Abstract
Background
Cardiogenic shock (CS) is an acute complex condition leading to morbidity and mortality. Vasoactive medications, such as vasopressors and inotropes are considered the cornerstone of pharmacological treatment of CS to improve end-organ perfusion by increasing cardiac output (CO) and blood pressure (BP), thus preventing multiorgan failure.
Objective
A critical review was conducted to analyze the currently available randomized studies of vasoactive agents in CS to determine the indications of each agent and to critically appraise the methodological quality of the studies.
Methods
PubMed database search was conducted to identify randomized controlled trials (RCTs) on vasoactive therapy in CS. After study selection, the internal validity of the selected studies was critically appraised using the three-item Jadad scale.
Results
Nine studies randomized 2388 patients with a mean age ranged between 62 and 69 years, were identified. Seven of studies investigated CS in the setting of acute myocardial infarction (AMI). The studies evaluated the comparisons of norepinephrine (NE) vs. dopamine, epinephrine vs. NE, levosimendan vs. dobutamine, enoximone or placebo, and nitric oxide synthase inhibitors (NOSi) vs. placebo. The mean Jadad score of the nine studies was 3.33, with only three studies of a score of 5.
Conclusions
The evidence from the studies of vasoactive agents in CS carries uncertainties. The methodological quality between the studies is variable due to the inherent difficulties to conduct a study in CS. Vasopressors and inotropes continue to have a fundamental role given the lack of pharmacological alternatives.
1. Introduction
Shock as a final pre-terminal state in many diseases is widely classified, according to the underlying mechanisms into, cardiogenic (e.g. acute myocardial infarction (AMI) or myocarditis), hypovolemic (i.e. fluid loss either internal or external), obstructive (e.g. cardiac tamponade or pulmonary embolism) and distributive (e.g. septic shock or anaphylaxis)Citation1,Citation2. The most common form of shock is septic (62%), then cardiogenic (16%), hypovolemic (16%), other forms of distributive shock, and finally obstructive shock (2%)Citation2. In-hospital mortality rate of shock in general is about 38%Citation3, and varies depending on the shock type. For example, mortality rate of septic shock ranges from 46Citation3,Citation4 to 61%Citation5 and that of cardiogenic shock (CS) from 40%Citation6 up to about 50%Citation7,Citation8.
Cardiovascular diseases (CVD) are the most common cause of CSCitation9. Cardiac diseases that impair, either in isolation or in combination, the function of myocardium, pericardium, conduction system, or valves, will lead to an acute hemodynamic instabilityCitation10. Apart from the cardiac etiologies, CS may occur due to other systemic illnesses, such as lung injury, sepsis, or other inflammatory conditionsCitation9,Citation11. The pathogenesis of CS is broadCitation9, which ranges from low cardiac output (CO) advanced chronic heart failure (HF) to a de novo CSCitation12. AMI is the most common causeCitation9,Citation12 and accounts for approximately half of CS casesCitation13. It has also been reported that CS complicated about 5–15% of AMI casesCitation8,Citation9,Citation13–18. Prior to the coronary revascularization era, the mortality rates in patients with CS ranged between 72 and 81%Citation19. Despite the advances in hemodynamic support devices and reperfusion techniquesCitation13–15, the in-hospital mortality remained highCitation13 (24.6 − 50%)Citation14,Citation15,Citation17–20, and has not changed since the publication of the SHOCK study in 1999Citation8. In a large population-based observational study over eight years (2003–2010), the incidence of CS complicating ST-segment elevation myocardial infarction (MI) increased from 6.5 to 10.1% (ptrend <.001). There was an increase in early revascularization rate (30.4–50.7%, ptrend <.001) and intra-aortic balloon pump (IABP) use (44.8–53.7%, ptrend <.001). Whereas, there was a significant decline in the in-hospital mortality (44.6–33.8%, ptrend <.001; adjusted odds ratio (OR) 0.71; 95% confidence interval (95% [CI], 0.68–0.75)Citation17. CS is generally recognized as a state of low CO due to acute left ventricular (LV) dysfunction and hypotension, leading to a life-threaCitation9,Citation11,Citation13,Citation14. There is no universal definition of CS in literatureCitation9,Citation14. It is defined as a clinical condition of persistent hypotension (i.e. systolic blood pressure (SBP) <90 mmHg, or need for catecholamine support to maintain SBP ≥90 mmHg) despite volume replacementCitation6,Citation8–10,Citation12,Citation18, with clinical features of end-organ hypoperfusion (i.e. altered mental status, cold/clammy skin or extremities, elevated serum creatinine, urine output <30 mL/h, or lactate >2.0 mmol/L)Citation6,Citation8–10,Citation12. Hemodynamic criteria, although not mandatory, can assist in confirming the diagnosis of CS, and usually include cardiac index (CI) <1.8 L/min/m2 without support or <2.2 L/min/m2 with supportCitation8,Citation10,Citation13, and elevated LV filling pressures (i.e. pulmonary capillary wedge pressure (PCWP) ≥15 mmHgCitation8–10 or >18 mmHg)Citation13.
Vasoactive medications, such as vasopressors and inotropes are considered the cornerstone of pharmacological treatment of CSCitation10,Citation12,Citation21, and are administered in about 90% of the patientsCitation22. They improve end-organ perfusion by increasing CO and blood pressure (BP)Citation12. However, they should not be used at high doses and for prolonged durations because they increase oxygen consumption of the myocardium and induce vasoconstriction that elevates the afterload and impairs the microcirculationCitation15,Citation22. Since the publication of the SHOCK trialCitation8, an immediate coronary revascularization has been recommended in patients presenting with AMI and complicated by CSCitation9,Citation10,Citation12,Citation23–25. In the setting of coronary revascularization, the pharmacologic vasoactive agents and mechanical circulatory support are considered the only therapeutic options available for the hemodynamic supportCitation11,Citation15. Despite the wide use of vasopressors and inotropes and the long experience with such drugs (i.e. since 1950s)Citation13, there are few studies available to help in guiding the drug selection as an initial and subsequent choice in CSCitation10,Citation13. As such, a critical review was conducted to analyze the currently available randomized controlled trials (RCTs) of vasoactive agents in patients presenting with CS to determine the indications of each drug and to systematically evaluate the methodological quality of these studies.
2. Methods
2.1. Search strategy
An electronic PubMed literature search of pertinent studies was independently conducted by two authors on 31 March 2020. The search aimed to find clinical trials performed with at least one group treated with an inotropic or vasopressor drug in critically ill patients with CS. The MeSH terms included: “Cardiogenic Shock”, “Cardiotonic Agents”, “Vasoconstrictor Agents”, “Vasodilator Agents”, and the individual agents (epinephrine, norepinephrine [NE], vasopressins, dobutamine, dopamine, amrinone, enoximone, milrinone, simendan, and “N(G)-monomethyl-arginine acetate”). Terms that indicated other causes of shock (anaphylactic, distributive, hypovolemic, neurogenic, obstructive, septic, and vasodilatory) were excluded. Terms were combined using Boolean operators “AND”, “OR”, and “NOT” to refine the search. The search was limited to “Humans” and “Clinical Trial”. Another literature search was conducted on 22 May 2020 to search for the registered clinical studies on CS using the United States (US) National Institutes of Health Registry (http://clinicaltrials.gov/).
2.2. Study selection and data extraction
The references obtained from the literature search were examined at a title/abstract level for relevance. Potentially relevant studies were retrieved as full articles. A manual search of the reference lists of the retrieved articles and pertinent reviews and meta-analyses was also performed to identify further studies. The selected studies enrolled adult patients and had a random allocation to treatment and comparison. The exclusion criteria included duplicate publications, non-adult studies, retrospective trial designs, conference posters, proceedings, and case reports or series. The data from the included studies were extracted for information about author name, publication year, study objective(s), sample size, inclusion/exclusion criteria, relevant definitions, interventions, comparators, outcomes, results, limitations, and conclusions.
2.3. Quality assessment
The internal validity of the selected studies was critically appraised using Jadad scale, a validated tool to evaluate the methodological rigor of RCTs. The three-item scale examines the following aspects of a trial: randomization and its description, double-blinding and its description, and patient disposition (i.e. dropouts or withdrawals). For each aspect, one point is awarded if present. An additional point is awarded for or deducted from the randomization and the double-blinding scores if their methods are appropriate or not, respectively. The five-point score ranges from 0 to 5, with scores between 0 and 2 indicating poor quality, and scores between 3 and 5 indicating good qualityCitation26–28. An expanded version of the scale (i.e. modified Jadad scale), is a six-item scale that addresses some of the limitations of the original scale with a score ranges from 0 to 8 with higher score indicates better quality. The additional items include description of inclusion/exclusion criteria, assessment of adverse effects, and description of statistical analysis with a point awarded for each if presentCitation29. The quality assessments of the selected studies were independently conducted by two authors with divergences resolved by a discussion with a third author and then having a consensus. For the purpose of this review, the validated three-item Jadad scale was used to compare and discuss the methodological quality of the included studies. However, the modified scale with three additional elements was also presented to provide a comprehensive quality overview of each study. The modified scales are not valid nor reliable unless they are validated and tested for reliability. Moreover, the presence of the following important aspects was stated for each study, allocation concealment, intention-to-treat analysis (ITT), and justification of the sample sizeCitation30.
3. Results
3.1. Study screening
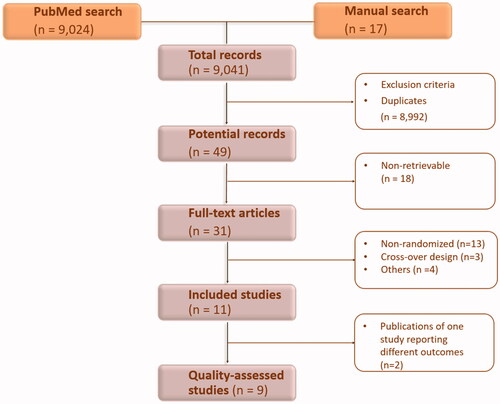
The electronic and manual literature searches resulted in a total of 9041 records that were screened at the title/abstract level. After excluding 8992 articles for irrelevance and duplication, the yield was 49 potential studies. Of these, 38 studies were excludedCitation31–68. The 11 remaining studies were identified as eligible for the inclusion in the review. Nine of them were assessed for methodological qualityCitation69–79, because twoCitation73,Citation74 of the 11 studies were additional publications of one studyCitation72 that reported different outcomes. The process of study selection and exclusion is presented in .
3.2. Study characteristics
The main characteristics of the selected studies are presented in . Of the nine studies, one was a Phase II dose-ranging studyCitation78 and two analyzed the patients presenting with CS in predefined subgroups analysis (289 of 1740 patients)Citation69,Citation76. The nine studies randomized 2388 patients (937 patients with CS) from 174 sites in total, mostly in Europe between 1999 and 2016. All the studies enrolled patients between 2003 and 2010, except two of them. One between 1999 and 200277 and the second between 2011 and 2016Citation71. The mean age of patients ranged between 62 and 69 years. There was variation in patient volume with seven studies were of small sample size ranging from 22 to 79. There were slight variations in the etiologies of CS between the studies. Of the nine studies, a total of sevenCitation71,Citation72,Citation75–79 investigated CS in the setting of AMI. Of the seven studies, four had clear definition of AMI in term ST-segment elevation or depression, elevated cardiac markers, and/or presence of a new left bundle branch blockCitation75,Citation77–79. Five studiesCitation71,Citation72,Citation75–77 mandated percutaneous coronary intervention (PCI), while in two studies, PCI was performed in 90%Citation78 and up to 97%Citation79 of the patients. In three studiesCitation75,Citation77,Citation78 all patients received IABP, while in another three it was used in 28%Citation76 and 90%Citation79 of patients, or at physician’s discretionCitation72. The definition of CS in the nine studies included hypotension, clinical signs of tissue hypoperfusion, and/or diagnostic criteria utilizing invasive cardiac monitoring to measure CI and PCWP. The primary endpoints of the studies included either clinical endpoints (mortality) or surrogate markers of hemodynamic stability. Mortality at 28 − 30 d was reported in four studiesCitation69,Citation75,Citation77,Citation79, while the other studies investigated various hemodynamic or echocardiographic parameters, such as mean arterial blood pressure (MAP), CI, CO, and wall-motion score index (WMSI)Citation70–72,Citation76,Citation78. In the eight studies that reported left ventricular ejection fraction (LVEF)Citation70–72,Citation75–79, the estimated mean LVEF across the studies was 28.75% (standard deviation [SD] = 6.0). Of these, only one studyCitation76 reported a mean LVEF of >35%.
Table 1. Summary of retrieved studies.
3.3. Vasoactive therapy
Three studies investigated vasopressor and/or inotropic medicationsCitation69–71, three tested the inodilator, levosimendanCitation72–76, and three studied the nitric oxide synthase inhibitors (NOSi)Citation77–79. The comparisons evaluated the efficacy of NE vs. dopamineCitation69, epinephrine vs. NE with or without dobutamineCitation70,Citation71, levosimendan vs. dobutamineCitation72–74, enoximone (i.e. phosphodiesterase inhibitor [PDEi])Citation75 or placeboCitation76, and NOSi vs. placeboCitation77–79.
3.3.1. Vasopressors/inotropes
3.3.1.1. Epinephrine vs. norepinephrine
Two small studies compared epinephrine to NE or NE-dobutamine in CS complicating AMI (the OptimaCC study)Citation71 or in HF patients without MICitation70, respectively. In the latter setting, both drugs improved the hemodynamic parameters compared to baseline such as MAP and CI (p < .01). However, in the epinephrine group, heart rate (HR) was significantly higher (p < .05) than in NE-dobutamine group and was associated with arrhythmia in three patients. Arterial lactate concentrations decreased in NE-dobutamine group (p < .01), while increased in the epinephrine group (p < .01) when compared to baseline values. Lactate level, lactate/pyruvate ratio, and PCO2 gap were significantly higher in the epinephrine group as well. No clinical outcomes have been investigated in this pilot studyCitation70. In the OptimaCC, a study with a robust methodological design, the changes in CI, systolic and diastolic arterial pressure, MAP, evolution of stroke volume index, and cardiac power (CPO) index were not significantly different between the two arms. However, there was a significant increase in the mean HR with epinephrine use only (p = .031), explained as having a more potent ß1-receptor activity. The metabolic changes that were statistically different between the groups included, higher metabolic acidosis (p = .0004) and lactate level (p < .0001) in the epinephrine group, whereas, increased arterial pH and decreased lactate level in the NE group. With regards to safety outcomes, there was no statistically difference in the incidence of arrhythmia between the groups (41% vs. 33%; p = .56). The study was stopped prematurely due to higher incidence of refractory CS in the epinephrine group (37 vs. 7%; p = .011). Although the relatively small sample size and early termination, the difference in refractory CS was statistically and clinically significant. The 60-d mortality did not differ significantly between epinephrine (52%) and NE (37%) groups (p = .25) with a trend toward higher mortality in association with epinephrine use on Day 7 (p = .08). In addition to a significant increase in the composite of death and extracorporeal life support (ECLS) use at Day 7 (p = .031) in the epinephrine armCitation71. Cautious interpretation, however, is warranted given the lack of power for such outcomes, not being pre-specified endpoints, and the lack of standardized criteria for ECLS initiationCitation19,Citation71.
3.3.1.2. Norepinephrine vs. dopamine
De Backer et al., in their rigorous SOAP II study, recruited 1679 patients and compared dopamine to NE as a first-line vasoactive agent selection in any shock state, such as cardiogenic, septic, and hypovolemic. There was not a significant difference in 28-d mortality (52.5 vs. 48.5%; OR 1.17, 95% CI 0.97 − 1.42; p = .10) between the study arms with more arrhythmic events in the dopamine group. Overall, 18.4% of the patients had arrhythmia with atrial fibrillation (AF) as the most common type which occurred in 86.1% of those patients. More patients experienced arrhythmia in the dopamine group than in the NE group (24.1 vs. 12.4%, p < .001). Consequently, the drug discontinuation rate due to arrhythmia was higher in the dopamine group (6.1 vs. 1.6%, p < .001). Dopamine was associated with a higher 28-d mortality rate (p = .03) in the predefined subgroup analysis of patients who presented with CS (n = 280)Citation69. However, the study was not powered to test the difference in patients with CS, thus the results of the CS subgroup should be interpreted with caution. In addition, the lack of operationalized definition of CS, evaluation across the hemodynamic phenotypes of CS, or information about MI or HF variables, could have confounded the findings of the studyCitation10.
3.3.2. Inodilators
Three studies of this review randomized 115 patients to receive levosimendan and compare it to dobutamineCitation72–74, enoximoneCitation75, or matching placeboCitation76 in the setting of AMI. In primary PCI-treated patients (n = 22) who developed CS secondary to severe LV systolic dysfunction, levosimendan was compared to dobutamine to evaluate their effects on hemodynamic measuresCitation72, LV diastolic functionCitation73, and long-term survivalCitation74 in three separate publications of one study. After 24 h of therapy, levosimendan was consistently better in increasing CI, LVEF (55 vs. 45%, p = .003), and CPO. There was no difference in the PCWP reduction, HR increase, change in the SBP, or serious adverse effects between the groups. As a mathematical product of CO and MAP, CPO is an indicator of cardiac contractility and ventricular–vascular coupling. It was considered a prognostic predictor of survival in CS in previous studiesCitation72. Improvement in echocardiographic diastolic measures, from baseline to 24 h after initiation of drug infusion, included significant reduction in isovolumetric relaxation time (from 116 to 70.4 ms, p < .001) and significant increase of the E/A ratio (from 0.6 to 1.4, p < .001) in patients on levosimendan. In contrary there were non-significant changes in the aforementioned measures in patients on dobutamine (from 114.7 to 102 ms and from 0.7 to 0.9, respectively)Citation73. The short-term improvement in the hemodynamic and echocardiographic measures did not translate into long-term clinical benefit (i.e. 12-month mortality)Citation74. This open-label study, however, had a small sample size to have a meaningful implication and was not powered to investigate mortality outcomeCitation72–74. Fuhrmann et al. in another small study (n = 32)Citation75 reported a higher survival rate at 30 d when compared levosimendan to enoximone (68.7 vs. 37.5%, p = .023). The main cause of death was a progressive and refractory HF, while multiorgan failure occurred only in the enoximone group and was responsible for death in 25% of patients in this study arm. Except for mixed venous oxygen saturation, there was a trend toward improvement in CI and LV stroke work index that did not reach statistical significance. Furthermore, the onset of rhythm disorders did not differ between the groups. This single-center, open-label study was terminated after an interim analysis that accepted a lower difference of effect between arms, which would have affected the power of the study. Husebye et al., in the LEAF study (n = 61)Citation76, demonstrated that levosimendan in patients with AMI complicated by symptomatic HF significantly improved regional contractility. The authors reported significant change in WMSI from baseline to Day 5 (effect size 20.13, 95% CI 0.255–0.013, p = .031). There was no statistically significant difference in secondary outcomes such as changes in NT-proBNP levels, time to major adverse cardiovascular events, time to first rehospitalization for HF, or six-month all-cause mortality. For the safety outcomes, there were more episodes of hypotension in patients on levosimendan compared to those on placebo (p = .029), with no difference in the use of vasopressor between the two groups. There was a lack of significant difference in the rates of sinus tachycardia, ventricular arrhythmias, AF, or ischemic episodes. In this study, only few patients presented with CS (19%). Patients tolerated levosimendan therapy and had similar safety and efficacy outcomes as compared to the whole study population.
3.3.3. Nitric oxide synthase inhibitors
Three studies in this review randomized 507 patients to receive NOSi and compared it against placebo or supportive care in patients presented with AMI complicated by a persistent or refractory CSCitation77–79. In the LINCS study (n = 30), Cotter et al. reported the occurrence of 30-d mortality in 27% of patients on NG-Nitro-L-Arginine-Methyl Ester (L-NAME) as compared to 67% on supportive care alone (p = .008). The cause of death in most of the cases was during the first week due to shock or multiorgan failure. The one-month and one-week survival rates in the L-NAME group were 73% and 80% compared with 33% and 40% in the supportive care group, respectively. Treatment with L-NAME resulted in statistically significant benefit in other secondary outcomes, such as increase in MAP and urine output, and decrease in times on IABP support and mechanical ventilation without excess of adverse eventsCitation77. Tilarginine (L-N-monomethyl-arginine [L-NMMA]) resulted in modest early increase in MAP and did not show benefit in term of 30-d mortality benefit when was investigated in SHOCK-2, a Phase II dose-ranging study. However, the study was not powered to assess the effect on mortalityCitation78. Consequently, the TRIUMPH study, an international, multicenter RCT was designed and powered to investigate the effect of tilarginine on 30-d mortality. Although tilarginine caused a greater increase in SBP at two hours compared to placebo (12.0 vs. 7.0 mmHg; p = .001), it had no effect on 30-d (48 vs. 42%; risk ratio 1.14, 95% CI 0.92–1.41; p = .24) or six-month all-cause mortality rates, either in the overall group or in any prespecified subgroups. In 78% of patients, the cause of death was of cardiac origin with 50% of them due to pump failure. In addition, tilarginine was well-tolerated but had no effects on the resolution or duration of CS, 30-d myocardial reinfarction, 30-d New York Heart Association functional class, or renal function. As a result, based on futility analysis, the study was terminated early after the enrollment of 398 patients of the planned sample size of 658 patientsCitation79.
3.4. Quality assessment
The mean Jadad score of the nine studies of this review is 3.33 (SD = 1.65). The Jadad scores of each study are provided in . The scores were rated by two authors with an agreement on the scores of all the studies except two before resolving the discrepancies. The Jadad scores show variation in research quality ranging from 1 to 5. Three studies were deemed high-quality methodologiesCitation69,Citation71,Citation76 that received a score of 5 because of appropriate randomization, double-blinding, and patient disposition. The three studies varied in size, vasoactive therapy comparisons, and primary outcomes. However, two studies (SOAP II and LEAF)Citation69,Citation76 did not have CS as a sole inclusion criterion, instead patients presenting with CS were analyzed in pre-defined subgroups. The two studies that had a Jadad score of 4, were Phase II (SHOCK-2)Citation78 and Phase III (TRIUMPH)Citation79 studies of tilarginine. The latter study was stopped due to futility. By excluding the aforementioned two studiesCitation78,Citation79, the mean Jadad score of the other seven studies was not affected (3.28; SD = 1.79). One studyCitation75 was graded a score of 3 due inadequate reporting of the randomization and double-blinding processes. The remaining three studiesCitation70,Citation72,Citation77 scored less favorably, with Jadad scores of 2 or less and were deemed poor methodological design burdened by inadequate randomization and blinding. One pilot studyCitation70 was given 2 points for the use of randomization and blinding. An open-label studyCitation72 was given 1 point for reporting dropouts, and the last oneCitation77 received a score of 1 for the use of randomization. The quality of the nine studies did not differ when they were evaluated using the modified Jadad scale with a mean score of 6.11 (SD = 1.83). The nine studies described the inclusion/exclusion criteria and the methods of statistical analysis, whereas, seven studiesCitation69,Citation71,Citation72,Citation76–79 reported adverse effects assessment. Of the nine studies, threeCitation70,Citation72,Citation77 did not conceal allocation, threeCitation70,Citation72,Citation75 did not state whether the statistical analysis was based on ITT principle, and threeCitation70,Citation72,Citation77 did not justify the sample size.
Table 2. Jadad scale.
3.5. Registered clinical studies
The search of the US National Institutes of Health Registry using “cardiogenic shock” as a broad term, resulted in 102 studies. Six studies were found for the recruitment status of “Suspended”, “Terminated”, and “Withdrawn”. Whereas, 96 studies were identified for the status of “Recruiting”, “Not yet recruiting”, “Active”, “Not recruiting”, “Completed”, “Enrolling by invitation”, or “Unknown status”. As a result, ten registered studies have been identified. Of them, one has been terminated. An additional registered study has been identified by manual search. A summary of the registered studies for the vasoactive agents in CS is provided in .
Table 3. Registered clinical trials.
4. Discussion
In this critical review, 11 studies of vasoactive therapy in CS were characterized and nine of them were evaluated for methodologic quality. The identified pharmacologic vasoactive therapy was categorized into three groups: vasopressors/inotropes, inodilators, and NOSi. The vasoactive agents may have been associated with hemodynamic beneficial effect but not with mortality benefit. However, levosimendan as add-on therapy improved survival as compared with enoximone but did not improve long-term survival when compared to dobutamine. In contrary, dopamine use was associated with higher mortality and adverse events rates. Epinephrine was associated with a transient lactic acidosis, higher HR and arrhythmia, and inadequate gastric mucosa perfusion. While writing the manuscript, a meta-analysisCitation80 of randomized (n = 6) and observational (n = 13) studies was published and did not show mortality reduction when vasopressors and inotropic agents were used in AMI complicated by CS. However, the analysis pooled data from heterogenous studies in many different aspects.
A meta-analysis presented in a clinical reviewCitation22 using RCTs of the PCI era, found a lower risk of mortality (relative risk 0.70, 95% CI 0.54–0.91) with NE compared to epinephrine or dopamine in the vasopressor subgroups using data from three studiesCitation69–71. Another meta-analysis of individual patient data investigated the association between epinephrine and short-term mortality in patients (n = 2583) presented with CS of any cause. It was reported that the incidence of epinephrine use was 37% and the mortality rate was 49%. The meta-analysis concluded that epinephrine was correlated with 3-fold increase in the risk of mortality compared to other agents (OR 3.3, 95% CI 2.8–3.9) with an adjusted mortality risk of OR of 4.7 (95% CI 3.4–6.4). The association remained robust even after propensity score matching (OR 4.2, 95% CI 3.0–6.0)Citation21. In the CardShock study (n = 219) that prospectively enrolled patients with CS, the 90-d mortality rate was 41% and vasopressor and/or inotropes were used in 94% of the patients. Most of the patients (75%) received NE as compared to those received (21%) epinephrine. In a multivariable logistic regression, epinephrine was independently associated with increased mortality risk (OR 5.2, 95% CI 1.88–14.7; p = .002) which did not change after adjustment or after propensity score adjustment (OR 3.3, 95% CI 1.4–7.7; p = .006). Furthermore, epinephrine was associated with worsening of cardiac and renal biomarkerCitation81. On the other hand, Morici et al. argued from their real-life experience and in contrary to the current opinion that epinephrine may still have a role in the treatment of the low output state. The authors discussed that epinephrine did not cause an increase in HR (101 ± 18.4 at baseline vs. 106 ± 17.6 at the infusion peak), nor in life-threatening arrhythmia. Moreover, it was used as a “pharmacologic bridge” to cardiac transplantation, or a more advanced, intense medical therapy. In comparison to other agents, dopamine required high doses to achieve comparable hemodynamic target at the expense of increasing the HR. Whereas, NE increased peripheral resistance which in turn increased the afterload. In addition, levosimendan required a vasopressor due to its hypotensive effect. However, their work was based on their center’s case-seriesCitation82. After the publication of the SOAP II study, dopamine was no longer preferred as an initial agent in CS. Historically, until the publication of a study by Bellomo et al.Citation83 and two subsequent meta-analysesCitation84,Citation85, it has been thought that dopamine has favorable effects on renal function. As such, administration of a low-dose dopamine in critically ill patients at risk of renal failure did not show significant renal protectionCitation83–85. A meta-analysis comparing dopamine with NE in CS, that did not include SOAP II study, showed a mortality rate at 28 d of 50.3% vs. 29.7% (risk ratio 1.611; 95% CI 1.219–2.129, p < .001; pheterogeneity = .01), arrhythmic events rate (i.e. AF, VF, and ventricular tachycardia) of 29.65% vs. 8.34% (risk ratio 3.426; 95% CI 2.130–5.510, p < .001; pheterogeneity = .875), and gastrointestinal reactions rate of 70.59 vs. 12.86% (RR, 5.474; 95% CI 2.917–10.273, p < .001; pheterogeneity = 0), respectively. Patients who were on dopamine had a significantly higher HR without significant differences in MAP, CI, lactic acid, and urine volume as compared to those on NECitation86. Dopamine exerts an indirect beta-adrenergic effect through the release of neural NE, which may explain the differences between dopamine and NE in patients with CS where there is a depletion in the neurotransmitters, leading to an attenuated response to the indirect adrenergic agentsCitation87. A study found that 75% of patients with CS were treated with NE, while 26% and 21%with dopamine and epinephrine, respectivelyCitation81. Dobutamine increases CO, decreases LV filling pressure, improves cardiac contractility, and is commonly administered with NECitation13. When the hemodynamic effects of dopamine and dobutamine were compared in 13 patients with CS, there were clear differences in their mechanisms of increasing BP and in their effects on LV filling pressure. Dobutamine significantly improved CI and stroke index (p < .05), while dopamine significantly increased LV filling pressure (p < .001) with no differences in other parameters, such as HR, MAP, or systemic vascular resistance (SVR)Citation49. In addition, dopamine and dobutamine, in mechanically ventilated CS patients (n = 8) produced comparable increases in CO. However, dopamine caused higher oxygen consumptionCitation52. Vasopressin is usually used in refractory septic shock as an add-on to support BP. In the VASST study (n = 778), there was no significant difference between low-dose vasopressin and NE in terms of the rates of mortality or serious adverse eventsCitation88. In a retrospective study of patients (n = 36) who had CS complicating MI, vasopressin significantly increased MAP without changing PCWP, CI, urine output, or inotrope requirementsCitation61. In this review, there were no RCTs identified on vasopressin in CS patients. However, there is a Phase IV RCT (NCT02118467) that has vasopressin in one of the treatment arms. The study is investigating vasoactive agents in any shock state with pre-specified analysis for various subgroups of patients including those with different etiologies of shock including CS (i.e. septic, cardiogenic and hypovolemic) ().
Inodilators, such as levosimendan or PDEi improve myocardial contractility and have the potential to induce vasodilation without increasing oxygen requirements. Their evidence in CS is still limitedCitation22. In an observational hemodynamic study (n = 25), levosimendan as a bail-out therapy improved right ventricular (RV) dysfunction in AMI patients presenting with CS as compared to usual care, including dobutamine and NE. It significantly increased CI, enhanced RV CPO index, and decreased pulmonary vascular resistance without changes in central venous pressure or mean pulmonary artery pressureCitation54. In a meta-analysisCitation15 of 17 studies (n = 729), levosimendan after coronary revascularization was associated with significant mortality reduction (OR 0.40, 95% CI 0.21–0.76; p for overall effect .005, pheterogeneity = .33, I2 = 12%), and secondary endpoints improvement, such as CI, length of intensive care stay, AF rate, and troponin I levels. However, only two studies of the meta-analysis pertained to patients with CSCitation74,Citation75. A recent meta-analysis of five non-RCTs (n = 557) concluded that levosimendan use in CS may reduce all-cause mortality (risk ratio 0.62, 95% CI 0.44–0.88; pfor effect = .007, I2 = 36%) and facilitate successful weaning from veno-arterial extracorporeal membrane oxygenation (risk ratio 1.42, 95% CI 1.12–1.8; pfor effect = .004, I2 = 71%)Citation89. An evidence, however, from an RCT should confirm the findings. In this review, there were no RCTs identified for milrinone, a commonly used PDEi in HF, in the literature search. A subgroup analysis of an old study (n = 40) in patients with severe HF was conducted for 18 patients with severe cardiac pump dysfunction with only three patients of them were in CS. The study examined the systemic and pulmonary arterial hemodynamics before and after milrinone infusion. Including the patients with CS, milrinone led to an overall improvement in hemodynamics (e.g. CI and PCWP) without producing pronounced decrease in BPCitation53. In another old study in CS (n = 20), dopamine/milrinone combination was compared to a standard regimen of dopamine/dobutamine. The former combination was beneficial in reducing the pre- and after-load, in addition to the myocardial oxygen demand but at expense of decreasing MAPCitation40. An ongoing randomized study will compare milrinone vs. dobutamine in a heterogeneous group of critically ill patients, including those presenting with CS (ClinicalTrials.gov Identifier: NCT03207165) (). Finally, the role of enoximone in CS was described earlier in 13 patients with persistent CS despite dobutamine use. It was used as an add-on agent and resulted in significant increases in CI and stroke volume index, and significant decrease in PCWP without a change in MAP. Twelve of the 13 patients survived the CS event, and five patients discharged alive from hospitalCitation64. Three years prior to the publication of the TRIUMPH study, another international RCT of NOSi 546C88 (or tilarginine) in septic shock was also terminated prematurely due to increased 28-d mortality (59 vs. 49%; p < .001)Citation90. Moreover, a feasibility study (ClinicalTrials.gov Identifier: NCT00782652) on inhaled nitric oxide for the treatment of CS due to RV infarction has been terminated after a year of its start date due to slow enrollment ().
It has been established that basic intensive care unit management of CS unresponsive to fluid therapy includes vasopressors and inotropes ( and ), in addition to other therapy, to prevent or treat multiorgan failureCitation9,Citation12,Citation18,Citation22,Citation91,Citation92. Vasodilators are sometimes used as wellCitation9. Vasopressor agents increase myocardial contractility and SVR through beta- and alpha-adrenergic receptors, respectivelyCitation2. Whereas, inotropes/inodilators (e.g. dobutamine, enoximone, milrinone, amrinone, and levosimendan) increase myocardial contractility and reduce SVR for LV unloadingCitation9. At early stages, inodilators may be needed due to elevated SVR (i.e. a compensatory response to sustain BP and organ perfusion). Subsequently, as the SVR decreases, due to the mounting systemic inflammatory response, only vasopressors can counteract the reduced SVR and usually at higher dosesCitation9,Citation11. Thus, along the different stages of CS, the choice of the vasoactive agent may vary from pure inotropic agent or inodilator support to a combination of inotropes and vasopressorsCitation11. Reestablishing adequate macro- and micro-circulation to maintain oxygen supply at the cellular level, and modulating the systemic inflammatory response to prevent cellular damage is the ultimate goal to avoid multiorgan system dysfunction. Because once the cellular damage is irreversible, any additional intervention has no mortality benefitCitation9. The current international guidelines do not have a universal agreement on the first-line vasoactive agent in CS (). None of the guidelines have graded NE a Class I recommendationCitation10,Citation12,Citation18,Citation23,Citation92–94. Two guidelines have opted an individualized approach based on CS etiology and/or phenotypeCitation10,Citation23, which would explain the continuous use of either NE or dopamine in 50% of CS cases and clarify the absence of unified recommendations. It is recognizable that evidence supporting many of the guidelines’ recommendations is weak or lackingCitation95. The current limited evidence on vasoactive agents use in CS resulted in wide knowledge gaps and potential areas of research, such as the definition of an optimal vasoactive agent, optimal timing of treatment, combination of different vasoactive agents, and testing of agents across various CS etiologies and severitiesCitation9,Citation11,Citation14,Citation95,Citation96.
Figure 2. Targets of vasoactive agentsCitation11,Citation13,Citation22.
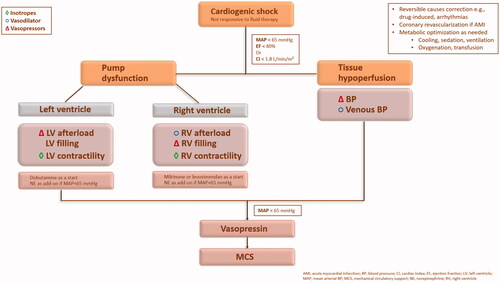
Table 4. Vasoactive agentsCitation10,Citation12,Citation18,Citation91,Citation92.
Table 5. First-line vasoactive agent in guidelines.
The assessment of methodological quality using a reliable and valid instrument (e.g. Jadad scale) is essential to capture the variations in the quality of the studies which may affect the overall conclusion of the resultsCitation30. Quality assessment investigates the validity constructs of a study which includes the internal validity (i.e. study’s methods), external validity (i.e. study’s results), and statistical analysisCitation97. The elements that have shown to change the treatment effects are lack of randomizationCitation98, inadequate allocation concealmentCitation99,Citation100, absence of blindingCitation100, and inadequate sample sizeCitation101,Citation102. The quality across the studies in this review was variable. Randomization and blinding are challenging in the context of CS. Thus, such obstacles would probably be reflected in the slow enrollment that causes study termination or withdrawal. Basic methodological standards support the consideration of other elements that may empirically influence the quality of the study. Elements including appropriate patient disposition description, inclusion, and exclusion criteria definitions, statistical analysis, outcomes objectivity, ITT, and baseline comparability can affect the quality of a study as well. However, this was not supported by respective studiesCitation30.
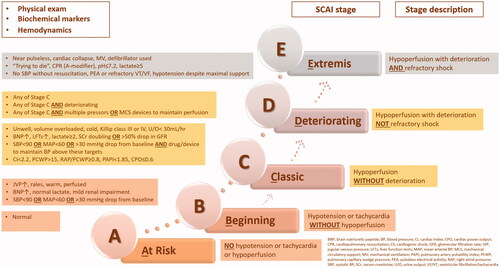
This review has some limitations. The included studies were generally of small volume and most of them investigated CS in the setting of AMI and LV dysfunction. There were slight variations in the etiologies and definitions of the CS. The primary endpoints of the studies focused on short-term mortality and surrogate markers of hemodynamic or echocardiographic parameters. Randomized studies on vasoactive therapy are generally difficult to perform in critically ill population, who are often excluded from contemporary studies. The challenge commences from obtaining an informed consentCitation10,Citation14. Data of the studies on CS are usually extrapolated from chronic HF studies which sometime have heterogeneity, poor quality and other limitations, leading to conflicting conclusionsCitation82. There are few adequately-powered RCTs on CS that have completed patient recruitment with the planned patients’ numberCitation10. The results of any study should be interpreted with caution in the presence of an inadequate power and sample sizeCitation101,Citation102. A larger study with adequate follow-up duration enhances the robustness and generalizability of the results. Large sample size is important for the detection of small differences in the effects. Elements needed to calculate sample size should include, the estimated outcomes in each group and the type I and type 2 error levelsCitation103. In this review, the sample size of the studies is considered small in general with volumes of less than 100, which may render the results inaccurate. The SOAP II study has a large sample sizeCitation69, however, the study was powered for shock patients in general and lacked specific important data on CS. The setting of CS is heterogeneous in many aspectsCitation11, including the heterogeneous population. Thus, therapeutic benefit, clinical outcomes, and prognosis may fluctuate in different patient subsets based on CS etiologies, CS hemodynamic phenotypes, severity of CS, and presence of comorbiditiesCitation19,Citation20. In some instances, there could be a delay in starting treatment after the onset of hemodynamic instability, which could influence the clinical presentation of the patientCitation11. Moreover, sometimes very sick patients may not fully benefit from the treatment, while others may improve with or without treatment. The presence of a standardized classification system would help in the differentiation between patient subgroups and the identification of the differences between studies. The Society for Cardiovascular Angiography and Interventions (SCAIs)Citation20 has proposed a simple, novel, consensus-based classification schema for CS risk stratification that has been endorsed by several societies. The classification describes five CS stages starting from A “at risk” to E “extremis”, i.e. from pre-hospital providers to intensive care staff. The stages in between are B “beginning”, C “classic”, and D “deteriorating”. The descriptors of each stage include physical exam (or bedside finding), biochemical markers, and hemodynamic parameters (). The general and non-rigid definition of each stage is practical in accommodating the clinical parameters variabilities upon patient presentationCitation104. Several studies have validated the SCAI classification in the acute settingsCitation104–110. Jentzer et al.Citation104 retrospectively investigated the construct validity of the SCAI classification at the time of cardiac intensive care unit admission and found a robust association between the SCAI CS stages and hospital mortality in heterogenous critically ill patients (n = 10,004). Upon further analysis of the hospital survivors (n = 9096)Citation106, the aforementioned group demonstrated that the SCAI classification at admission predicted the post-discharge mortality as well. Similarly, Schrage et al.Citation107 reported independent association of the SCAI classification and the 30-day mortality in patients presented with CS or large AMI (n = 1004). Baran et al.Citation108 were the first to prospectively show that initial SCAI stage was predictive for survival in unselected critically ill patients (n = 166). Moreover, they found that the reassessment of SCAI stage at 24 h has refined the prognosis. The SCAI shock classification has also been validated retrospectively in specific subsets of patients, i.e. after out of hospital cardiac arrest (n = 393)Citation109 and AMI (n = 300)Citation110. Additionally, from an analysis of a nationwide registry, Thayer and colleagues have validated the SCAI classification in the prediction of in-hospital mortality in patients presenting with CS (n = 1414). They have also demonstrated the association of both escalated SCAI stages and in-hospital mortality with worsening venous congestionCitation105. Using complete hemodynamic data that is derived from an early placement of a pulmonary artery catheter was associated with a lower in-hospital mortality in patients with CS as compared to incomplete or no data useCitation111. Finally, in this review, there were variations in the primary outcomes reported. Since CS is associated with high death rate, in-hospital mortality as an endpoint would be more appropriate and more beneficial than surrogate marker of the hemodynamic stability. Taken together, a “one-size-fits-all” approach in CS cannot warrant optimal management and safe generalizability. Therefore, an individualized approach may be more appropriate.
Figure 3. SCAI shock stagesCitation20,Citation104.
5. Conclusion
CS is an acute complex condition leading to morbidity and mortality. Despite the advances in coronary revascularization and mechanical circulatory support, the rates of mortality due to CS are still high. The evidence for the use of vasoactive agents in CS carries uncertainties. The analyses of available studies demonstrate benefits of some agents over others without a robust evidence for an absolute first-line agent. This is probably reflected on the various international guidelines’ recommendations. The methodological quality between the studies is variable, owning to the diversity in CS etiologies and phenotypes and to the inherent difficulties to conduct a study in the setting of CS. Patients enrollment is a challenge when adequately powered study is a must for reliable results. Appropriate blinding and randomization cannot always be guaranteed. The methodological limitations in most of the studies may render the results inconclusive. Overall, the quality of CS studies may underperform. The crucial goal in the management of CS is to prevent tissue hypoperfusion and the consequent multiorgan dysfunction by maintaining the hemodynamic stability. Given the lack of therapeutic alternatives, vasopressors and inotropes continue to play a fundamental role. According to the best available evidence and the balance between the risk and benefit of vasoactive agents, epinephrine is disregarded as an initial option, and tilarginine is considered futile in CS. The use of NE alone or in combination with an inodilator (e.g. dobutamine or levosimendan) as appropriate may be suggested. Nonetheless, the choice of an initial vasoactive agent is still controversial. There is a need for adequately powered and well-designed studies to address the current controversies and explore the unanswered clinical questions.
Transparency
Declaration of funding
The publication of this article was funded by the Qatar National Library.
Declaration of financial/other relationships
The contents of the article and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None stated.
References
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734.
- Gamper G, Havel C, Arrich J, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2016;2(2):CD003709.
- Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the sepsis occurrence in acutely ill patients (SOAP) study. Crit Care Med. 2006;34(3):589–597.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- Alberti C, Brun-Buisson C, Chevret S, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med. 2005;171(5):461–468.
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296.
- Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448–454.
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625–634.
- Schumann J, Henrich EC, Strobl H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1(1):CD009669.
- van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American heart association. Circulation. 2017;136(16):e232–e268.
- Squara P, Hollenberg S, Payen D. Reconsidering vasopressors for cardiogenic shock: everything should be made as simple as possible, but not simpler. Chest. 2019;156(2):392–401.
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200.
- Amado J, Gago P, Santos W, et al. Cardiogenic shock: inotropes and vasopressors. Choque cardiogénico – fármacos inotrópicos e vasopressores. Rev Port Cardiol. 2016;35(12):681–695.
- Shaffer A, Sheikh O, Prasad A. Cardiogenic shock: a systematic review of clinical trials registered with clinicaltrials.gov. J Invasive Cardiol. 2020;32(4):E86–E96.
- Maharaj R, Metaxa V. Levosimendan and mortality after coronary revascularisation: a meta-analysis of randomised controlled trials. Crit Care. 2011;15(3):R140.
- Goldberg RJ, Samad NA, Yarzebski J, et al. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340(15):1162–1168.
- Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. JAHA. 2014;3(1):e000590.
- Ibanez B, James S, Agewall S, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC. Eur Heart J. 2017;39(2):119–177.
- van Diepen S. Norepinephrine as a first-line inopressor in cardiogenic shock: oversimplification or best practice? J Am Coll Cardiol. 2018;72(2):183–186.
- Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37.
- Léopold V, Gayat E, Pirracchio R, et al. Epinephrine and short-term survival in cardiogenic shock: an individual data meta-analysis of 2583 patients. Intensive Care Med. 2018;44(6):847–856.
- Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683.
- O’Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice. Circulation. 2013;127(4):e362–e425.
- Amsterdam EA, Wenger NK, Brindis RG, et al. AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228.
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
- Moher D, Jadad AR, Nichol G, et al. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Clark HD, Wells GA, Huët C, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20(5):448–452.
- Wolfson C, Perrault A, Demers L, et al. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord. 2001;12(3):232–236.
- Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175.
- Coma CI, López-Sendón J. Efecto hemodinámico de la dobutamina en el shock cardiogénico [Hemodynamic effect of dobutamine in cardiogenic shock]. Rev Esp Cardiol. 1981;34(6):483–489. ‐
- Aroński A, Kübler A, Sliwinski M, et al. Die Anwendung von Dopamin bei schock und Herzinsuffizienz [The use of dopamin in shock and heart failure (author's transl)]. Anaesthesist. 1978;27(4):183–186.
- Belskii NE, Pilipenko VA, Roshchin SI, Shira AI, et al. Use of nitroglycerin in the treatment of acute heart failure and cardiogenic shock in patients with myocardial infarction. Cor Vasa. 1987;29(2):89–97.
- Iversen S, Mayer E, Hake U, et al. Efficacy of phosphodiesterase inhibitor enoximone in management of postcardiotomy cardiogenic shock. Scand J Thorac Cardiovasc Surg. 1992;26(2):143–149.
- Nathan CR, Dowling LE. S. Dopamine versus norepinephrine in the treatment of shock. CJEM. 2011;13(6):395–397.
- Bahloul M, Tounsi A, Ben Algia N, et al. Does change of catecholamine use improve the outcome of patients with shock admitted to intensive care unit? Am J Ther. 2014;21(5):358–365.
- Anshelevich IV, Prutkin AF, Rubin AI, et al. Primenenie nitroglitserina v kompleksnom lechenii kardiogennogo shoka u bol'nykh infarktom miokarda [Use of nitroglycerin in the complex treatment of cardiogenic shock in patients with myocardial infarction]. Kardiologiia. 1988;28(4):87–89.
- Korewicki J, Ostrzycki A, Opolski G. Własne doświadczenia w leczeniu wstrzasu kardiogennego w przebiegu świezego zawału serca dopamina i nitroprusydkiem sodu [Our experience with the treatment of cardiogenic shock in recent myocardial infarction with dopamine and sodium nitroprusside]. Kardiol Pol. 1984;27(5):345–351.
- Trojnar R, Kutarski A, Kedra WA, et al. Zastosowanie dobutaminy w leczeniu wstrzasu zawałowego [Use of dobutamine in the treatment of post-infarction shock]. Wiad Lek. 1983;36(7):531.
- Meissner A, Schmelzle T, Simon R. Differentialtherapie des kardiogenen Schocks mit Dopamin/Milrinon im Vergleich zu Dopamin/Dobutamin [Differential therapy of cardiogenic shock with dopamine/milrinone in comparison with dopamine/dobutamine. Z Kardiol. 1996;85(11):839–846.
- Wang GJ, Guo YJ, Hou J, et al. Comparison of the effect of dopamine and norepinephrine on cardiogenic shock. Chin J Pract Med. 2011;38:75–76.
- Qiu HX, Lin HL, Gu Y, et al. Comparison of dopamine and norepinephrine on treating patients with cardiogenic shock caused by coronary heart disease. Chin J Mod Drug Appl. 2013;7:139–140.
- He YH, Chen L, Jiao YL, et al. The contrast between dopamine and norepinephrine in the treatment of cardiogenic shock. Northern Pharma. 2014;2:51.
- Gu XF. Contrastive analysis of the therapeutic effect of IABP with dopamine and norepinephrine for treating acute myocardial infarction with cardiogenic shock. Chin J Mod Drug Appl. 2014;13:98–99.
- Tan ZH. The comparison of dopamine and norepinephrine for treating cardiogenic shock. Mod Diagn Treat. 2016;27:67–68.
- Xiong RC, Yu Z, Sun J, et al. Efficacy of dopamine and norepinephrine in patients with cardiogenic shock. Chin J Mult Organ Dis. 2016;15:919–922.
- Li JD, Liu LX, Guo M. Effects of norepinephrine and dopamine on haemodynamics and tissue perfusion in patients with cardiogenic shock. Med Theory Pract. 2015;28:3171–3173.
- Jin X. The comparison between dopamine and norepinephrine in shock. Chin Tradit Med Technol. 2014;1:186.
- Francis GS, Sharma B, Hodges M. Comparative hemodynamic effects of dopamine and dobutamine in patients with acute cardiogenic circulatory collapse. Am Heart J. 1982;103(6):995–1000.
- Fowler MB, Timmis AD, Crick JP, et al. Comparison of haemodynamic responses to dobutamine and salbutamol in cardiogenic shock after acute myocardial infarction. Br Med J (Clin Res Ed). 1982;284(6309):73–76.
- Timmis AD, Fowler MB, Chamberlain DA. Comparison of haemodynamic responses to dopamine and salbutamol in severe cardiogenic shock complicating acute myocardial infarction. Br Med J (Clin Res Ed). 1981;282(6257):7–9.
- Richard C, Ricome JL, Rimailho A, et al. Combined hemodynamic effects of dopamine and dobutamine in cardiogenic shock. Circulation. 1983;67(3):620–626.
- Klocke RK, Mager G, Kux A, et al. Effects of a twenty-four-hour milrinone infusion in patients with severe heart failure and cardiogenic shock as a function of the hemodynamic initial condition. Am Heart J. 1991;121(6 Pt 2):1965–1973.
- Russ MA, Prondzinsky R, Carter JM, et al. Right ventricular function in myocardial infarction complicated by cardiogenic shock: Improvement with levosimendan. Crit Care Med. 2009;37(12):3017–3023.
- Delle Karth G, Buberl A, Geppert A, et al. Hemodynamic effects of a continuous infusion of levosimendan in critically ill patients with cardiogenic shock requiring catecholamines. Acta Anaesthesiol Scand. 2003;47(10):1251–1256.
- Inglessis I, Shin JT, Lepore JJ, et al. Hemodynamic effects of inhaled nitric oxide in right ventricular myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2004;44(4):793–798.
- R R, Jr, Tesarová J, Pechman V, et al. The effects of short-term norepinephrine up-titration on hemodynamics in cardiogenic shock. Physiol Res. 2010;59(3):373–378.
- van Diepen S, Reynolds HR, Stebbins A, et al. Incidence and outcomes associated with early heart failure pharmacotherapy in patients with ongoing cardiogenic shock. Crit Care Med. 2014;42(2):281–288.
- Cotter G, Kaluski E, Blatt A, et al. L-NMMA (a nitric oxide synthase inhibitor) is effective in the treatment of cardiogenic shock. Circulation. 2000;101(12):1358–1361.
- Russ MA, Prondzinsky R, Christoph A, et al. Hemodynamic improvement following levosimendan treatment in patients with acute myocardial infarction and cardiogenic shock. Crit Care Med. 2007;35(12):2732–2739.
- Jolly S, Newton G, Horlick E, et al. Effect of vasopressin on hemodynamics in patients with refractory cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2005;96(12):1617–1620.
- Sicignano A, Bellato V, Riboni A, et al. Enoximone in infusione continua nel trattamento dell' ischemia miocardica acuta con sindrome da bassa gittata [Continuous infusion of enoximone in the treatment of acute myocardial ischemia with low output syndrome]. Minerva Anestesiol. 1994;60(3):109–113.
- Bussmann WD, Wehrheim HG. Therapie des kardiogenen schocks mit dobutamin und nitroglycerin [Therapy of cardiogenic shock with dobutamine and nitroglycerin. Dtsch Med Wochenschr. 2008;108(34):1273–1280.
- Vincent JL, Léon M, Berré J. The role of enoximone in the treatment of cardiogenic shock. Cardiology. 1990;77(3):21–33.
- Myburgh JA, Higgins A, Jovanovska A, et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34(12):2226–2234.
- Ichai C, Soubielle J, Carles M, et al. Comparison of the renal effects of low to high doses of dopamine and dobutamine in critically ill patients: a single-blind randomized study. Crit Care Med. 2000;28(4):921.
- Morici N, Oliva F, Ajello S, et al. Management of cardiogenic shock in acute decompensated chronic heart failure: The ALTSHOCK phase II clinical trial. Am Heart J. 2018;204:196–201.
- Salem R, Mebazaa A. Nitric oxide inhibition rapidly increases blood pressure with no change in outcome in cardiogenic shock: the TRIUMPH trial. Crit Care. 2007;11(3):136.
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789.
- Levy B, Perez P, Perny J, et al. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011;39(3):450–455.
- Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173–182.
- García-González MJ, Domínguez-Rodríguez A, Ferrer-Hita JJ, et al. Cardiogenic shock after primary percutaneous coronary intervention: effects of levosimendan compared with dobutamine on haemodynamics. Eur J Heart Fail. 2006;8(7):723–728.
- Dominguez-Rodriguez A, Samimi-Fard S, Garcia-Gonzalez MJ, et al. Effects of levosimendan versus dobutamine on left ventricular diastolic function in patients with cardiogenic shock after primary angioplasty. Int J Cardiol. 2008;128(2):214–217.
- Samimi-Fard S, García-González MJ, Domínguez-Rodríguez A, et al. Effects of levosimendan versus dobutamine on long-term survival of patients with cardiogenic shock after primary coronary angioplasty. Int J Cardiol. 2008;127(2):284–287.
- Fuhrmann JT, Schmeisser A, Schulze MR, et al. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction. Crit Care Med. 2008;36(8):2257–2266.
- Husebye T, Eritsland J, Müller C, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail. 2013;15(5):565–572.
- Cotter G, Kaluski E, Milo O, et al. LINCS: L-NAME (a NO synthase inhibitor) in the treatment of refractory cardiogenic shock: a prospective randomized study. Eur Heart J. 2003;24(14):1287–1295.
- Dzavík V, Cotter G, Reynolds HR, et al. Effect of nitric oxide synthase inhibition on haemodynamics and outcome of patients with persistent cardiogenic shock complicating acute myocardial infarction: a phase II dose-ranging study. Eur Heart J. 2007;28(9):1109–1116.
- Alexander JH, Reynolds HR, Stebbins AL, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297(15):1657–1666.
- Karami M, Hemradj VV, Ouweneel DM, et al. Vasopressors and inotropes in acute myocardial infarction related cardiogenic shock: a systematic review and meta-analysis. J Clin Med. 2020;9(7):2051. Published 2020 Jun 30.
- Tarvasmäki T, Lassus J, Varpula M, et al. Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care. 2016;20(1):208.
- Morici N, Sacco A, Oliva F, et al. Epinephrine for acute decompensated heart failure and low output state: friend or foe? Int J Cardiol. 2011;149(3):384–385.
- Bellomo R, Chapman M, Finfer S, et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) clinical trials group. Lancet. 2000;356(9248):2139–2143.
- Friedrich JO, Adhikari N, Herridge MS, et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142(7):510–524.
- Kellum JA, Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29(8):1526–1531.
- Rui Q, Jiang Y, Chen M, et al. Dopamine versus norepinephrine in the treatment of cardiogenic shock: a PRISMA-compliant meta-analysis. Medicine (Baltimore). 2017;96(43):e8402.
- Port JD, Gilbert EM, Larrabee P, et al. Neurotransmitter depletion compromises the ability of indirect-acting amines to provide inotropic support in the failing human heart. Circulation. 1990;81(3):929–938.
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887.
- Burgos LM, Seoane L, Furmento JF, et al. Effects of levosimendan on weaning and survival in adult cardiogenic shock patients with veno-arterial extracorporeal membrane oxygenation: systematic review and meta-analysis. Perfusion. 2020;35(6):484–488.
- López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32(1):21–30.
- Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8(8):e011991.
- Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the acute cardiovascular care association of the European society of cardiology. Eur Heart J Acute Cardiovasc Care. 2020;9(2):183–197.
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
- Yancy CW, Jessup M, WRITING COMMITTEE MEMBERS, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–e327.
- van Diepen S, Thiele H. An overview of international cardiogenic shock guidelines and application in clinical practice. Curr Opin Crit Care. 2019;25(4):365–370.
- He B, Kong L, Ge J. Cardiogenic shock: dopamine or norepinephrine? It's a question. Cardiol Plus. 2017;2:1–4.
- Verhagen AP, de Vet HC, de Bie RA, et al. The art of quality assessment of RCTs included in systematic reviews. J Clin Epidemiol. 2001;54(7):651–654.
- Chalmers TC, Celano P, Sacks HS, et al. Bias in treatment assignment in controlled clinical trials. N Engl J Med. 1983;309(22):1358–1361.
- Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613.
- Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412.
- Freiman JA, Chalmers TC, Smith H, Jr, et al. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. N Engl J Med. 1978;299(13):690–694.
- Moher D, Dulberg CS, Wells GA. Statistical power, sample size, and their reporting in randomized controlled trials. JAMA. 1994;272(2):122–124.
- Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869–23. Published 2010 Mar
- Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117–2128.
- Thayer KL, Zweck E, Ayouty M, et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9):e007099.
- Jentzer JC, Baran DA, van Diepen S, et al. Admission Society for Cardiovascular Angiography and Intervention shock stage stratifies post-discharge mortality risk in cardiac intensive care unit patients. Am Heart J. 2020;219:37–46.
- Schrage B, Dabboura S, Yan I, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96(3):E213–E219.
- Baran DA, Long A, Badiye AP, et al. Prospective validation of the SCAI shock classification: Single center analysis. Catheter Cardiovasc Interv. 2020;96(7):1002–1339.
- Pareek N, Dworakowski R, Webb I, et al. SCAI cardiogenic shock classification after out of hospital cardiac arrest and association with outcome. Catheter Cardiovasc Interv. 2021;97(3):E288–E297. https://doi.org/10.1002/ccd.28984.
- Hanson ID, Tagami T, Mando R, et al. SCAI shock classification in acute myocardial infarction: insights from the National Cardiogenic Shock Initiative [published online ahead of print, 2020 Jul. Catheter Cardiovasc Interv. 2020;96(6):1110–1137.
- Garan AR, Kanwar M, Thayer KL, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail. 2020;8(11):903–913.