also, many of the studies identified in Wade et al.Citation12 reported large response rates, yet were not identified in that prior analysis as warranting special consideration, though such studies – even if they are not randomized and well controlled – may provide valid dataCitation15.
For the reasons stated above, studies using a within-patient study design and rated by Wade et al. as at high or uncertain RoB and passing a Delfini critical appraisal were included. In addition, studies rated by Wade et al.Citation12 as being at high or uncertain RoB were retained in this current review if they reported large response rates (defined as 40% or greater). Finally, searches for studies on topical formulations of aluminum and zirconium were also conducted, as these were not considered by Wade et al.
Studies not meeting inclusion criteria were rejected following title and/or abstract review; full text was retrieved for studies selected for consideration for inclusion. All studies were found in PubMed and hand searches of retrieved study reference lists.
Evidence evaluation and grading
Critical appraisals were performed for all efficacy studies selected for inclusion and rated for RoB. Each eligible study was assessed for RoB using tools created by Delfini Group based on standard evidence-based medicine principles and have been used by many groups – the tools are freely available at www.delfini.org. With rare exception, critical appraisal findings were documented only sufficiently to reach a grade; for example, if a lethal threat to validity was found, that threat was considered enough to reach a rating of "high risk of bias" and render documentation of further threats unnecessary.
However, in some studies with very large response rates, despite being rated as at high RoB, the validity and clinical usefulness were taken into consideration; in these instances, an explanation for any such exceptions was provided.
Wade et al.Citation12 was assessed for validity and clinical usefulness using a Delfini critical appraisal tool developed for assessing quality of data (http://www.delfini.org/delfiniTools.htm#catool).
All studies considered for inclusion in Wade et al.Citation12 were reevaluated for potential critical appraisal in this analysis (note that in Wade et al.Citation12, findings were summarized regardless of whether the study was rated as at high or unclear RoB). Key findings regarding efficacy, quality of life, satisfaction and safety were summarized narratively. The RoB ratings for individual clinical trials were assigned by Delfini as “Low,” “Moderate,” Borderline’, “High” and “Unclear” (). For strength of evidence (SOE) classification, rankings assigned by Delfini for synthesized evidence were “High,” “Moderate,” “Borderline,” “Low,” and “Unclear” ().
Results
Of 191 studies eligible for screening, 159 were excluded because they had insufficient information to appraise, were at a high RoB, were superseded by a more recent study, or existed as a poster with no corresponding manuscript. A total of 32 studies were eligible for critical appraisal for validity and clinical usefulness, and all 32 met critical appraisal criteria and were included in the present analysis ().
Overall summary of findings
Only three studies – a report of two clinical trials of glycopyrronium cloth (2.4%)Citation16 and one trial of botulinum toxin A injections for the treatment of axillary hyperhidrosisCitation17 were rated as being at “low” RoB. The overall SOE for most interventions for efficacy was weak with a large degree of heterogeneity in elements such as the study size, population studied, therapeutic interventions, study duration, endpoints reported, and effect sizes. SOE of pharmacological treatments and medical device interventions used to treat different types of hyperhidrosis are reviewed and summarized by focal region and type of intervention in and briefly discussed below (detailed descriptions of individual trials appear in the following section, “Individual Treatment Summaries”)Citation16–45. Efficacy and safety findings, including effect size estimates for pharmacological treatments and medical device interventions are summarized in .
Figure 4. Overall summary of SOE ratings and evidence synthesis for unique studies identified for appraisal (n = 32)*.
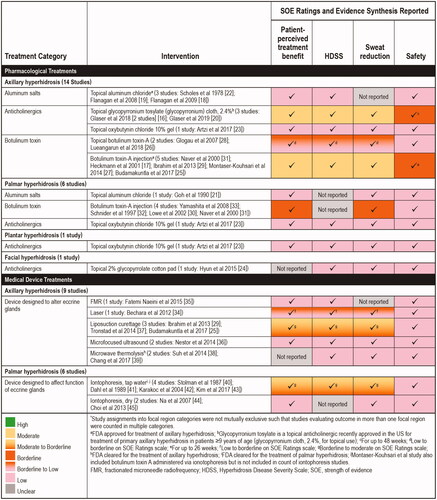
Table 1. Summary and risk of bias for aluminum salt studies.
Table 2. Summary and risk of bias for anticholinergic agents.
Table 3. Summary and risk of bias for botulinum toxin type A studies.
Table 4. Summary and risk of bias for medical devices that alter eccrine glands.
Table 5. Summary and risk of bias for iontophoresis studies.
Efficacy findings – strength of evidence ratings
An overall summary of SOE with respect to efficacy outcomes is provided below. Of note, many of the studies had very small sample sizes (N < 50), which may lead to reporting of inflated treatment effect estimatesCitation46, and calls into question whether there was sufficient statistical power to detect significant differences between groups.
Axillary hyperhidrosis (23 studies): SOE ratings for topical glycopyrronium cloth (2.4%) and botulinum toxin A injections were “moderate” – the highest rating of any therapeutic agents included in this review. A “borderline to moderate” rating was assigned to liposuction curettage. The SOE rating for topical botulinum toxin A and laser treatments was “low to borderline.” The SOE rating was “low” for topical aluminum chloride and topical oxybutynin chloride 10% gel. SOE ratings were “low” for botulinum toxin administered via iontophoresis, microwave thermolysis, microfocused ultrasound and fractionated microneedle radiofrequency ().
Palmar hyperhidrosis (12 studies): The highest SOE rating for pharmacological treatments for palmar hyperhidrosis was “borderline,” achieved by botulinum toxin A injections. The SOE rating for tap water iontophoresis was “borderline to moderate.” The SOE rating was “low” for topical aluminum chloride, topical oxybutynin chloride 10% gel, and dry iontophoresis ().
Plantar hyperhidrosis (1 study): The SOE rating was “low” for topical oxybutynin chloride 10% gel ().
Facial hyperhidrosis (1 study): The SOE rating for topical 2% glycopyrrolate-impregnated cotton pads was “low” ().
Safety findings
The majority of trials did not provide sufficient evidence to draw firm conclusions about safety. Some studies provided few safety details and in others there was no mention of adverse events. In addition, the short duration of many studies did not allow for sufficient follow-up for reporting adverse events. The overall SOE for safety was rated as “low” for all interventions except glycopyrronium cloth 2.4%Citation16,Citation20 and botulinum toxinCitation17, which were rated “borderline.” These were larger trials of higher methodological quality and longer duration (up to 48 weeks for glycopyrronium cloth 2.4% and up to 26 weeks for botulinum toxin A). Although safety information reported in the reviewed studies is included here, readers are directed to other sources for more complete safety details.
Comparative efficacy and safety findings
There is insufficient evidence to draw firm conclusions regarding the comparative efficacy and safety of commonly used therapies included in this review for the treatment of axillary, palmar, plantar, and facial hyperhidrosis. Therefore, clinicians and patients will need to consider the SOE regarding benefits and harms for each intervention along with important contextual elements when making treatment decisions.
Individual treatment summaries
Pharmacological treatments
Efficacy and safety findings for each included pharmacological intervention (aluminum salts, anticholinergics, and botulinum toxin) are summarized in .
Aluminum salts
It has been proposed that the metal ions in aluminum salts reduce sweating by damaging epithelial cells along the lumen of the sweat duct, thereby creating a plug that obstructs sweat glandsCitation47. Four studies were included that investigated aluminum salts for the treatment of axillary and palmar hyperhidrosis (no studies were identified for zirconium compounds). Of these four studies, three studies with aluminum salts were identified via the database searchCitation18,Citation19,Citation21 and were rated as at “unclear,” “borderline to high” or “high” RoB, respectively, but with large response rates. The fourth studyCitation22 was identified using a hand search of retrieved study reference lists and was rated at “high” RoB, but with a large response rate. SOE for efficacy, quality of life, and patient satisfaction for aluminum salts (aluminum chloride hexahydrate) for treatment of both axillary and palmar hyperhidrosis was “low.”
In axillary hyperhidrosis, two trialsCitation18,Citation19 provided striking inconsistencies in Hyperhidrosis Disease Severity Scale (HDSS) response rates for aluminum chloride (33% vs. 72%, ). Data for palmar hyperhidrosis were limited to one small, within-patient study of limited duration and lacked the use of standardized scales to measure reduction in sweat productionCitation21.
Anticholinergics
Anticholinergics reduce sweat production by blocking acetylcholine, the chemical messenger that triggers sweat glands to produce perspirationCitation48. Topical formulations include glycopyrronium tosylate (GT) pre-moistened cloth (QBREXZACitation7, FDA-approved for use in primary axillary hyperhidrosis), two compounds not commercially available (glycopyrrolate wipes and glycopyrrolate-impregnated cotton pads), and oxybutynin chloride gel. This review includes five studies focused on topical anticholinergics across a range of focal regions (). Of note, no studies assessing the efficacy of oral anticholinergic drugs were found to meet our inclusion criteria.
Two reports, representing three studies, of GT in primary axillary hyperhidrosis were included: Glaser et al. reported two studiesCitation16 rated at “low” RoB and with large response rates, and Glaser et al. reported an observational open-label extension phase of those studies for safetyCitation20. Based on these trials, approximately three-fourths of patients met the response criteria (defined as ≥50% reduction in axillary sweat production) within one month of starting treatment. One within-patient study of facial hyperhidrosis from the Wade et al.Citation12 review, reevaluated in the current review was rated as “moderate” RoBCitation24. This trial reported a reduction in the rate of sweat production at the forehead of 36.68 ± 11.41% on day 10 (p<.025). A fifth study (palmar and plantar regions) was obtained from the database search for updatesCitation23, and was rated at “moderate” RoB with large response rates.
The highest quality of evidence (“moderate” for efficacy, quality of life, and satisfaction), representing the largest studies to date in patients with axillary hyperhidrosis, evaluates topical GT pre-moistened cloth: two trials (N = 697) demonstrated that most patients will experience a meaningful reduction in axillary sweating at 4 weeks. In those trials, the most commonly reported treatment-emergent adverse events were dry mouth, application site pain, mydriasis, oropharyngeal pain, and headache, and two serious adverse events were reported with GT (mydriasis and dehydration)Citation16. A 44-week open-label extension study is the longest clinical safety trial available for topical GT and found that no new safety signals emerged, with demonstrated consistency across efficacy outcomesCitation20. The remaining studies evaluating the use of topical anticholinergics consist of “low” SOE for efficacy, quality of life, satisfaction, and safety.
Typical anticholinergic-related adverse events that have been reported by patients in studies of anticholinergics include dry mouth, urinary retention, constipation, blurred vision, impaired taste, rapid heart rate and heart palpitations. An unproven causal association between oral anticholinergic drugs (tertiary amines) and dementia has been reported in elderly patients receiving long-term, high-dose therapy for indications other than hyperhidrosisCitation49. Other sources with a focus on anticholinergic safety should be consulted for detailed safety information.
Botulinum toxin
Botulinum toxin reduces sweating by blocking nerve signals responsible for producing perspirationCitation50, and onabotulinum toxin A (BOTOXCitation8) is FDA-approved for treatment of severe axillary hyperhidrosis. Ten studies on the efficacy and safety of botulinum toxin in hyperhidrosis treatment are included in this review (six in axillary hyperhidrosis, three in palmar hyperhidrosis, and one in both; ). The literature search yielded more studies for botulinum toxin type A injections than for any other treatment; studies evaluated use of botulinum toxin A in both axillary and palmar hyperhidrosis. The evidence for the efficacy of botulinum toxin type B studies did not meet our inclusion criteria.
Specifically, two studies in axillary hyperhidrosisCitation25,Citation26 were both rated at “moderate” RoB and with large response rates. Another axillary studyCitation17 was obtained from Wade et al. and was rated as “low” RoB with large response rates. The axillary study by Montaser-Kouhsari et al.Citation27 was excluded by Wade et al. as not relevant but was included in this review because we disagreed with that assessment and was rated as “moderate” RoB with large response rates. The remaining six studies are of a within-patient design and were included in Wade et al. but are reevaluated hereCitation28–33 all were rated at “moderate” RoB with large response rates with the exception of Glogau et al., which was rated at “borderline” RoB.
Although results vary across studies, there is sufficient evidence to conclude that botulinum toxin A injections are effective in reducing sweat production in the axillae by more than 50% for 6 months or longer. The study of highest quality for botulinum toxinCitation17 (n = 145) reported a nearly 90% decrease in mean rate of sweat production at two weeks; the rate was reduced to approximately 65% at 24 weeks (open label after 2 weeks), with nearly all (98%) subjects stating they would recommend this therapy.
There is insufficient evidence to draw firm conclusions regarding the safety of botulinum toxin A injections for treatment of axillary hyperhidrosis. Data from short-term, follow-up studies and other reports suggest that patients are likely to experience injection-site pain, which is at times severe; other non-severe adverse events have been reported. In the double-blind, placebo-controlled study with injectable botulinum toxin ACitation17, transient adverse effects included headache, muscle soreness of the shoulder girdle, increased facial sweating, and axillary itching.
The use of topical botulinum toxin A has also been studied to a limited extent in axillary hyperhidrosis. Although no commercial formulation is currently available, evidence from two trials suggests that topical application of botulinum toxin A may provide short term sweat reduction (sweat production was reduced by approximately 20% to more than 50% at 2 weeks), lasting approximately 6 weeks with acceptable patient satisfactionCitation26,Citation28. In addition, there is insufficient evidence to draw firm conclusions regarding the safety of topical application of botulinum toxin A for the treatment of axillary hyperhidrosis.
Evidence to support the use of botulinum toxin A injections for the treatment of palmar hyperhidrosis is more limited, but suggests that patients may achieve a reduction of approximately 25–50% or more in palmar sweating for three weeks to six monthsCitation30–33 (). There is insufficient evidence to draw conclusions regarding the safety of botulinum toxin A injections for the treatment of palmar hyperhidrosis. Reported adverse events across the palmar trials include hand pain, finger numbness, thumb and finger weakness, excessively dry hands, indigestion/heartburn and slight transient reduction of power of finger grip lasting 2–5 weeks. There is uncertainty about major or long-term adverse events. Efficacy, quality of life, and patient satisfaction were rated as borderline; SOE for safety was rated as low.
Medical device treatments
Medical devices used to treat primary hyperhidrosis are designed to alter eccrine glands (curettage, laser therapy, microwave, fractional needle radiotherapy and ultrasound therapies) or affect their function (iontophoresis). This review includes 15 studies on these devices, nine in axillary and six in palmar hyperhidrosis. Efficacy and safety findings for each included medical device are summarized in and . There is insufficient evidence to draw conclusions regarding the efficacy and safety of curettage, laser therapy, fractionated microneedle radiofrequency, microwave therapy or ultrasound therapy compared to other available therapies for the treatment of axillary hyperhidrosis.
Fractionated microneedle radiofrequency
Fractional microneedle radiofrequency (FMR) is a recently developed, minimally invasive method for delivering thermal energy to the interface between the epidermis and subcutaneous tissue. This technology uses rapid penetration with microneedles, which causes irreversible thermolysis of apocrine and eccrine sweat glands without destroying the epidermis. One single-blind, within-patient, right-left comparison study in axillary hyperhidrosis was included in the present analysisCitation35 (moderate RoB). Efficacy, quality of life, patient satisfaction, and safety SOE were rated as “low” ().
Lasers
Evidence from one within-patient, unblinded, randomized trial evaluating five cycles of an 800 nm diode laser compared to no treatment in adults with axillary hyperhidrosis was included in the present analysisCitation34. A significant decrease in sweating rate was observed on both the laser-treated sites and the untreated sides. Post-treatment biopsy results were not consistent with tissue destruction. Efficacy, quality of life, and patient satisfaction were rated as low to borderline, and safety SOE was rated as “low” ().
Micro-focused ultrasound
Micro-focused ultrasound waves cause vibration of tissues resulting in heating and destruction of axillary sweat glands. Two randomized, within-patient, double-blind, sham-controlled pilot studies of micro-focused ultrasound plus visualization in axillary hyperhidrosis were included in the present analysisCitation36. Efficacy, quality of life, patient satisfaction, and safety were rated as having “low” SOE ().
Liposuction curettage
Liposuction curettage is performed at the dermal–subcutaneous interface using a liposuction device and a sharp, rasping-type cannula to damage the sweat glands. Three studies evaluating curettage were included in the present analysisCitation29,Citation25,Citation37 (), two of which were comparator studies of curettage and botulinum toxinCitation29,Citation25 (studies also are included in for botulinum toxin arm). Efficacy, quality of life, and patient satisfaction were rated as “borderline” to “moderate”; safety was rated as having “low” SOE. There is sufficient evidence to conclude that liposuction curettage for the treatment of axillary hyperhidrosis is effective in reducing sweat production by 30% to over 80% for 6 months or moreCitation25,Citation29,Citation37. There is insufficient evidence to draw conclusions regarding long-term safety of subcutaneous curettage for the treatment of axillary hyperhidrosis but limited short-term evidence suggests that patients should expect to experience axillary discomfort, soreness or pain. Other reported adverse events include dysesthesias, hyperpigmentation, “bothersome” scar formation, focal hair loss, subcutaneous fibrotic bridles, seromas, wound infection, bleeding, hematoma and skin necrosis.
Microwave thermolysis
Microwave therapy devices (e.g. miraDry, FDA-cleared for treatment of primary axillary hyperhidrosis) focus heat at the skin-subcutaneous tissue interface causing irreversible thermolysis of apocrine and eccrine sweat glands. This review included two case reports for evidence synthesis of safety dataCitation38,Citation39 (); no studies met criteria for efficacy evaluation. Efficacy, quality of life, patient satisfaction, and safety were rated as having “low” SOE. Suh et al.Citation38 and Chang et al.Citation39 each present a case report for ulnar and median nerve injury following microwave thermolysis, and in one patientCitation39, significant sensory and motor deficits had not resolved at the 6-month follow-up.
Iontophoresis
Iontophoresis typically delivers electrical current (15–20 mA) through tap water to treat hyperhidrosis but very limited evidence suggests it can be performed “dry.” While the mechanism of action is not known, hypotheses include inhibition of nerve transmission, as well as inhibition or obstruction of sweat flow by altering pH or ion deposition in sweat ducts. This review includes seven studies on the use of iontophoresis to treat hyperhidrosis: six in palmar hyperhidrosis (four tap water iontophoresisCitation40–43, two dry iontophoresisCitation44,Citation45 and one in axillary hyperhidrosisCitation27 comparing the effect of botulinum toxin solution administered via iontophoresis to injected botulinum toxin; all had large response rates.
Tap water iontophoresis
Based on evidence from four trials, there is sufficient evidence (SOE rating of “borderline to moderate”) to conclude that tap water iontophoresis for the treatment of palmar hyperhidrosis is effective in producing a clinically meaningful reduction in sweat production (reported reductions of 30–90% after 1–4 weeks of treatment and lasting several weeks to several months) with acceptable patient satisfactionCitation27,Citation40–43. Reduction in sweating maybe maintained by repeat treatments every few days to 2 weeks. No valid evidence was found regarding the use of tap water iontophoresis for the treatment of axillary or plantar hyperhidrosis.
There is insufficient evidence to draw conclusions regarding the safety of iontophoresis for the treatment of palmar hyperhidrosis but limited short-term evidence suggests that patients are likely to experience minor discomfort. Reported adverse events include transient tingling, erythema and vesiculation of the skin. There is uncertainty about major or long-term adverse events. Efficacy, quality of life, and patient satisfaction were rated as “borderline” to “moderate,” while safety SOE was rated as “low.”
Dry iontophoresis
Based on the two studies included in the present analysesCitation44,Citation45 efficacy, quality of life, patient satisfaction, and safety SOE were rated as “low.”
Combined drug therapy and iontophoresis
Based on the single study included in the present analysisCitation27 (axillary hyperhidrosis), the evidence for combining drug therapy with iontophoresis is inconclusive.
Regardless of the type of iontophoresis used, there is insufficient evidence to draw conclusions regarding the efficacy, satisfaction and safety of iontophoresis compared to other available therapies for the treatment of palmar hyperhidrosis.
Discussion
This report provides an evidence-based review of commonly used interventions for the treatment of primary hyperhidrosis. The efficacy and safety evidence provided in this review can be used to optimize treatment strategies for patients suffering from primary hyperhidrosis. To make optimal decisions, healthcare providers and patients should be informed of not only a study’s results, but also the quality of evidence supporting the results. Considering the quality of the evidence when examining the results of studies is important because bias tends to favor the treatment being studied, and lower quality studies are more likely to report inaccurate results; highly biased studies may distort results by more than 50%Citation51–53. Shared decision-making and the use of decision support materials (often called patient decision aids) improve decision-making around many different preference-sensitive clinical choices. The SOE information included in this review, together with summarized efficacy and safety data, will be helpful in creating decision support materials, which have been shown to improve shared decision-making discussionsCitation54,Citation55.
A key aspect of this analysis was a critical appraisal of studies of aluminum/zirconium compounds, as this was not a component of the prior Wade et al. analyses (no studies of zirconium salt compounds met our inclusion criteria). In addition, evidence from studies with large response rates and/or those that used a within-patient design were considered. Overall, there was a lack of high-quality of evidence to support use of treatments for primary hyperhidrosis; the SOE was highest for topical glycopyrronium cloth (2.4%) and botulinum toxin A injections for the treatment of axillary hyperhidrosis, which increases our confidence in the reliability of reported results for these two interventions. There were more studies identified in axillary hyperhidrosis than in other regions of the body ().
Although there were only two studies investigating topical glycopyrronium cloth for the treatment of axillary hyperhidrosis, they were of “moderate” quality with large groups, good designs and execution for both efficacy and safety, and reported consistent results. In these two key clinical trials for glycopyrronium cloth, most treatment-emergent adverse events were transient and reversible. Anticholinergic side effects are a potential risk with glycopyrronium cloth treatment, and Glaser et al.Citation16 noted that patients who do not wash their hands following application may inadvertently transfer the drug to another body area such as the eyes. Authors concluded that unilateral ophthalmologic events of mydriasis and blurred vision were most likely due to local exposure, whereas anticholinergic side effects such as dry mouth and urinary hesitation were likely a result of systemic exposure.
With respect to botulinum toxin A, the cumulative amount of reliable data relevant to treatment of axillary hyperhidrosis increases confidence in the results. Some studies for botulinum toxin A injections and topical treatments were of “low to borderline” quality, and most clinical trials reviewed did not provide sufficient evidence to draw firm conclusions about safety due to lack of reporting, small sample size, limitations of study design, and short study duration. Even when safety evidence is of low quality, reported adverse events and serious adverse events along with the quality of the evidence provide valuable information to healthcare providers and patients. We found two case studies of patients diagnosed with severe median and ulnar nerve injury following microwave thermolysis whose symptoms either did not resolve at a 6-month follow-up or resolved only after intensive physical therapy for 6 monthsCitation38,Citation39.
In addition to considerations of efficacy and safety, patients should also be made aware of the differences between treatment modalities that may affect their treatment choices (e.g. potential discomfort or pain, ease of application, inconvenience, cost, and ease of adherence). Patients should be provided with information sufficient to assist them in making informed decisions that satisfy their personal healthcare needs, values and preferences.
Another critical aspect of this analysis was a thorough evaluation of studies that utilized a within-patient design (in keeping with the most commonly used terminology in Wade et al.; many other synonyms exist). In this review, all but one of the studies using within-patient design were also shown to have large response rates. For the context of this analysis using hyperhidrosis studies, an understanding of the strengths of within-patient trials compared to trials utilizing a between-group design is particularly important, as the within-patient design is frequently encountered in studies of hyperhidrosis and allows comparison of treatments involving right and left axillae, palms or soles within the same patient.
When evaluated with criteria used for between-group trials, the within-patient design is likely to be rated as “at high risk of bias” for several reasons, including that they tend to be characterized by small populations and frequently lack randomization and blinding. In traditional between-group trials, such as RCTs, selection and bias are key considerations when assessing a study for RoB. However, for many dermatological conditions, including primary hyperhidrosis, within-patient designs may in fact have certain advantages in terms of establishing balanced study groups (i.e. those with balanced baseline clinical and demographic characteristics). In within-patient trials, selection bias may be reduced because one side of the body is compared to the opposite side in the same person, and demographic and clinical prognostic variables are similar or equal. For example, potential differences in age, sex, genetics, laboratory values, comorbidities and other prognostic variables are presumably less of a threat to validity using a within-patient design because there is only one body with two comparison sites. We found very little evidence in the medical literature regarding the strengths and weaknesses of the within-patient study design as there is little guidance available on critical appraisal of such studies. To facilitate our review, we developed a list of considerations (as distinguished from criteria), which is provided in Supplementary Materials (p. 81).
Some of these considerations were derived from a brief literature review, consideration of crossover-design studies, general considerations, and our knowledge of study bias and how it may affect reported results. Further well-designed and executed studies comparing outcomes from within-patient studies to outcomes from between group designs for the same conditions and treatments will be required to understand how within-patient studies might differ from between-group studies in terms of validity considerations. If the strength of the within-patient study design is confirmed, fewer study subjects would be required which would result in lowered exposure and inconvenience to study subjects, reduced study costs and minimized distortion of results due to bias.
Conclusions
Optimal treatment choice depends upon understanding the quality of the evidence regarding each treatment’s efficacy and safety (considerations of convenience, cost, etc. may also be important but are beyond the scope of this review). In this systematic review, we evaluate the efficacy, health-related quality of life, satisfaction, and safety of commonly used interventions in the treatment of primary hyperhidrosis of the head, axilla, palms, and soles in children and adults. Information provided in this review will be of use to patients and other decision-makers. We also provide detailed information about the quality of the hyperhidrosis evidence in addition to the reported results of included studies.
In hyperhidrosis, as in other clinical conditions, treatment decisions should be patient centered. At this time, because of the quality of evidence, only imprecise estimates of effect are possible for most commonly employed treatments for hyperhidrosis, and statements about comparative effectiveness are not possible.
Transparency
Declaration of funding
This work was funded by Dermira Inc., a wholly-owned subsidiary of Eli Lilly and Company.
Declaration of financial/other relationships
MES and SAS: In accordance with Taylor & Francis policy and our ethical obligation as researchers, we are reporting that Dermira, Inc., a wholly owned subsidiary of Eli Lilly and company, funded this research. We have no other financial disclosures or conflicts to report. KKG: is employed by Dermira, Inc., a wholly owned subsidiary of Eli Lilly and Company. A reviewer on this manuscript has disclosed that they are a board member of international hyperhidrosis society. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
Medical writing support for this manuscript was provided by Amel Alqadah, PhD and Jennifer Hepker, PhD and Merrilee Johnstone, PhD of Prescott Medical Communications Group (Chicago, IL), with financial support from Dermira, Inc, a wholly owned subsidiary of Eli Lilly and Company.
Supplemental Material
Download PDF (1.3 MB)Notes
1 Dermira Inc, Menlo Park, CA
2 Allergan, Inc., Irvine, CA
3 Miramar Labs, Inc., Santa Clara, CA
References
- Doolittle J, Walker P, Mills T, et al. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res. 2016;308(10):743–749.
- Glaser DA, Hebert A, Pieretti L, et al. Understanding patient experience with hyperhidrosis: a national survey of 1,985 patients. J Drugs Dermatol. 2018;17(4):392–396.
- Hamm H, Naumann MK, Kowalski JW, et al. Primary focal hyperhidrosis: disease characteristics and functional impairment. Dermatology. 2006;212(4):343–353.
- Naumann MK, Hamm H, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218–1226.
- Kamudoni P, Mueller B, Halford J, et al. The impact of hyperhidrosis on patients’ daily life and quality of life: a qualitative investigation. Health Qual Life Outcomes. 2017;15(1):121.
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669–680.
- QBREXZA® (glycopyrronium) cloth [prescribing information]. Menlo Park (CA): Dermira Inc.; 2018. Available from: http://pi.dermira.com/QbrexzaPI.pdf
- BOTOX® (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use [prescribing information]. Irvine (CA): Allergan, Inc.; 2011. Available from: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190620-BOTOX-100-and-200-Units-v3-0USPI1145-v2-0MG1145.pdf
- Wechter T, Feldman SR, Taylor SL. The treatment of primary focal hyperhidrosis. Skin Therapy Lett. 2019;24(1):1–7.
- Ioannidis JPA, Stuart ME, Brownlee S, et al. How to survive the medical misinformation mess. Eur J Clin Invest. 2017;47(11):795–802.
- Wade R, Rice S, Llewellyn A, et al. Interventions for hyperhidrosis in secondary care: a systematic review and value-of-information analysis. Health Technol Assess. 2017;21(80):1–280.
- Wade R, Llewellyn A, Jones-Diette J, et al. Interventional management of hyperhidrosis in secondary care: a systematic review. Br J Dermatol. 2018;179(3):599–608.
- PICO annotation; 2020; [cited 2020 Jun 30]. Available from: https://training.cochrane.org/resource/cochrane-information-specialists-handbook/12-pico-annotation
- Collaboration TC. Methodological expectations of Cochrane Intervention Reviews (MECIR): standards for the reporting of plain language summaries in new Cochrane Intervention Reviews; version 1; 2013; [cited 2019 Aug 22]. Available from: https://methods.cochrane.org/sites/default/files/public/uploads/pleacs_2019.pdf
- Glasziou P, Chalmers I, Rawlins M, et al. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007;334(7589):349–351.
- Glaser DA, Hebert AA, Nast A, et al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: results from the ATMOS-1 and ATMOS-2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2019;80(1):128–138e2.
- Heckmann M, Ceballos-Baumann AO, Plewig G. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488–493.
- Flanagan KH, Glaser DA. An open-label trial of the efficacy of 15% aluminum chloride in 2% salicylic acid gel base in the treatment of moderate-to-severe primary axillary hyperhidrosis. J Drugs Dermatol. 2009;8(5):477–480.
- Flanagan KH, King R, Glaser DA. Botulinum toxin type a versus topical 20% aluminum chloride for the treatment of moderate to severe primary focal axillary hyperhidrosis. J Drugs Dermatol. 2008;7(3):221–227.
- Glaser DA, Hebert AA, Nast A, et al. A 44-week open-label study evaluating safety and efficacy of topical glycopyrronium tosylate in patients with primary axillary hyperhidrosis. Am J Clin Dermatol. 2019;20(4):593–604.
- Goh CL. Aluminum chloride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol. 1990;29(5):368–370.
- Scholes KT, Crow KD, Ellis JP, et al. Axillary hyperhidrosis treated with alcoholic solution of aluminium chloride hexahydrate. Br Med J. 1978;2(6130):84–85.
- Artzi O, Loizides C, Zur E, et al. Topical oxybutynin 10% gel for the treatment of primary focal hyperhidrosis: a randomized double-blind placebo-controlled split area study. Acta Derm Venereol. 2017;97(9):1120–1124.
- Hyun MY, Son IP, Lee Y, et al. Efficacy and safety of topical glycopyrrolate in patients with facial hyperhidrosis: a randomized, multicentre, double-blinded, placebo-controlled, split-face study. J Eur Acad Dermatol Venereol. 2015;29(2):278–282.
- Budamakuntla L, Loganathan E, George A, et al. Comparative study of efficacy and safety of botulinum toxin a injections and subcutaneous curettage in the treatment of axillary hyperhidrosis. J Cutan Aesthet Surg. 2017;10(1):33–39.
- Lueangarun S, Sermsilp C, Tempark T. Topical botulinum toxin type a liposomal cream for primary axillary hyperhidrosis: a double-blind, randomized, split-site, vehicle-controlled study. Dermatol Surg. 2018;44(8):1094–1101.
- Montaser-Kouhsari L, Zartab H, Fanian F, et al. Comparison of intradermal injection with iontophoresis of abobotulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2014;25(4):337–341.
- Glogau RG. Topically applied botulinum toxin type A for the treatment of primary axillary hyperhidrosis: results of a randomized, blinded, vehicle-controlled study. Dermatol Surg. 2007;33(s1):S76–S80.
- Ibrahim O, Kakar R, Bolotin D, et al. The comparative effectiveness of suction-curettage and onabotulinumtoxin-A injections for the treatment of primary focal axillary hyperhidrosis: a randomized control trial. J Am Acad Dermatol. 2013;69(1):88–95.
- Lowe NJ, Yamauchi PS, Lask GP, et al. Efficacy and safety of botulinum toxin type A in the treatment of palmar hyperhidrosis: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2002;28(9):822–827.
- Naver H, Swartling C, Aquilonius SM. Palmar and axillary hyperhidrosis treated with botulinum toxin: one-year clinical follow-up. Eur J Neurol. 2000;7(1):55–62.
- Schnider P, Binder M, Auff E, et al. Double-blind trial of botulinum A toxin for the treatment of focal hyperhidrosis of the palms. Br J Dermatol. 1997;136(4):548–552.
- Yamashita N, Shimizu H, Kawada M, et al. Local injection of botulinum toxin A for palmar hyperhidrosis: usefulness and efficacy in relation to severity. J Dermatol. 2008;35(6):325–329.
- Bechara FG, Georgas D, Sand M, et al. Effects of a long-pulsed 800-nm diode laser on axillary hyperhidrosis: a randomized controlled half-side comparison study. Dermatol Surg. 2012;38(5):736–740.
- Fatemi Naeini F, Abtahi-Naeini B, Pourazizi M, et al. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: a sham control study. Australas J Dermatol. 2015;56(4):279–284.
- Nestor MS, Park H. Safety and efficacy of micro-focused ultrasound plus visualization for the treatment of axillary hyperhidrosis. J Clin Aesthet Dermatol. 2014;7(4):14–21.
- Tronstad C, Helsing P, Tonseth KA, et al. Tumescent suction curettage vs. curettage only for treatment of axillary hyperhidrosis evaluated by subjective and new objective methods. Acta Derm Venereol. 2014;94(2):215–220.
- Suh DH, Lee SJ, Kim K, et al. Transient median and ulnar neuropathy associated with a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2014;40(4):482–485.
- Chang CK, Chen CY, Hsu KF, et al. Brachial plexus injury after microwave-based treatment for axillary hyperhidrosis and osmidrosis. J Cosmet Laser Ther. 2017;19(7):439–441.
- Stolman LP. Treatment of excess sweating of the palms by iontophoresis. Arch Dermatol. 1987;123(7):893–896.
- Dahl JC, Glent-Madsen L. Treatment of hyperhidrosis manuum by tap water iontophoresis. Acta Derm Venereol. 1989;69(4):346–348.
- Karakoc Y, Aydemir EH, Kalkan MT. Placebo-controlled evaluation of direct electrical current administration for palmoplantar hyperhidrosis. Int J Dermatol. 2004;43(7):503–505.
- Kim DH, Kim TH, Lee SH, et al. Treatment of palmar hyperhidrosis with tap water iontophoresis: a randomized, sham-controlled, single-blind, and parallel-designed clinical trial. Ann Dermatol. 2017;29(6):728–734.
- Na GY, Park BC, Lee WJ, et al. Control of palmar hyperhidrosis with a new "dry-type" iontophoretic device. Dermatol Surg. 2007;33(1):57–61.
- Choi YH, Lee SJ, Kim DW, et al. Open clinical trial for evaluation of efficacy and safety of a portable "dry-type" iontophoretic device in treatment of palmar hyperhidrosis. Dermatol Surg. 2013;39(4):578–583.
- Dechartres A, Trinquart L, Boutron I, et al. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346(14):f2304.
- Holzle E, Braun-Falco O. Structural changes in axillary eccrine glands following long-term treatment with aluminium chloride hexahydrate solution. Br J Dermatol. 1984;110(4):399–403.
- Brackenrich J, Fagg C. Hyperhidrosis. Treasure Island (FL): StatPearls; 2020.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407.
- Grunfeld A, Murray CA, Solish N. Botulinum toxin for hyperhidrosis: a review. Am J Clin Dermatol. 2009;10(2):87–102.
- Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613.
- Poolman RW, Struijs PA, Krips R, et al. Reporting of outcomes in orthopaedic randomized trials: does blinding of outcome assessors matter? J Bone Joint Surg Am. 2007;89(3):550–558.
- Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412.
- Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–312.