ABSTRACT
This paper offers a first step to analysing sub-sector variation in firms’ learning and the types of leads or lag in industrial regulation in the Indian health industry, one of the world’s largest and broadest suppliers in critical generics, vaccines, and diagnostics. Sub-sector variation in an industry’s learning and regulation trajectory has received relatively little attention in economic development literature and has potentially important consequences for the design of the industrial policy. Our argument rests on the transfer of complexity of learning in a sub-sector to generic industrial regulations. The paper appeals to evolutionary and institutional (E-I) approaches in economics, which have made significant contributions in improving the understanding of how firms learn, and applies a qualitative heuristic focused on co-evolving institutional domains to extract some insights from the dynamics of the diagnostics and devices sector. The paper finds that although firms continue to learn and innovate, persistent regulatory challenges to firms are generated by the misapplication of industrial policies to diagnostics and devices that were intended for pharmaceuticals and vaccines. Our findings suggest sub-sector specific changes are needed on value priorities for policy design, use, and regulation of diagnostics and devices in healthcare.
1. How firms learn in economic development: regulation design in industrial policy
Economic development scholarship has placed a heavy premium on institutional gains arising from the efforts of private and public firms in building their technological capabilities. This paper contributes a conceptual framework and preliminary novel methodology to address regulatory gaps in industrial policy using India’s health industry, one of the world’s largest and perhaps most diverse. Our argument rests in the transfer of complexity of learning in a sub-sector to rules of industrial organization and industrial policy. Our interest is in addressing the question about the conditions under which existing regulation shapes the learning of firms in industrial sub-sectors such as medical devices and diagnostics. Medical devices and diagnostics cover screening, diagnosis, and treatment. They may include a range of engineering instruments and medical in vivo and in vitro diagnostics (IVD) such as cardiac stents, bone caps, catheters, metal prosthetics, x-ray machines and similar instruments. These devices are valuable in determining the efficacy of a course of therapy or in conducting pharmaceutical research, making them an increasingly important sub-sector in the health industry.
When firms in healthcare industries learn and develop capabilities across time, they do so shaped by norms, guidelines, industry rules, governmental regulations that influence the direction and degree of building technological capabilities. For firms that successfully navigate this wider institutional environment, economic development benefits follow. A sub-sector lens avoids the challenge of seeking a single ‘correct’ way to contrast countries in the health industry. From an evolutionary perspective, institutional change occurs not through single institutions but through bundles of interacting norms and rules that may go together at any time in history and geography (Srinivas Citation2012). Regulatory goals become a pivotal element of industrial policies to shape the multiplicity of rules for pricing, competition, or product variety. Economic development then emerges as a context-specific transformation of learning as firms navigate these regulatory goals and industrial policies. While it is tempting to compare countries by their health industry, disease and technology specificity may drive sub-sector priorities and firms’ responses to any regulation. Some intermediate heuristics can help sort through institutional types and national industries with more direct inferences and judgements regarding the consequences for development (Srinivas, Prasad, and Rao Citation2020).
Sub-sector variation helps clarify why regulation exists and how it can be better designed to bring health and industrial goals closer together. Prior research demonstrates that adjoining countries in the same sub-sector may show substantial variation in building health industry capabilities even if they are broadly agreed on the goals: in Tanzania and Kenya (Mackintosh et al. Citation2018), and those with similar institutional legacies of laws and policies, may diverge in one industry (Russo and Banda Citation2015). Therefore, the importance of local production capability and technological learning depends on the types of learning that firms undertake of export competitors, but also from domestic competitors and often collaborators or those in adjacent sub-sectors. Thus, vaccines and diagnostics firms in cervical cancer may well be potential ecosystem collaborators offering complementary products and services. However, if competition is considered an essential industrial policy ethos for a country, and early screening of a disease a central health policy goal, then whether or not to require and how to regulate competition among early screening diagnostics firms is likely to be an essential element of planning and policy. Such priority issues, many driven by different value premises, do generate challenges for regulatory design if not explicitly discussed and resolved.
Consequently, the wider economics scholarship built on cross-industry learning has highlighted how countries advance through manufacturing, issues that have served well to analyse economic development, maybe poor explanations for why some sub-sectors in the same industry do much better than others and what this implies. The health industry’s unique importance to an economy requires special attention: firms learn and change within an – usually national – institutional context where regulatory changes may be slow, and they may face unique international regulatory features related to quality, safety, efficacy or costs. Firms may sometimes learn irrespective of these national and international regulations, but they may struggle to convert such learning into production and innovation gains. Health industry exporters are also regulated by importer country regulations. Some evolutionary-institutional approaches include systemically bringing health and industrial systems together and determining their mutual institutional overlaps and divergences using a co-evolutionary, combinatorial approach (Srinivas Citation2012); others rest on uncertainties and evolutionary features of system demand (Hodgson Citation1988); some underscore local production capabilities in specific geographies (Mackintosh et al. Citation2018); interventions to define market structure such as intellectual property rights or price regulations (Chaudhuri Citation2005); others on public planning and administrative processes to encourage problem-solving (Russo and Band Citation2015); generics upgrading and market dynamics (Kale Citation2018); or the political economy under which specific stakeholders can improve the health industry’s social mandate (Kale and Wield Citation2019; Papaioannou et al. Citation2016). This vast body of scholarship, primarily built over the last decade and a half, reveals significant country similarities and differences in how industrial capabilities may be converted into health gains.
While the development literature is clear on the challenge, the economics debate on how firms learn can also benefit from the sub-sector lens: the less or more viable organizational routines require attention (Nelson and Winter Citation1982); so do relational firm-level managerial and project execution features (Amsden Citation1989; Lall Citation1983); as does the selection impact of technology and industrial policy (e.g. Lall and Pietrobelli Citation2005), and the specific hurdles of technology transfer and innovation in developing countries (Arocena and Sutz Citation2000; Srinivas and Sutz Citation2008; Chataway et al. 2014). The domestic planning processes require improvement to identifying and adapting the gains in learning within firms to unmet needs and demand, with the evolutionary nature of demand requiring special considerations, including improved regulatory design (Srinivas Citation2018). While industry associations can mediate some of this identification and matching industrial capabilities to domestic demand, national experiences show differences in how the relational process emerges and takes root (Papaioannou et al. Citation2016). However, the healthcare technology industries in some developing countries suffered from the lack of industry associations and their representation in government policymaking, severely affecting the development of opportunities for local firms. Similarly, other non-state actors can play a critical role in acting as centres for investment, technology transfer, and training, as more diffuse ‘knowledge intermediaries’ and in building programmatic ties with the state to represent underserved populations.
The paper is therefore structured as follows. Section 2 briefly discusses the regulatory design and institutional theory. In Section 3, we turn next to the emerging production successes occurring in India, already seen as “supplier to the world’, and whose gains in pharmaceuticals have led to mixed fortunes in medical devices and diagnostics. The methodology, therefore, qualitatively differentiates within-sector variation that tracks the difficulties of translation of learning gains from the highly successful pharmaceuticals sector and vaccines and to struggles of medical devices and diagnostics. We conclude with some implications for regulatory policy and the use of specific industrial policy instruments.
2. Evolutionary-Institutional (E-I) approaches
2.1. Institutional change as a complex, combinatorial process
Differences in learning across sub-sectors matter for a regulatory design that treats the health industry as one institutional story. Consequently, while the theories of learning-induced economic development offer robust explanations for institutional change of a particular type, i.e. single-industry/multiple countries and single-country/multiple industries, they are more hesitant about those that combine two or more institutional domains or those that involve varying degrees of sub-sector differentiation in a single industry. Frameworks of comparative national health industries and their characteristics are analysed to reveal specific institutional contrasts (Malerba and Nelson Citation2012; Srinivas Citation2012; Kale Citation2019; Kale and Little, Citation2007; Mackintosh et al. Citation2016). Iconic health examples such as the Jaipur Foot prosthetic or the Hib vaccine emerged from NGOs or the state by generating detailed health assessments of needs and demand (Srinivas Citation2018).
In evolutionary-institutional explanations, technological capabilities are selected by their policy environment. Including some effect from regulatory lag or catch-up means that as regulators better understand how firms are changing and what incentives or pressures to use, data may demonstrate if they assist firms to grow and innovate, do nothing to help, or worse, place obstacles in the paths of firms. Firms function within a wider institutional environment, and their organizational strategies change with this environment, and their routines endogenise in various ways in the uncertain environment (Nelson and Winter Citation1982). Institutional variety, such as the several ways of building technological capabilities, becomes a key explanatory requirement to understand development (Srinivas Citation2020). Rather than an inevitable progressive system that linearly moves regulation forward, the combinatorial aspects of co-evolution of distinct institutional domains may create open-ended sub-systems and unexpected pathways in which policy selection effects can manifest (Srinivas Citation2020). Focused on single industry sectors, this may be more precisely termed ‘industry trickle up’ since scholarship is less clear in explaining how gains in some sub-sectors of a single industry benefit other sub-sectors such as pharmaceuticals, vaccines, and medical devices (including diagnostics) or ‘trickle horizontally’.
Even amongst those evolutionary perspectives that recognize the benefits of a neo-Schumpeterian perspective, sub-schools offer diverse value propositions for learning goals and the importance of processes and protagonists in building equitable and cohesive versus fragmented systems (Papaioannou and Srinivas Citation2019). Elsner (Citation2017), for example, point to promising cross-fertilization and useful commonalities between different Evolutionary-Institutional (E-I) methods, which include System Dynamics, (Evolutionary) Game Theory, Simulations, Social Fabric Matrix approach using network analysis and graph theory, and others. Thus E-I approaches are not value-neutral either in methods or theory (Elsner Citation2017) because they may include policy design, conflict of means and end, and fundamental issues about technology selection and contingent technology progressiveness (Srinivas Citation2012). Any improvement in methods will require more attention to how specific learning and regulatory roadblocks and the complex paths emerging from industrial policies as currently designed. The more technological advances occur, the more challenging a domestic political economy becomes, not less, as one might assume (Srinivas Citation2020). Therefore, we should expect more sub-sector differentiation to emerge and more regulatory fine-tuning within industrial policy even while some standardization occurs as countries industrialize.
2.2. Sector and sub-sector heuristics: regulation and E-I methods in the context
The case of the Indian health industry (‘Supplier to the World’) and three sub-sectors – pharmaceuticals, vaccines, and diagnostics, permits closer scrutiny of the underlying economic assumptions. The methodology here differentiates within-sector variation that tracks the difficulties of translation of learning gains from the highly successful pharmaceuticals sector to vaccines and medical devices and diagnostics. The argument rests on institutional cohesion and the difficulty of transferring the complexity of learning in a sub-sector to rules of industrial organization.
This section applies a qualitative heuristic (Srinivas Citation2012) to demonstrate the different learning pathways of medical device firms. The heuristic offers a qualitative way to trace different evolutionary features of the sub-sectors and their need for improved regulatory design. The paper uses the triad explained below. It then systematically uses ‘snapshots’ of industrial evolution in one country across the sub-sectors to illustrate the challenge of regulatory design. The authors draw on significant experience in the several sub-sectors and rely on ongoing secondary data and validation from detailed open-ended semi-structured interviewing with medical device and diagnostic firms in India from 2019 to 2021. It involved interviewing over 30 representatives from organizations across diagnostics, biotech, pharmaceuticals, policy think tanks, universities and research institutions, non-profit enterprises and hospitals. While the methods are not quantitatively supported or traditionally triangulated, by definition, they are judiciously contrasted with details from firms’ actual experiences and other interviews, including those from hospitals and other buyers. The experiences are also consistent with other projects in which the authors are involved in which diagnostics firms have struggled with existing regulatory design in their scale-up and expansion. The analysis finds that the methods used in this preliminary way resolve some forms of regulatory lag, which remain unresponsive to how firms learn, and specific challenges they face in one sub-sector of medical devices and diagnostics.
The following steps are followed:
Step 1 The three sub-sectors are briefly analysed to differentiate distinct learning paths for firms in broadly similar regulatory environments.
Step 2 The paper then addresses the case study of the medical devices (devices and diagnostics) sub-sector in more detail. Tables provide the specific types of technological capabilities being built, the industrial regulation lag, and challenges for firms. Specific Indian medical guidelines and regulations are discussed in terms of their implications for market creation and regulation of diagnostics, e.g. affordability and the technical standards and procurement to achieve this.
Step 3 An ongoing multi-country project on cancer care provides interview composites of diagnostic firms and their challenges. These are briefly discussed to point to some of the regulatory issues that firms face and how learning and innovation may emerge and could be strengthened.
3. Qualitative heuristics in the health industry
3.1. Step 1. Co-evolving, combinatorial learning paths and regulations
The methodology employed here uses a qualitative heuristic initially developed for a one-country, one-industry analysis to assess evolutionary snapshots of institutional bundles. The heuristic of the institutional ‘triad’ (Srinivas Citation2012, 8) breaks the analysis and periodization of the health industry into three distinct institutional domains: production (1), demand (3), and delivery (2) ().
Figure 1. Institutional triad. Adapted from (Srinivas Citation2012, 8).
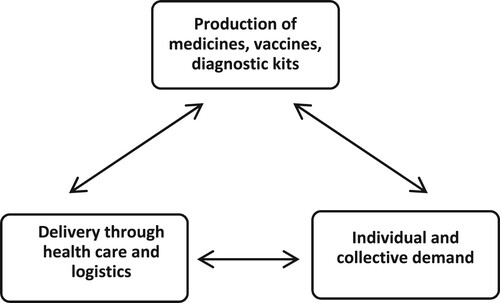
The real-world implications of the co-evolution of these domains reveal an interdependency across the spheres of industrial policy (seen as 1) and health policy (broadly seen as encompassed in 2 and 3). The ‘markets’ of the health industry can thus be described through the three co-evolving domains of production, demand, and delivery, in which industrial development literature has well described the production elements of manufacturing, testing, prototyping of technological capabilities but struggles to connect analytically and programmatically to the demand and delivery political economy of health policy.
The measurement of introduction, lead or lag of policies and how firms learn in conjunction or their absence show that qualitative heuristics can play some conceptual utility for the health industry (Srinivas Citation2012, Citation2016, Citation2020), and evolutionary theorizing has also proceeded along such lines (Elsner Citation2017). ‘History-friendly’ analyses have been mainly relegated to simulations of industrialized economies and industry sectors such as semiconductors and pharmaceuticals (e.g. Malerba and Nelson Citation2012). Unlike simulations, which are sensitive to initial conditions and models of evolution, the qualitative heuristic here is built on the assumption that institutional variety is multidimensional and an ongoing process through which the co-evolution of industrial and health policy occurs. It is intended to build on ‘appreciative theorising’ (Nelson and Winter Citation1982). Thus, from roughly the 1950s to the 2000s, the evolution of India’s health industry with successive capabilities in generic pharmaceuticals, vaccines, and biotechnology capabilities can be captured in a series of national and international ‘snapshots’. We combine this with secondary qualitative data and corroborate its technical features with authors, with economics, S&T policy, and experience working in the Indian diagnostics industry.
Our methods recognize the following practical, administrative features of note in the heuristic above: ‘industrial policy’ (1) is often administered in national ministries of industry and commerce or finance and is traditionally concerned with diverse planning elements for industrial dynamism: set-up, investment, approvals, targets, subsidies or other fiscal instruments, trade tariffs and non-tariff barriers such as technical standards. It also considers compliance with various regulatory functions, including quality and safety, pricing, labour, and different grades and frequencies of checking that the permitted functions undertake to build and maintain a firm or an industry’s standing. As can be seen, features of ‘industrial policy’ may substantially overlap with ‘health policy’ (2,3), although the nature of such overlap is institutionally demarcated and enacted in distinct ways. Notably, and importantly, to differentiate this heuristic versus others, political economy considerations such as democracy itself, or centralized or devolved nature of bureaucracies, can be more explicitly addressed, a point that quantitative evolutionary strategies such as simulation of sub-sector growth may struggle with, but game-theoretical approaches may also offer. These overlapping goals within political economy about health priorities and value assumptions about process design may include items such as appropriate pricing (e.g. ‘affordable medicines’) or safety (which may include technical standards such as laid out by the relevant Food, Drug, or Chemical regulations in the country and specific to the industry).
3.2. Step 2. India’s sub-sector dynamics: pharmaceutical learning and learning from pharmaceuticals
No industry has more resoundingly contributed to such gains in capabilities in select LMICs (low and middle-income countries) than the health industry, and no country with perhaps more evidence of this than India (Lall Citation1987; Sahu Citation1998; Kale and Wield Citation2019; Srinivas Citation2012). While the early industrial policy goals for this industry emphasized infant industry protections (Sahu Citation1998) and later patent policies also played important roles, they were structured through bundled policy instruments to induce a range of industrial capabilities. Specific policy instruments such as canalization – protected imports and procurement of raw ingredients of chemicals, for example – did double duty as regulatory levers to induce a set of long-term capabilities in the national interest (Sahu Citation1998). Srinivas (Citation2006, Citation2016) underscored the challenge of how advances in technological capabilities initially driven by state goals, ironically through considerable public, then private sector success, made policy priorities less obvious and more challenging to regulate. Success in technological capabilities induced more challenges in deploying industrial policy instruments and made regulation more complex. Greater technological capabilities generate new stakeholders and expectations for the provision of goods and services.
Furthermore, in democracies, where accountability and transparency may be under more significant pressure, technological learning in the health industry may have led to greater pressures on access or other demands and made regulation's goals more contested and the path less clear (Srinivas Citation2012; Mackintosh et al. Citation2016). Problem-framing and solving, relatively clear in a ‘first market environment’, led to considerable success for India's path today; yet for both pharmaceuticals and vaccines, different types of export inducements and search and learning successes in a ‘second market environment’ from the early 1970s onward where significant export capabilities were built, led to contrasting consequences for domestic health regulation (Mackintosh et al. Citation2016). Price controls have been a continuous feature of many Indian policies, with mixed effects over classes of problems (Chaudhuri Citation2019). In particular, ‘3 Ws’ (WHO, WTO and Waxman-Hatch) in a second market environment played significant roles in shaping Indian exports (Srinivas Citation2006, Citation2012). WHO guidelines and procurement strategies in close conjunction with national extended programmes of immunization led to more precise institutional networks of developing country vaccine manufacturers and considerable induced technical standards upgrading and rewards from international procurement. Industrial policies are usually applied as bundles of policy instruments that combine and exert effects on firms in various ways, and learning may not be a priority of policy design.
Applying the heuristic to this sub-sector reveals a complex policy pattern from the 1950s-1990s that is simplified and represented as follows to show dominant (not exclusive) combinations.
In , an extrapolation of the heuristic is captured across time in ‘snapshots’ that depict how generic pharmaceuticals under heavy regulatory oversight and industrial clarity of national goals, advanced by the dominance of 2 + 3 dominating 1 in the first market environment (FME). For the second market environment (SME), the growing dominance of technological capabilities in 1, primarily through attractive export markets, is dominated by foreign health, not industrial policy inducements through welfare state buying and institutional procurement. This resulted in the dominance of 1 over institutional norms of 2 and 3. In this explanation, the state is not a passive inducer and responder, or what evolutionary scholars would term a selection environment. Rather, the state actively intervenes to different degrees in all three institutional domains in the heuristic, and all three institutional domains are increasingly ‘industrial’ in organization, not merely the realm of manufacturing production (1). One finding is that the nature of Indian problem-solving has become far more complex over time as a technological advance has occurred in a democracy, and problem-solving in some respects weakened in the Third Market Environment and later as state-supported axes of production (1) became very successful (Srinivas Citation2012, Citation2016) This critical interventional role of the state has been termed by Mazzucato (Citation2018) as entrepreneurial arguing that the state can proactively promote the emerging high growth but high-risk areas by funding the most uncertain phase of the research and even overseeing the commercialization of products or services. The analysis, however, implies a more complex role and challenge for the state. For instance, continued dependence on Chinese APIs continues.
Figure 2. Snapshots for pharmaceuticals. Source: Srinivas (Citation2012) and drawn from primary data collection experience and extensive secondary analysis of the sector.
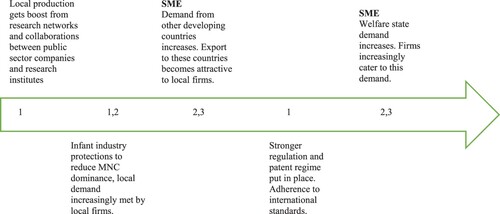
Similarly, regulation is not adaptive to changing demand and delivery shifts. Although delivery (2) in the triad becomes increasingly technologically driven and industrialized (e.g. radiology tests, telemedicine, tele-diagnostics, pathology labs using new business models for just-in-time delivery, or other modifications) and thus itself capable of industrial gains, regulatory design has not adapted. Similarly, consumption/demand (3), especially in growing welfare states, uses technological capabilities to identify people, establish the legitimacy of claims, calculate benefits, and apportion the interface with delivery (Srinivas Citation2016, Citation2020). The importance of these dynamics precedes the challenges for Indian medical devices and diagnostics.
As countries have industrialized, the institutional design of technical upgrading and the building of technological capabilities has increasingly depended on exports. The successes of countries, such as India, in pharmaceutical and vaccines, have underscored the importance of manufacturing improvements that were rewarded by export markets. This was not a feature, however, of every major policy goal. On the contrary, while the successes of the early years were indisputable, the successful combination of policy inducements and regulatory controls of the infant industry ‘first market environment’ (FME) created their own challenges in turn. As public firms became critical repositories of capabilities even if not always highly efficient, private firms began to grow through a multiplicity of strategies, often by direct migration from public sector firms, but also in joint ventures, technology transfer, and later, from universities and research institutes. While the FME set the stage for remarkable successes for public and private firms alike, a Second Market Environment (SME) defined the export conditionalities and tight standards under which private firms began to proliferate. These included the electrical, electronics, telecommunications, original equipment and brad supply chains in India, as well as networks of generic machine tools and moulding manufacturers that appear to have become especially isolated in separate industrial policies for medical devices and diagnostics.
3.3. Vaccines
Vaccine markets and their regulations are dissimilar from both pharmaceutical markets and devices. This is because vaccines for communicable diseases build on network principles, and those for paediatric effects build on compounding effects of immunity over time. Programmatically, vaccines required depend on effectiveness through crowding in use, and network spread requires low cost or zero cost to patients. This implies that a range of vaccine characteristics that exemplify the technological capabilities of vaccine suppliers follow a considerably different economic path from diagnostics or medicines. In vaccines, heavy subsidies are meant to ensure low or zero cost to consumers, and the subsidies and procurement initiatives can and have been effective in health policy (affordability, access, spread of use, safety) and industrial policy (technological capabilities in R&D, manufacture, technical standards and safety and quality concerns). A major differentiating factor for firms in vaccines and generic pharmaceuticals is how demand and delivery is structured and regulated. In vaccines, demand occurs primarily through procurement by government or international agencies and delivery through a range of programmes and organizations. This organized demand can also contribute resources to upgrade critical technology features of firms. In generic pharmaceuticals, the economic arguments are different since immunity, and its network economics are not relevant. Thus, for different reasons, demand and delivery structures and organizations evolve differently from vaccines. While industrial policies for boosting production continue to matter in both cases, the differentiating features across sub-sectors of demand and delivery do matter.
Indian regulators and health policy experts have known mainly how to answer these questions after many years of iterative learning and failures (Srinivas Citation2006, Madhavi Citation2003), although clear instances of unclear market rules, heavy-handed state intervention, and problems with pricing have all been witnessed. Madhavi (Citation2003) argues that private vaccine manufacturers have often lobbied to include their vaccines into the extended programme of immunization, a questionable practice. Broadly conceived, however, health goals and industrial upgrading occurred alongside through the efforts of a wider array of national and international stakeholders.
The SME began to define the differences in outcomes between sub-sectors of the health industry. Pharmaceuticals and vaccines began to require different regulatory strategies within and beyond the second market environment and have broadly managed to succeed, e.g. between on and off-patent medicines, for essential vaccines, national and international procurement as an industrial policy tool solved some major hurdles as an effective demand-side regulatory instrument for production improvements while attending to safety, quality, and affordability outcomes (Srinivas Citation2006). Pharmaceuticals and vaccines from India were thus both successful in supplying the world because, in the first and second market environments, industrial policy instruments strategically or unwittingly also worked as standards and quality upgrading regulatory instruments, with price controls serving to further the norms of market variety and competition rather than stifling them, and benefiting health policy directly. It can be argued that as private firms became more cash-rich and experienced export successes, the national controls became less effective, and the differences became more visible between production-inducing instruments of industrial policy and those of regulation and control.
As scholarship has shown, the growing technological learning has required increased experience in coordinating institutional variety of multiple markets, the difficulty of transferring learning across products and processes, of experimenting with new business models and designing in situ new regulatory frameworks for domestic capabilities with few relevant foreign experiences (Kale Citation2019). However, the challenge even for more successful countries such as India or Brazil has been how to convert technological capabilities and manufacturing success into robust institutional gains across sectors and institutional domains beyond production successes. Indeed, as Srinivas (Citation2012) has shown, Indian production successes have made the domestic political economy challenges more acute as both firms, and the state have struggled to embed the R&D and manufacturing with demand and delivery institutional domains within the country. Diverse market environments provided an explanatory framework for the norms and rules to explain institutional change over time and the difficulty of forcing the convergence of industrial and health policies.
Industry sub-sectors have different dynamics and require overt plans, especially those in the national interest, where national missions, WTO rules, and business clarity and industry association coordinating could be best exploited. Industrial policies can appear common to sub-sectors, but in fact, as policy bundles can rapidly advance some versus others, even when all sub-sectors are considered important. In pharmaceuticals, India strategically used aggressive bundles of industrial policies – procurement, intellectual property, fiscal incentives, etc. – but the same bundles without differentiation may adversely affect other sub-sectors. In vaccines, for example, procurement design itself requires minute tweaking to fine-tune incentives for technological upgrading but has broadly aligned with donors’ intent (Srinivas Citation2006). However, in boosting pharmaceuticals, countries have very unevenly used procurement design to pressure donors to align with domestic priorities (Chataway et al., 2014). Encouragingly, many countries are now achieving a hard-won R&D, prototyping and manufacturing capability in specific aspects of the health industry such as generic pharmaceuticals, yet struggle to convert these capabilities into gains in other health industry sub-sectors such as vaccines, or medical devices and diagnostics.
3.4. The medical devices and diagnostics sub-sector
Notwithstanding India’s success in boosting creation and manufacture of Covid-19 medical diagnostics: including the difficult task of compressed timeline for generation of quality and safety regulation and creation and commercialization of diagnostics kits along with industrial supply chains of testing and reporting, considerable uncertainty of market aims and industrial policy plagues long-term regulatory design (Srinivas, Prasad, and Rao Citation2020). Precisely because of India’s success in the creation of Covid-19 diagnostics and vaccines, sub-sector variations in regulatory design have become more pronounced.
While the health industry is usually analysed under one umbrella of policies and regulations, urgent questions are being raised about the distinctiveness of devices and diagnostics. Some key differences exist among healthcare technology industries relating to the mechanism of action, role of users, nature of regulation and product development process (). Unlike pharmaceuticals and vaccines, the performance of medical devices depends not only on the device itself but also on how it is used, which has implications for setting up a regulatory framework for the medical device and diagnostics industry.
Table 1. Differences between medical devices, diagnostics and pharmaceuticals (Global Medical Technology Alliance Citation2015 and author modifications).
The regulatory framework in healthcare technology sectors primarily shares similar claimed objectives: to ensure a level playing field for global trade and access to liberalized markets, enhance human well-being, and secure health promotion (Altenstetter Citation2014). More detailed sub-sector analysis raises the challenge of how such diverse goals might be reconciled. The regulations for any industry represent a specific and critical institution as they frame the norms, rules, customs and routines (both formal and written, or, more often, informal and internalized). They govern every aspect of the industry. The regulatory framework influences the structure, function, and behaviour of organizations in the industry.
In contrast to pharma and biotech products, medical devices and diagnostics have an intended primary mode of action on the human body, and in contrast to that of medicinal products, it is not metabolic, immunological, or pharmacological. As a result, medical devices and diagnostics worldwide are classified based on their safety requirements. Several other criteria are also considered to evaluate the potential risk: degree of invasiveness, duration of contact, affected body system, and local versus systemic effects.
The comparative analysis of medical device regulations in advanced countries suggests that the emergence of innovative technologies, a globally operating industry and locally delivered healthcare are key drivers of medical device regulation (Altenstetter Citation2014). However, medical devices and diagnostics have several unresolved questions for how to frame and address technological advances and under what conditions the state can establish clear-cut and credible inducements and regulations for this sub-sector, in many respects an industry in its own right.
3.5. Evolving Indian medical device guidelines and regulation
The Indian Ministry of Health and Family Welfare (MoHFW) and the Central Drugs Standard Control Organization (CDSCO) are the main regulatory bodies responsible for overseeing the governance of medical devices and diagnostics. The CDSCO exercises regulatory control over importing drugs, devices, and diagnostics and approves new medical products and clinical trials.
Before 2005, medical devices in India were largely unregulated. Indian medical device manufacturers voluntarily attained certifications from the Bureau of Indian Standards (BIS) but primarily for low-tech instruments. The demand for high-tech instruments was met with imports following trade liberalization in the 1980s and 1990s, with 70% of the market remaining import-driven (Kale and Wield, Citation2019). Even with access to new devices, a largely unregulated market, explained in part by the government’s ‘limited understanding of how medical devices work’, left consumers unprotected and presented roadblocks for innovation (Kale Citation2019).
An incident in 2004 at a hospital in Mumbai triggered the debate and development of Indian medical device regulations. In response, the government amended the Drugs and Cosmetics Act 1940 (D&C Act) and the Drugs and Cosmetics Rules 1945 (Rules) to cover ten specific medical devices. The primary objective of the D&C Act is to promote safe and effective healthcare by regulating the import, export, manufacture, distribution, and sale of drugs, cosmetics, and (now) devices.
This early Indian medical device regulation model was based on drug regulation (D&C Act) before splitting off from it. The inherent differences between drugs and devices make uncritical application of the drug regulatory model for device governance significantly challenging. In the Indian case, this conflation of medical devices and diagnostics with pharmaceuticals led to severe licensing inconsistencies and delays. In 2009, the D&C Act was amended to include a discrete (albeit extremely limited) chapter on medical devices.
The most significant initiative towards comprehensive regulation for medical devices came from the introduction of Medical Devices Rules, 2017 (MDR). The establishment of the Medical Device Rules, 2017, brought a novel departure in the regulation of medical devices and diagnostics. Whereas a notified list of devices was previously classified as drugs under the DCA, based on consultations with the Drugs Technical Advisory Board (DTAB), the revisions contained in the Medical Device Rules, 2017 present a more comprehensive regulatory framework for medical devices of various classifications. The shows that drugs and devices are a concurrent subject, with both state and central government involvement in licensing and regulation.
Table 2. Medical devices and diagnostics: Regulatory lags, challenges and impact on a triad of institutional domains.
The MDR rules, 2017 introduced a risk-based classification system, regulatory standards, proper manufacture licensing requirements, shelf life restrictions, quality management system and more focused clinical regulations. These rules also seek to nurture a culture of self-compliance by medical device manufacturers and create a robust ecosystem for all stakeholders, including innovators, manufacturers, providers, consumers, buyers, and regulators. Medical devices have been divided into four categories based on their risk type – Class A, B, C and D, where A and B covers low-risk devices such as diagnostic equipment and C and D cover high-risk devices such as implantable devices. It was notified that the central agencies would be involved in approving devices in C and D categories.
In 2020, the Indian government further amended the rules providing a new definition for medical devices and introducing a new chapter for registration of medical devices by their respective manufacturers and importers. The medical device (amendment) rules, 2020 also exempted the 37 categories of already regulated or notified medical devices from the requirement of registration introduced by the new chapter. The medical device amendment (2020) rules aimed to ensure that every medical device, either manufactured in India or imported, has quality assurance before it can be distributed/sold in the market.
Where they exist, Bureau of Indian Standards (BIS) product standards are to be followed. Where these are not available, those set by the ISO or IEC should be observed. However, the BIS continues to have relevant certifications for low-tech devices, with all remaining devices borrowing product standards from either the ISO or IEC, pointing to an ever-present lacuna in policy preparedness, awareness, and foresight customize standards and incentivise indigenous, innovative manufacturers.
There were further issues with medical device regulations and policy frameworks that created challenges for technological capability development for the Indian industry. These are discussed in the next section.
3.6. Problems with medical device and diagnostic regulatory frameworks
The passing of Medical Device Rules, 2017 or the medical device amendment (2020) rules did not alter the categorization and treatment of the medical devices and diagnostics as drugs and continuing mismatch between regulation and products. There are still three significant issues with the new medical device regulation; the first pertains to the division of regulatory enforcements power between the state and central agencies, while the second relates to categorizing of devices and diagnostics under the Drugs and Cosmetics Act, 1940 and third is concerned with governance structure for the implementation of the regulation.
First of note is the role that states play in sales/distribution licenses for all classes of drugs. Regarding manufacturing licenses, low-risk devices (classes A&B) are under states’ purview, whereas higher-risk devices (C&D) sit under the Central Licensing Authority. This division of responsibilities between central and state agencies has led to issues of creating appropriate incentives for innovation, additional delays and roadblocks and lack of capacity at the state and central level to monitor class C&D devices.
Second, it is argued that medical devices and diagnostics need a new regulatory act rather than a modification of the existing act designed for the governance of drugs and cosmetics. As Rajiv Nath (Citation2019), Forum Coordinator of the Association of Indian Medical Device Industry (AiMED), has specified that within diagnostics and devices as well, there is a wide variety of products and:
you cannot have the same penalty for a manufacturing failure of a pair of spectacles as for a contact lens or for an intraocular lens. Patient safety is more complex with devices where the same are ‘a shared responsibility of the manufacturer, medical practitioners, product user and the regulator.
A third critical issue concerns the governance structure set up to implement and interpret the rules as the existing regulatory system is proving to be inadequate. In some instances, the existing system has resulted in MNCs flouting standards by pushing ‘pre-owned’ or ‘second hand’ medical and diagnostic medical equipment to be used in private clinics and hospitals with no assessments done on their levels of accuracy or safety.
The National Health Mission, particularly the Free Diagnostic Service Initiative (FDSI) in India, provides further rationale for a robust regulatory framework and investment strategies for medical devices and diagnostics. However, the industrial policy incentives, guidelines, and regulatory roadblocks remain problematic according to the Association of Indian Medical Device Industry (AiMED), which states that ‘key strategic aspects of the Road Map discussed with the Indian Medical Devices Industry Associations are missing’ (Nath Citation2019). AiMED seek the regulation reflecting not the permit system of India’s pharmaceutical regulatory environment but instead seeks regulations that mimic international best practices of voluntary compliance (Sarin Citation2018).
3.7. Step 3. Firm-level case studies
Three brief case composites of Indian startup firms in medical devices and diagnostics are presented here. The composites created offer anonymity but provide some context in which technological advances occur in specific regulatory contexts.
Firm A is an instrumentation specialist firm that helps the screening and treatment strategies for a type of cancer by improving optics instrumentation. In principle, if the regulatory design were well adapted to the concerns of innovative technology firms such as Firm A, it could be a global market leader in this area. It is new to the market and is currently engaged in extensive clinical networks and prototyping of instruments. Firm A's pathway has come from scientific and instrumentation breakthroughs from precision improvements to measurement in cancer and miniaturization of instrument design.
Firm B is focused on combining multiple organizational and technological innovations that enhance the Point of Care (PoC) quality of service. Firm B also uses artificial intelligence to improve precision in diagnostics and, in principle, can work across sub-sectors and industries from its platform analysis approach to large data.
Firm C is a startup involved in developing PoC diagnostics to detect non-communicable diseases (NCDs), anaemia and monitoring wellness parameters. It is focused on creating diagnostics that are portable, easy to use and can be used in resource-constrained environments.
All three firms have the potential to be global leaders in their product segments. However, these firms face the following regulatory challenges lagging their technological challenges that can be understood using the triad:
It faces unclear regulations about access to tissues and patients-and its ecosystem of clinics, hospitals to access patients-is shaped by regulations created with good intentions but unclear processes in fast-changing contexts about ethics, documentation, and approval timelines. (1 shows progress, but 1 and 2 in the triad are delinked). The founder of Firm A highlights the impact of unclear regulations on the growth of his business,
‘In India, the regulatory structure is such that new technologies are hard to come here. I worked in a company and they wanted to do a trial at RCC. They couldn’t do it because the Ethics Committee didn’t approve of it because the device wasn’t certified. The device had to be approved by the drug controller, who said he didn’t have anything to approve – they said, ‘Get it from ICMR’. They won’t do anything without these kinds of approvals.’.
The burden of proof for its products and services is regulated by clinical trials requirements designed primarily for approval of pharmaceuticals. (1 and 2 of the triad are linked but driven by regulations written for pharmaceuticals)
For all three firms, unlike pharmaceuticals or vaccines which have no components, medical device and diagnostic instrumentation improvements are often dependent on materials, size of instruments, and different scale and accuracy of measurement considerations of component elements. The compounding of accuracy or errors of component instruments and parts use a single product or process regulations that serve other sub-sectors. (There may be multiple operational triads for components of devices and diagnostics. For instance, a ventilator or a CT scan may have many parts, each of which has a different set of regulations governing their presumed relationship of 1 with 2 or 1 with 3).
For all three firms, procurement guidelines for hospitals to buy such instrumentation have no adaptability in modifying the procurement process to address institutional change. This may mean encouraging buyers to comply with approval guidelines while providing incentives such as subsidies or scalable bulk orders that recognize a medical device or diagnostic's novelty, speed of solution, or ability to address an urgent problem. This leaves firms to have the experience or expertise to negotiate, manage the procurement while satisfying the business imperative. For entrepreneurs of startups, this can become a burden. (Explicit subsidy hurdles to 2 exists that complicates the tie between 1 and 3). The CEO of Firm B points out,
‘We have tried getting through to the system. Even after we met and presented in front of the Health Secretary, Principal Secretary, it didn't translate into access into the health system where it can run as a pilot, implement it etc., completing the loop hasn't happened. Maybe as a startup, we also don’t know how to navigate the system’.
Public hospitals, although with priorities for low-cost diagnosis and treatment, do not help these firms in medical devices and diagnostics any more than private hospitals. Their systems may offer potential network benefits for firms if they successfully win a tender. In practice, the process may be cumbersome and opaque, making it simpler to negotiate with single private institutional buyers at a time. (Public access rationale for linking 1 and 3 may be so cumbersome that firms in 1 may need to seek private firms in 2 directly). The founder and CEO of Firm A elaborates on challenges due to lack of appropriate ecosystem,
‘After initial seeding, which helps startups develop a proof of concept but from the proof of concept to the product actually being used by beneficiaries or customers – there is a long journey that these startups are required to traverse through. What many of us felt is that there are not enough linkages in the ecosystem for entrepreneurs to get from point A to Z’.
Firms such as B and C that have many innovations in principle should be well rewarded. However, business organization improvements such as Point of Care (PoC) innovations that enhance the screen and treat turnaround time to technological innovations in both hardware and software are even less likely to have a clear benefit from regulations designed for pharmaceuticals or vaccines. (More innovative firms in 1 do not necessarily experience greater reward; more innovations seem to complicate relations of 1 with 2,3 rather than reduce them).
Within the policy constrained environment, these firms have shown signs of technological learnings. These firms have developed the products using local resources, created production capabilities to manufacture at a scale and compete in a market dominated by the MNCs. One of the critical aspects of technological learning for these firms is creating business models that help manage policy vacuums and economic uncertainties. For example, the CEO of firm B comments highlighting technological learning,
‘So most of the science was actually driven by the availability of resources. In the UK, it was about how we could access samples, and in India, it was more about techniques and what we could actually get.
4. Analysis and discussion: E-I methods heuristics and the medical devices and diagnostics subsector
The Indian medical device and diagnostic industry has been hampered by inadequate trade, industrial and regulatory policy frameworks ().
Table 3. Interaction of 3 institutional domains in 3 health sub-sectors (Srinivas Citation2012; Kale Citation2019).
The highlights the inadequacy of the current regulatory framework, a tax structure that incentivises imports against indigenous manufacturing, procurement rules favour MNCs and the failure of DPCO to resolve affordability issues. Imports are expensive and impact the availability of low-cost, effective, safe, and locally suited medical devices. Our analysis suggests that medical devices and diagnostics have had an unclear relationship between innovation and affordability for at least four reasons: there have been no clear price regulations until recently that forced suppliers and retailers to drop their prices; the institutional domain of industrial policy for medical devices is arguably nascent, and policies that shape innovation are delinked from those of pricing; the medical devices sector has minimal procurement and insurance which can narrowly direct suppliers to designated pricing segments, and the diverse types of firms and non-profits in medical devices offer varied pathways to pricing-some hospitals and clinics even providing entirely free medical devices and related healthcare.
We apply the heuristic to analyse Indian medical devices and in vivo and in vitro diagnostics. When combined with findings from the three case study firms, as the prior sections have shown, very different challenges ensue ().
Figure 3. Snapshots of the medical device and diagnostic industries. Source: Adapted from Srinivas (Citation2012, 8) by the authors
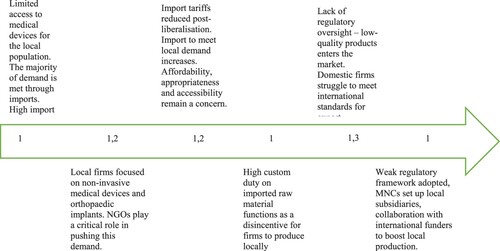
There are at least three-fold gaps for medical devices compared to pharmaceuticals and vaccines:
Significant differences with pharmaceuticals and vaccines on the production side, with large imports continuing and growing but unevenly regulated domestic technological capabilities;
Significant differences in demand support: lack of large single institutional procurers, whether domestic or foreign such as exist with pharmaceuticals and especially with vaccines.
Absence of strong infant industry protections alongside upgrading requirements similar to the First Market Environment and Second Market Environments for India, but strong price controls being introduced on certain types of devices and diagnostics before this type of demand opportunity exist. On the other hand, with a spreading coverage of state-level and central health insurance schemes, greater coverage of medical devices is in contrast to the experience of pharmaceuticals and vaccines.
In such a framework, the distinctive learning paths of pharmaceuticals and vaccines can be explained through different institutional arrangements built over time that forced the market conditions and non-market institutions to cohere closely and resulted in industrial and health policy instruments moving closer together. For example, welfare procurement markets shaped Indian generic drug manufacturers differently from vaccine manufacturers and international institutional procurers. These specific differences in two different manufacturing domains – generics and vaccines – reveal findings of the importance of differentiating between the traditional economics of industrial policy design and becoming improved manufacturers, and the institutional reform and slow learning required to modify innovating, growing, and mature, established elements of manufacturing (Srinivas Citation2020).
4.1. Discussion: institutional learning in the Indian health industry
This paper has viewed regulations as a fundamental aspect of industrial policy’s selection effects on the institutional variety that exists in any sector and shapes firms’ learning environment. The experience of the medical devices and diagnostics sub-sector in India was analysed for the first time in contrast to the country’s pharmaceuticals and vaccines experiences that are now well analysed and emphasizes the potential for evolutionary-institutional methods and their insights. Consistent with prior scholarship on Indian pharmaceuticals, the analysis of medical devices and diagnostics reveals several dimensions of institutional learning challenges that remain in India, arguably, one of the late industrial world's most successful examples of R&D and manufacturing success. These challenges include
Technological capabilities in medical devices have emerged into a regulatory environment that was designed and equipped for pharmaceuticals.
These capabilities, although pronounced for R&D and manufacturing, has struggled without the institutional learning environments of demand (insurance, procurement) or delivery (close ties to clinics and hospitals, or required use in primary health care).
State-led intervention is notably absent in this sub-sector relative to pharmaceuticals and vaccines despite the sectors potential health impact. Kale and Wield (Citation2019, 19) point out that setting industrial policy for medical devices is highly complex compared to pharma-biotech as it requires the involvement of a broader range of distinctive health institutions, regulatory institutions and industrial institutions.
Despite innovation being high in the Indian medical devices industry, there is no clear correlation between state-initiated policies to reduce production costs or consumer outcomes to increase affordability and access.
It is evident that methodologies that attempt different institutional combinations offer possible traction for such an evolutionary analysis. Most institutional change studies either approach the issue through formalism and econometric models, making it difficult to capture change in progress in countries for which data is emerging or whose institutional environments are significantly different.
The evolutionary, institutional perspective of the heuristic shows that industrial gains in manufacturing do not reflect a steady march to a finish line. Instead, cross-sector effects, loss of capacity over time, challenges of organizational adaptation, regulatory selection mechanisms, and firms’ survival pose distinct challenges to both the conceptual framework of economics and policy design toward a dynamic industrial organization in healthcare. As even industrialized countries demonstrate, there exist real challenges of loss of technological capabilities and the production political economy to nurture and retain capabilities (e.g. public sector vaccine manufacturing in the Netherlands, affordable medicines or suitable screened and tested devices in the US). For firms and policymakers, difficult questions emerge even in successful cases of production capability. The challenges are two-fold: to consolidate the manufacturing or R&D gains and ensure that they are converted into health gains, which is no easy task; second, to guide a ‘health industry’ in terms of regulation, governance norms, and export revenues, but to attend to the practicalities of diverse sub-sectors in this industry.
However, a country's regulatory framework is vital in addressing local health priorities, incentivising firms, and ensuring patient access to new technologies. The promotion of global harmonization can undermine the role that nation-states and national authorities should play in devising a regulatory framework suitable for local conditions (Kale Citation2019). It is interesting to note that out of the five countries/international agencies involved in setting up GHTF (Global Harmonisation Task Force), Japan is the only country to incorporate some parts of the technical standard requirements recommended by the GHTF into its legal order (Altenstetter Citation2014).
There are other arguments for the customization of regulatory design. Medical devices need to be designed in a contextually appropriate manner – nearly all devices present in developing countries have been designed for use in industrialized countries. Up to three-quarters of these devices do not function in their new settings and remain unused. Factors contributing to this are lack of needs assessment, appropriate design, robust infrastructure, spare parts when devices break down, consumables, a lack of information for procurement and maintenance, and trained healthcare staff. These issues are part of a broader problem in many countries: the lack of a medical device management system.
5. Conclusion
While institutional variety manifests in an evolutionary process of learning and dynamism in the industry, much more is required to understand significant differences across industry sub-sectors. Within-sector differences in the same country capture learning and rigidities translating to other sub-sectors in the same industry. Second, industrial policy response may be out of sync within and across some sub-sectors more than others.
By focusing on opportunities to extend methods of evolutionary, institutional economics to contend directly with within-sector differences in learning and regulatory lag, the paper has attempted to offer some resolution for how one might systematically map the different combinations and pathways adopted by sub-sectors of one country’s health industry. In doing this, we can move from more generic questions about infant industry status to specific policy menus that have been historically considered at the nation-state but could answer questions of particular policy reform, e.g. Food and Drug regulations. It has also made more evident the limited conditions in which an otherwise strong state that has assisted pharmaceutical and vaccine manufacturers has been far less helpful to medical device firms, raising the question of how regulatory ‘lag’ can be minimized and who has the power to do so. Should neither consumers nor the state but medical personnel or industry associations influences the design of regulations and inducements to firms? Furthermore, given the different periods in which specific combinations of the triad are evident in some sub-sectors and not others, what does it mean to have a ‘domestic’ industrial policy in a global market for diagnostics and increasingly global health guidelines on their use? If diagnostics are considered an essential part of the screen and treat alongside vaccines, they are quite different E-I starting conditions than if they are substitutes. Similarly, the triad strategy as part of a Systems Dynamics approach in E-I analysis can capture the geographic reach and market variety as core regulatory inducements in design. When diagnostic firms are successful exporters in highly restrictive markets, domestic regulators in health and industrial policy should consider whether they can automatically accept with few hurdles the technological capabilities and technical standards these firms represent or clarify what customization for local contexts is required.
There are wider questions to contextualize about medical devices and diagnostics as a high-impact sub-sector. There is clearly a need for a long-overdue specialized regulatory architecture exclusively focused on governing devices and diagnostics. Iterative learning stimulates technical competency and growing capabilities in institutional design, where the technological capabilities can be fully established to generate public benefit. This permits governments to help businesses initiate and expand critical industries that branch out and are increasingly embedded in the economy while generating dynamic new associated capabilities and sectors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amsden, A. 1989. Asia's Next Giant: South Korea and Late Industrialisation. New York: Oxford University Press
- Altenstetter, C. 2014. Medical Technology in Japan: The Politics of Regulation. New Brunswick, NJ: Transaction Publishers.
- Arocena, A., and J. Sutz. 2000. "Looking at National Systems of Innovation from the South." Industry and Innovation 7 (1): 55–75.
- Chaudhuri, S. 2005. The WTO and India's Pharmaceutical Industry. Delhi: Oxford University Press.
- Chaudhuri, S. 2019. “How Effective Has Been Government Measures to Control Prices of Anti-Cancer Medicines in India.” SSRN Electronic Journal. doi:10.2139/ssrn.3767833
- Elsner, W. (2017, January). "Social Economics and Evolutionary Institutionalism Today: Theoretical Components and 'Heterodox' Convergence in a Socio-Economic Perspective." In Forum for Social Economics (Vol. 46, No. 1, pp. 52–77). Routledge.
- Global Medical Technology Alliance. 2015. Differences between Medical Devices and Drugs, http://www.globalmedicaltechnologyalliance.org/papers/GMTA_Differences_Between_Devices_IVD_Drugs_RevFINAL_17July12-pdf-pdf.pdf.
- Hodgson, G. 1988. Economics and Institutions: A Manifesto for a Modern Institutional Economics. Cambridge: Philadelphia, Policy Press and University of Pennsylvania Press.
- Kale, D. 2018. “From Small Molecule Generics to Biosimilars: Technological Upgrading and Patterns of Distinctive Learning Processes in the Indian Pharmaceutical Industry.” Technological Forecasting and Social Change 145: 370–383.
- Kale, D. 2019. “From Small-Molecule Generics to Biosimilars: Technological Upgrading and Patterns of Distinctive Learning Processes in the Indian Pharmaceutical Industry.” Technological Forecasting and Social Change 145: 370–383.
- Kale, D., and S. Little. 2007. “From Imitation to Innovation: The Evolution of R&D Capabilities and Learning Processes in the Indian Pharmaceutical Industry.” Technology Analysis & Strategic Management 19 (5): 589–609.
- Kale, D., and D. Wield. 2019. “In Search of the Missing Hand of ‘Collaborative Action’: Evidence from the Indian Medical Device Industry.” Innovation and Development 9 (1): 1–23.
- Lall, S. 1983. The new Multinationals: The Spread of Third World Enterprises. New York: Wiley.
- Lall, S. 1987. Learning to Industrialise: The Acquisition of Technological Capability by India. New York: Macmillan Press.
- Lall, S., and C. Pietrobelli. 2005. “National Technology Systems in Sub-Saharan Africa.” International Journal of Technology and Globalisation 1 (3-4): 311–342.
- Mackintosh, M., G. Banda, W. Wamae, and P. Tibandebage, eds. 2016. Making Medicines in Africa: The Political Economy of Industrialising for Local Health. Springer.
- Mackintosh, M., J. Mugwagwa, G. Banda, P. Tibandebage, J. Tunguhole, S. Wangwe, and M. Karimi. 2018. “Health-Industry Linkages for Local Health: Reframing Policies for African Health System Strengthening.” Health Policy and Planning 33 (4): 602–610.
- Madhavi, Y. 2003. "The Manufacture of Consent? Hepatitis B Vaccinations." Economic and Political Weekly 38 (24): 2417–2424.
- Malerba, F., and R. R. Nelson, eds. 2012. Economic Development as a Learning Process: Variation across Sectoral Systems. Cheltenham: Edward Elgar Publishing.
- Mazzucato, M. 2018. The Entrepreneurial State. London: Penguin Books.
- Nath, R. 2019. “Why Medical Devices Need Their Own Law.” Financial Express, April 30th, https://www.financialexpress.com/opinion/why-medical-devices-need-their-own-law/1562997/.
- Nelson, R. R., and S. G. Winter. 1982. An Evolutionary Theory of Economic Change. Cambridge: The Belknap Press of Harvard University Press.
- Papaioannou, T., and S. Srinivas. 2019. “Innovation as a Political Process of Development: Are Neo-Schumpeterians Value-Neutral?” Innovation and Development 9 (1): 141–158.
- Papaioannou, T., A. Watkins, J. Mugwagwa, and D. Kale. 2016. “To Lobby or to Partner? Investigating the Shifting Political Strategies of Biopharmaceutical Industry Associations in Innovation Systems of South Africa and India.” World Development 78: 66–79.
- Russo, G., and G. Band. 2015. "Re-Thinking Pharmaceutical Production in Africa; Insights from the Analysis of the Local Manufacturing Dynamics in Mozambique and Zimbabwe." Studies in Comparative International Development 50 (2): 258–81.
- Sahu, S. K. 1998. Technology Transfer, Dependence and Self-Reliant Development in the Third World: The Machine-Tool and Pharmaceutical Industries in India. Westport, CT: Praeger.
- Sarin, R. 2018. ImplantFiles: Law on Medical Devices Has Waited 12 Years. https://indianexpress.com/article/india/implant-file-medical-device-regulation-bill-cdsco-5475513/.
- Srinivas, S. 2006. “Industrial Development and Innovation: Some Lessons from Vaccine Procurement.” World Development 34 (10): 1742–1764.
- Srinivas, S. 2012. Market Menagerie: Health and Development in Late Industrial States. Palo Alto CA: Stanford University Press.
- Srinivas, S. 2016. “Healthy Industries and Unhealthy Populations: Lessons from Indian Problem-Solving.” In Making Medicines in Africa, edited by M. Mackintosh, Geoffrey Banda, P. Tibandebage, and W. Wamae, 183–199. London: Palgrave Macmillan.
- Srinivas, S. 2018. “Evolutionary Demand, Innovation and Development1.” In Development with Global Value Chains: Upgrading and Innovation in Asia, edited by Nathan D, Tewari M, and Sarkar S, 349–379. Cambridge: Cambridge University Press.
- Srinivas, S. 2020. “Institutional Variety and the Future of Economics.” Review of Evolutionary Political Economy 1 (1): 13–35.
- Srinivas, S., R. K. Prasad, and P. Rao. 2020. The Clinical Foreground and Industrial Background: Customising National Strategy for COVID-19 Testing. IKD Working Paper No. 87 October, IKD centre (IKD) Research Group, The Open University UK, Milton Keynes. http://www.open.ac.uk/ikd/publications/working-papers/87.
- Srinivas, S., and J. Sutz. 2008. “Developing Countries and Innovation: Searching for a new Analytical Approach.” Technology in Society 30 (2): 129–140.
