Abstract
Traveler's diarrhea (TD) is caused by Escherichia coli in 30% of cases. We have developed a phage cocktail for prophylaxis of TD caused by E.coli, Shigella flexneri, Shigella sonnei, Salmonella enterica, Listeria monocytogenes or Staphylococcus aureus, and investigated its effectiveness against infection caused by the non-pathogenic Lac (-) strain of E.coli K12 C600 in animal and human trials. On the 6th day of both animal and human trials E. coli K12 C600 strain was detected in titer of 104 CFU/g of mice feces and 106 CFU/g of human feces in the control (untreated) groups, while it was not detected in the samples of either of the study (phage-treated) groups. These results have great significance because the original coliphages included in the cocktail have a broad host-range including ETEC, EAEC and EHEC strains which cause severe cases of TD.
Background and Aims
Traveler's diarrhea (TD) is a polyetiological clinical syndrome characterized by 3 or more unformed stools a day in people traveling into another country or climate zone, particularly in tourists. All of TD cases are caused by microorganisms entering the human body with fecally-contaminated food and water. Dysfunction of gastro-intestinal tract in tourists most frequently appears within the first 2 weeks after arrival. During the trip, depending on the region, 25% to 75% of tourists experience one or more episodes of diarrhea. Usually, TD doesn't last longer than one week, however, in 6–10% of cases it may continue for 2 weeks or longer. TD may be caused by changes in food and regimen in a new place of stay. Around 100 million people from developed countries travel annually to regions with a high risk of developing TD: Latin America, South-East Asia and Africa, which results in up to 40 million cases of TD per year. More than 80% of traveler's diarrhea cases are caused by a variety of bacterial enteropathogens: diarrhea-producing E.coli accounts for the majority of infections (15-40%), although Campylobacter (3-20%), Salmonella (3-15%), Shigella (3-10%) and some other bacteria have also been isolated from travelers.Citation1 It is known that probiotics may offer a safe and effective method for TD prevention.Citation2 Abedon T.S. et al. and Mai V. et al. in accordance with the definition of probiotics given by the World Health Organization, where they are described as having a healing effect on human organism when administered in adequate quantities, consider that it is possible to classify bacteriophage-based preparations as a new class of probiotics - phagebiotics.Citation3,4 We have developed a phage-based probiotic dietary supplement for prophylaxis of TD caused by E.coli (including ETEC, EAEC and EHEC strains), Shigella flexneri, Shigella sonnei and Salmonella enterica and have evaluated its effectiveness against infection caused by the non-pathogenic Lac (-) strain of E. coli in animal and human trials. We present the data of in-vivo model experiment that confirms effectiveness of the phage-based product against E.coli in this article. We have already tested the efficacy of our phages against Salmonella Enteritidis infection in outbred mice.Citation5
Materials and Methods
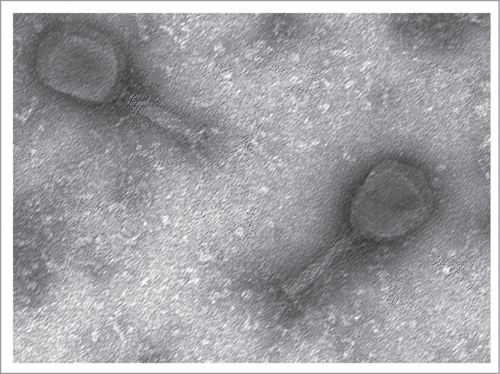
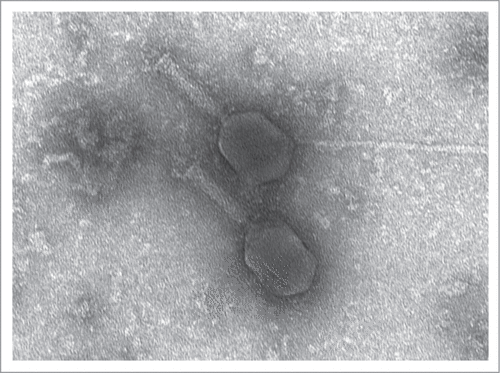
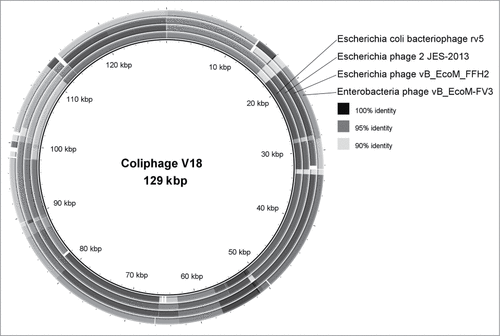
The phage-based probiotic dietary supplement consists of 7 bacteriophage strains active against the world's most wide-spread TD pathogens. The strains are: Escherichia coli EcD7 (polyvalent strain active against ETEC, EAEC, EHEC, Sh. flexneri and Sh. sonnei), Escherichia coli V18 (active against EHEC), Salmonella Typhimurium ST11, Salmonella Enteritidis SE40, Salmonella Infantis SI3, Staphylococcus aureus CH1, Listeria monocytogenes Lm1 at the titer of at least 106 PFU/ml. The complete characterization of these strains has been published earlier.Citation5 It reflected the origin of the phages, their lytic spectrum, maximum cultivation titers, molecular genetics analysis, including PCR-confirmation of absence of known pathogenic loci; virulent nature, proved by the absence of known integrases and the on-line program PHACTS (www.phantome.org/PHACTS); their difference from earlier known relative strains of bacteriophages. The latter was based on a full genome sequencing (Ion Torrent semiconductor sequencing) and bioinformatics analysis carried out in the Ubuntu 12.04 operating system based on the Linux core. De-novo automatic genome assembly was performed using NEWBLER (Roche Diagnostics) software which worked well in this operating system. The obtained data was visualized using the BRIG software.Citation6 The DNAs of coliphages EcD7 and V18, contained in the phage-based probiotic dietary supplement, compared with already known representatives of Myoviridae family are presented in .Footnote* These genetic maps allow us to demonstrate originality of EcD7 and V18 coliphages as they differ from already known phage maps were published in NCBI GenBank. Morphologically these coliphages also correspond to Myoviridae family ().
Figure 1. A comparison of the genome of the coliphage EcD7 with the affine phages of the T4-like viruses Phi1 and RB49.
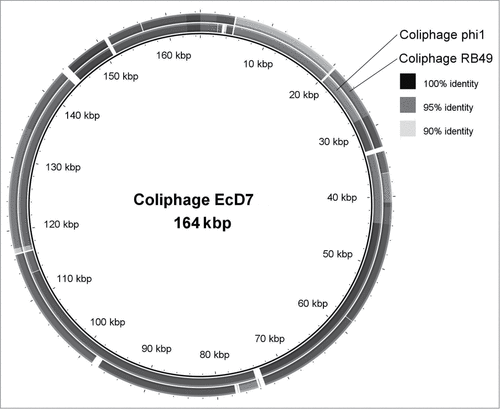
Figure 2. A comparison of the genome of the coliphage V18 with the affine phages of the Myoviridae rv5, 2 JES-2013, vB_EcoM_FFH2 and vB_EcoM-FV3.
Several tests were performed to characterize the bacteriophages' stability: resistance to increased temperature, pH variations and chloroform. The resistance of phages to increased temperature was observed in the range of 50–85°C. Plaque quantification from heated samples was carried out using Gratia double agar overlay method. We used chloroform treatment (and further centrifugation) as the cheapest method for elimination of bacterial cells and debris from phage-lysates to pre-sterilize them before microfiltration. Heating of phage-lysates is also a well-known pre-sterilization step. So, these characteristics are very important for future phage-based products producing. Phage-lysates were incubated for one hour at room temperature with buffer solutions calibrated to specific pH values to determine the bacteriophages' resistance to acidic and alkaline pH values. We estimated the titer of the phages in the phage-lysates after incubation using Gratia method.
The phage-based dietary supplement was in liquid form and additionally contained licorice root syrup, apple pectin and glycine. Licorice root syrup may be suitable for antispasmodic, enveloping properties and for improvement of organoleptic (taste) properties. Apple pectin may be suitable as a toxin sorbent and viscosity regulator. Glycine may be suitable as a stabilizer of phage titer in storage and also as an antioxidant.
The pharmacokinetics of the phage-based probiotic dietary supplement was studied on outbred miceFootnote* model (10 individuals) after single intragastric injection.
The acute and chronic toxicity of the phage-based probiotic dietary supplement was estimated in the same manner as previously described.Citation5 The acute toxicity was estimated using 2 species of laboratory animals: outbred mice and guinea pigs. The chronic toxicity assessment was carried out on 30 healthy white outbred mice. The phage-based probiotic dietary supplement in a dose of 0.5 ml was injected intragastrically once a day for 14 days. The 15 mice from the control group were injected intragastrically with 0.5 ml of physiological solution using the same technique. The pre-clinical trials were carried out according to Khabriev R.U.Citation7 We carried out all experiments using initially healthy mice. The mice had been under observation in quarantine during 2 weeks to check their health.
Using the method of model infection suggested by Drozdova O.M. et al.,Citation8 we conducted the following pilot experiment on 40 lab animals and 45 healthy volunteers: the control groups were infected with a non-pathogen strain of E. coli K12 C600 during 3 days in doses of 5 × 107 CFU/ml every 24 hours, and the study groups were subjected to identical infection, but they were treated with phage-based probiotic dietary supplement before, during and 24 hours after the infection period. Host range of both coliphages includes E. coli K12 C600. The animals and humans in the control groups took saline solution instead of phage-based probiotic dietary supplement following the same pattern. The animals and healthy volunteers selected for the experiment had no signs of presence of lactose-negative strains of E. coli in their initial stool samples (). To ensure a maximum accuracy of the experiment, the data of the microbiological study were verified by the molecular genetics method. E. coli cultures obtained from each dilution of the feces were washed off the surface of the dishes with a sterile saline solution for subsequent extraction of DNA from the bacterial suspension via sorption method and performing a PCR specific to the E. coli strain K12 C600 with end-point detection. We used the primers for the amplification which had been previously described by Kuhnert P. et al.Citation9 All animal experiments and human trials were approved by the Ethical Committee of Gabrichevsky Research Institute. Throughout the experiments, mice and guinea pigs were given food and water ad libidum. All of the human trials were carried out in accordance with the Declaration of Helsinki and with Russian national legislation.
Table 1. Design of a pilot study on modeling of E.coli TD
Results
The results of the bacteriophages' stability testing are presented in . These results show a possibility of oral administration of the phage-based probiotic dietary supplement taking into account a gastric acidity. It is well known that the pH values in stomach are shifted to the alkaline side in patients with acute intestinal infections. So, the pH range from reflects the real situation in gastrointestinal tract of these patients. The in vitro tests were confirmed by in vivo pharmacokinetics studies. The pharmacokinetics of the coliphages of the supplement is showed in the .
Table 2. Stability of bacteriophage strains
Table 3. Dynamics of phages persistence in mice intestines after single intragastric injection of the phage-based probiotic dietary supplement [lg(pfu)/g]
shows the results of acute toxicity experiments. After all these tests we found no chronic toxicity signs such as changes in their physiological condition or weight as well as on morphology of organs of the laboratory animals (liver, lungs, heart, thymus, spleen, base of tongue, lymph nodes, stomach, small intestine and colon, mesenterium and brain).
Table 4. Results of acute toxicity experiments of the phage-based probiotic dietary supplement
The results of the prophylactic effectiveness tests of the phage-based probiotic dietary supplement are presented in . The feces of the control groups showed lactose-negative E. coli on the 6th day of the experiment (). At the same time, in the feces of the animals and volunteers from the study groups only E. coli with normal fermentation properties was found (). Properties of normal E.coli include lactose-, rhamnose-, sorbitol-, lysine-positive reactions and citrate-, malonate-negative reactions.
Figure 5. Lactose-negative E. coli from the feces of the control (a) and lactose-positive E. coli from the study (b) groups on the 6th day of the experiment (Endo agar).
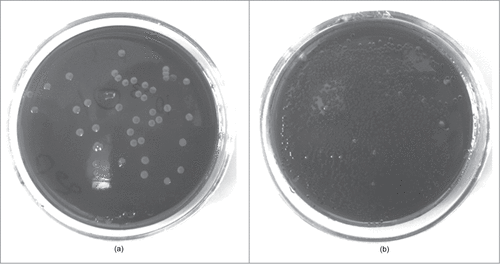
Table 5. Microbiological test results for feces
It is worth noticing, that in the stool samples obtained on the 6th day from the animals and humans of the study groups bacteriophages were present in 100% of cases, but seventy-two hours after the last intake (8th day of experiment) of the phage-based probiotic dietary supplement, coliphages were found in only 20% of the samples.
shows electrophoregrams, allowing to visualize the results of the amplification (horizontal electrophoresis of a PCR-product). The bands representing positive samples correspond to 969 bp amplicon (between 750 bp and 1000 bp ladder bands). Before the infection, the E. coli strain K12 C600 had been absent in all of the stool samples. On the 6th day of the experiment E. coli K12 C600 was identified in the samples from mice of the control group in the amount of up to 104 CFU/g of feces. At the same time it was not found in the samples from the study group. E. coli K12 C600 was identified in the samples from persons of the control group in the amount of up to 106 CFU/g of feces but it was not found in the samples from the study group. We used the PCR which is specific to all E. coli K12 strains with IS5 insertion but firstly K12 strains are not residents intestinal microflora of either humans and mice, and secondly mice and volunteers participating in the study had been checked for the presence of E. coli K12 strains in feces
Table 6. PCR-test results
Conclusions
Thus, the experimental model of an infectious process on lab animals and a small group of volunteers proves possible specific prophylaxis of traveler's diarrhea caused by E. coli, using the phage-based probiotic dietary supplement. These results have great significance because two original coliphages included in this phage-based probiotic dietary supplement have a broad host-range including O26, O55, O103, O104, O121, O125, O127, O128, O145, O146, O157 E. coli strains which cause severe cases of TD. The presented phage-based probiotic dietary supplement has been already registered in Russian Federation as a specialized product (RU.77.99.19.004.E.002820.02.15 dated 10.02.2015)Footnote* and is commercially available under the trademark “Foodphage.” Application of this phagebiotic dietary supplement can be an effective tool aimed to decrease TD incidence especially in high-risk areas.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Notes
* We are planning to submit the genome sequences of EcD7 and V18 coliphages to NCBI GenBank as soon as our international patent application will be published (International patent application No. PCT/RU2014/000483).
* We used the line of outbred white mice from the Laboratory Animals Nursery “Andreevka” (Federal State Budgetary Scientific Institution “Scientific Center of Biomedical Technologies”, http://www.scbmt.ru/).
* Register of certificates of EurAsEC countries, http://fp.crc.ru/evrazes/?type=list.
References
- Diemert DJ. Prevention and self-treatment of traveler's diarrhea. Clin Microbiol Rev 2006; 19(3):583-594; PMID:16847088; http://dx.doi.org/10.1128/CMR.00052-05
- McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis 2007; 5:97-105; PMID:17298915; http://dx.doi.org/10.1016/j.tmaid.2005.10.003
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage 2011; 1(2):66-85; PMID:22334863; http://dx.doi.org/10.4161/bact.1.2.15845
- Mai V, Ukhanova M, Visone L, Abuladze T, Sulakvelidze A. Bacteriophage administration reduces the concentration of Listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int J Microbiol 2010; 2010:624234. Article ID 624234; PMID:20652074
- Aleshkin AV, Volozhantsev NV, Svetoch EA, Verevkin VV, Krasilnikova VM, Bannov VA, et al. Bacteriophage cocktail in prophylaxis against food-borne infections. In: Mendez-Vilas A, editor. Worldwide research efforts in the fighting against microbial pathogens: from basic research to technological developments. Boca Raton: BrownWalker Press; 2013. p. 100-104.
- Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 2011; 12:402; PMID:21824423; http://dx.doi.org/10.1186/1471-2164-12-402
- Khabriev RU, ed. Guidelines for experimental (pre-clinical) studies of new pharmacological agents. 2nd ed. Moscow: Meditsina Publishers ltd; 2005.
- Drozdova OM, An RN, Chanishvili TG, Livshits ML. Experimental study of the interaction of phages and bacteria in the environment. J Microbiol Epidemiol Immunobiol 1988; 7:35-39; PMID:3055761
- Kuhnert P, Nicolet J, Frey J. Rapid and accurate identification of Escherichia coli K-12 strains. Appl Environ Microbiol 1995; 61(11):4135-4139; PMID:8526531