Abstract
The importance of ATP-Binding Cassette G1 (ABCG1) transporter in obesity was recently brought to light by recent findings uncovering its key role in adipogenesis with physiopathological consequences in human obesity. Thus, silencing of ABCG1 expression using an RNAi approach allows inhibition of adipocyte differentiation and maturation leading to reduction of fat mass growth in vivo in mice. Studies of ABCG1 in obese subjects validated its deleterious role in the context of obesity, suggesting that adipose tissue ABCG1 could be a potential therapeutic target in obese patients. Here, we discuss the potential feasibility of such strategy and provide a brief overview of critical issues to be considered.
Obesity is characterized by an excessive fat accumulation in adipose tissue (AT) leading to fat mass formation and weight gain. Adipose tissue is the site of energy storage and adipocytes, the most abundant cells in AT, are key cells performing this metabolic function.Citation1 Indeed, excessive food energy intake associated with a lack of physical activity strongly favors energy storage in adipocytes in the form of triglycerides (TG) and contributes to AT growth. A key step in this pathway involves Lipoprotein Lipase (LPL)-mediated lipolysis of TG-rich lipoproteins formed following meal consumption, leading to the generation of free fatty acids (FFA) which are taken up by adipocytes and stored in the form of TG. During fasting periods, stored TG can be hydrolyzed by specific lipases (adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoglyceride lipase (MGL)) in order to release FFA into the circulation, providing energy for other tissues.Citation2 AT expansion is considered as a healthy process consisting in the growth of AT through the recruitment of adipogenic precursor cells, i.e. hyperplasia, combined to an efficient angiogenesis and matrix remodeling. However, this very well-orchestrated machinery can be disorganized in pathological conditions during which expansion of AT is characterized by an enlargement of existing adipocytes, i.e., hypertrophy, an impaired angiogenesis, appearance of hypoxia, fibrosis, adipocyte death and inflammation.
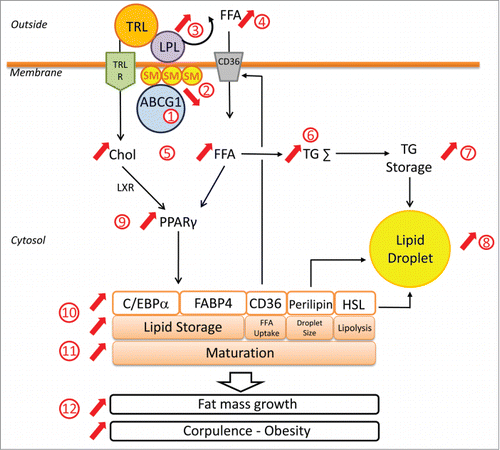
Very recently, we identified a new actor in TG storage in adipocyte which contributes to fat mass formation and development of obesity in humans.Citation3 Specifically, we reported that the membrane ATP-Binding Cassette G1 transporter (ABCG1) promotes LPL-dependent TG storage in adipocyte and activates adipogenic transcriptional program controlled by PPAR (peroxisome proliferator-activated receptor)γ (). Deciphering the underlying mechanism led us to conclude that ABCG1-mediated sphingomyelin (SM) efflux is required for optimal LPL activity and TG storage in adipocytes.Citation3 Moreover, our translational in vivo studies in mice and in obese patients uncovered the deleterious role of ABCG1 in AT formation and obesity, allowing us to suggest that ABCG1 can represent a novel therapeutic target in obesity.Citation6
Figure 1. Role of Adipocytes ABCG1 in regulating adipocyte differentiation and maturation. ABCG1 expression in adipocytes (1) promotes cellular sphingomyelin (SM) efflux and decreases SM-rich lipid raft formation (2). Low amounts of membrane SM ensures optimal LPL activity and hydrolysis of triacylglycerol-rich lipoproteins (TRL) (3), contributing to the release of free fatty acids (FA) (4). Released FFAs are then taken up by the cell (5) and used for triglycerides synthesis (6), leading to TG storage (7) and to increased lipid droplet size in adipocytes (8). Optimal LPL activity is also accompanied by TRL uptake, ensuing increase of intracellular cholesterol content (5). Abundance of intracellular cholesterol and fatty acid derivatives leads to both activation and expression of PPARγ (9) as well as upregulated expression of PPARγ-target genes, such as C/EBPα, FABP4, CD36, PERILIPIN and HSL (10) which are involved in adipocyte maturation (11). Indeed, PPARγ, C/EBPα and FABP4 are major players in lipid storage, whereas CD36, PERILIPIN and HSL control FA uptake, lipid droplet size and TG hydrolysis, respectively. As a consequence, ABCG1 in adipocytes promotes adipogenesis and contributes to increase fat mass growth, weight gain and development of obesity (12).
This hypothesis is reinforced by the previously unexpected observation that total ablation of Abcg1 in mice protects from diet-induced obesity as illustrated by reduced fat mass and body weight gain in Abcg1-/- animals as compared to control Abcg1+/+ littermates.Citation4 In agreement with the role of Abcg1 in adipocyte TG storage, adipose cell size was diminished in AT from Abcg1-deficent mice in this study, highlighting the importance of Abcg1 in this biological process. Taken together, these data identify Abcg1 as a Janus-faced metabolic switch which depends on the metabolic context. Thus, although Abcg1 protects from tissue lipid accumulation by promoting cellular free cholesterol efflux to HDL in a cholesterol-rich context, it favors cellular lipid storage, mainly TG, in a glycerolipid-rich environment (not glycerophospholipids) (). It is therefore appealing to speculate that ABCG1 might represent a promising pharmacological target in metabolic states characterized by excessive fat depots as observed in obesity. Methodological strategy used in our study led us to propose that an RNAi approach could be an efficient tool to silence Abcg1 expression in AT in obese patients or in individuals prone to develop morbid obesity in order to reduce fat mass formation and potentially body weight. Indeed, the RNAi strategy is an emerging therapeutic approach based on the use of small interfering RNA (siRNA) to silence target genes involved in human diseases. Because of their ability to specifically recognize a target gene sequence and to down regulate locally and temporally its expression, siRNAs constitute an exciting and promising therapeutic tool for diseases for which an effective treatment is currently unavailable or partially efficient, as it is presently the case for obesity. At the moment, several clinical trials are being conducted using local delivery of siRNAs in order to silence the expression of a specific target gene in diverse pathologies, such as age-related macular degeneration, diabetic macular edema, cancers (solid tumors, chronic lymphocytic leukemia, lung cancer, glioblastoma multiforme, metastatic melanoma), hepatitis B and C, HIV/AIDS, acute renal failure and hypercholesterolemia.Citation5 These trials illustrate the growing interest in such therapeutic strategy for silencing a disease-causing gene. One of the major obstacles is however the ability to deliver locally the siRNAs/shRNAs to the target tissue, AT in the case of ABCG1, without affecting unwanted tissues. Thus, a considerable effort is provided by expert pharmaceutical companies in this field to develop nanoparticles able to deliver siRNAs locally into a specific tissue. In this context, development of nanoparticles carrying siRNAs that silence ABCG1 expression and target specifically adipocyte in AT might therefore constitute a potentially interesting therapeutic approach in obesity.Citation6
Figure 2. ABCG1 is a Janus-faced metabolic switch. In a cholesterol-rich metabolic context, ABCG1 protects from tissue lipid accumulation by promoting cellular free cholesterol efflux (left side of the Janus face). By contrast, ABCG1 promotes cellular lipid storage in a fat-rich environment by controlling lipoprotein lipase activity (right side of the Janus face). TRL: Triglyceride-rich lipoproteins, FFA: Free fatty acids, LPL: lipoprotein lipase.

Although merely speculative at this stage, some critical points must be taken in account. First of all, the tissue specificity of the intervention is an important issue as Abcg1 has been reported to play important biological functions in other tissues where its inhibition could lead to undesired metabolic consequences. The role of Abcg1 in insulin secretion from mouse pancreatic β-cells is clearly the most critical one. Indeed, Sturek et al. elegantly showed that loss of Abcg1 expression impaired insulin secretion both in vitro and in vivo by altering granule cholesterol content and morphology in mouse β-cells.Citation7 Thus, decreased insulin secretion as well as impaired glucose tolerance were observed in Abcg1-/- mice fed a normal chow diet as compared to control Abcg1+/+ mice, whereas Abcg1 deficiency was without effect on insulin sensitivity.Citation7 Interestingly, a recent epigenome-wide association study among 837 nondiabetic participants in the Genetics of Lipid Lowering Drugs and Diet Network study suggested that cytosine guanine dinucleotide (CpG) methylation at CpG sites in ABCG1 locus in CD4+ T cells was significantly associated with insulin levels and HOMA-IR.Citation8 Those latter studies would suggest that pharmacological inhibition of ABCG1 could exert potentially deleterious effects on insulin resistance (IR), This possibility is however weakened by the observation that Abcg1-/- mice fed a high-fat diet did not exhibit glucose intolerance or liver steatosis, in contrast to control Abcg1+/+ littermatesCitation4, suggesting that total Abcg1 deficiency should rather protect against IR rather than deteriorate it. However, our analysis of ABCG1 SNPs in 1320 morbidly obese patients revealed that both rs1378577 and rs1893590 were not associated with HOMA index independently of BMI.Citation3 More importantly, the deleterious effect of ABCG1 SNPs on BMI was not accompanied by any effect on diabetes or HOMA index.Citation3 Moreover, it is to note that neither a loss-of-function mutation nor a common variant in the ABCG1 gene were associated with Type 2 diabetes in 40,600 individuals of the Copenhagen City Heart Study (CCCHS) and the Copenhagen General Population Study (CGPS).Citation9
Numerous observations validated the positive association between Abcg1 expression and AT formation and growth. Indeed, expression of Abcg1 was markedly increased during adipocyte differentiation in both mouseCitation3,4 and human adipocytes.Citation3 In addition, elevated Abcg1 expression was observed in AT from obese db/dbCitation10 or NZO miceCitation4 in comparison to wt mice. Finally, upregulated expression of ABCG1 in human AT was associated with increased fat mass in morbidly obese individuals.Citation3 Those data are consistent with the notion that Abcg1 represents a major player in adipogenesis and fat mass growth, the latter being illustrated by either total deletionCitation4 or AT-specific RNAi-mediated silencingCitation3 of Abcg1 expression in non-obese C57BL/6 mice which drastically hampered fat mass development in those animal following a high-fat diet. However, the potential usefulness of a therapeutic inhibition of Abcg1 must be evaluated in obese animals as such strategy would be initially envisaged in obese patients in order to hamper formation, or ideally, reduce size of preexisting excessive AT mass. To date, data are lacking in order to validate inhibition of Abcg1 expression in obese animals as an efficient approach to achieve such beneficial effects. Nevertheless, it is important to note that weight loss in obese individuals does not appear to be accompanied by a decreased ABCG1 expression in AT, as it could have been initially expected. Indeed, gene expression analysis of subcutaneous AT from obese patients following caloric restrictionCitation11-14 or surgeryCitation15 did not reveal any reduction of ABCG1 expression in AT after weight loss but instead detected an upregulation of this pathway in some cases.Citation16,17 These intriguing data suggest that ABCG1 can exert a more complex role in AT than that recently identified by us in adipocytes.Citation3 Thus, Edgel et al. reported that although Abcg1 expression was increased in AT of db/db mice during obesity, an elevation of Abcg1 expression was equally observed in those mice after caloric restriction.Citation10 Interestingly, analysis of abdominal AT macrophages from db/db mice under feeding conditions of either ad libitum or caloric restriction revealed an enhanced Abcg1 expression in those cells after caloric restriction concomitant to the elevation of intracellular cholesterol content as compared to ad libitum.Citation10
Together, these observations suggest that ABCG1 can exert a dual role in AT metabolism, promoting adipocyte differentiation and maturation during fat mass growth but contributing to its reduction during caloric restriction mediated by potential effects on AT macrophages. Ongoing experiments in our laboratory using mouse models with a conditional deletion of Abcg1 in either adipocyte or macrophages should help us to decipher molecular and cellular mechanisms underlying in these processes.
In conclusion, our recent findings bring to light ABCG1 as a potential candidate for pharmacological inhibition in the context of obesity; further investigations are required in order to explore the whole spectrum of ABCG1 actions in AT.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885–96; PMID:17139329; http://dx.doi.org/10.1038/nrm2066
- Kolditz CI, Langin D. Adipose tissue lipolysis. Curr Opin Clin Nutr Metab Care 2010; 13:377–81; PMID:20531174; http://dx.doi.org/10.1097/MCO.0b013e32833bed6a
- Frisdal E, Lay SL, Hooton H, Poupel L, Olivier M, Alili R, Plengpanich W, Villard EF, Gilibert S, Lhomme M, et al. Adipocyte Atp-binding cassette G1 promotes triglyceride storage, fat mass growth and human obesity. Diabetes 2015; 64:840–55; http://dx.doi.org/10.2337/db14-0245
- Buchmann J, Meyer C, Neschen S, Augustin R, Schmolz K, Kluge R, Al-Hasani H, Jürgens H, Eulenberg K, Wehr R, et al. Ablation of the cholesterol transporter adenosine triphosphate-binding cassette transporter G1 reduces adipose cell size and protects against diet-induced obesity. Endocrinology 2007; 148:1561–73; PMID:17194745; http://dx.doi.org/10.1210/en.2006-1244
- Kim SS, Garg H, Joshi A, Manjunath N. Strategies for targeted nonviral delivery of siRNAs in vivo. Trends in Mol Med 2009; 15:491–500; PMID:19846342; http://dx.doi.org/10.1016/j.molmed.2009.09.001
- The ABCG1 gene as a marker and a target gene for treating obesity (PCT/EP2011/073140). 2011
- Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC. An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic β cells. J Clin Invest 2010; 120:2575–89; PMID:20530872; http://dx.doi.org/10.1172/JCI41280
- Hidalgo B, Irvin MR, Sha J, Zhi D, Aslibekyan S, Absher D, Tiwari HK, Kabagambe EK, Ordovas JM, Arnett DK. Epigenome-wide association study of fasting measures of glucose, insulin, and HOMA-IR in the Genetics of Lipid Lowering Drugs and Diet Network study. Diabetes 2014; 63:801–7; PMID:24170695; http://dx.doi.org/10.2337/db13-1100
- Schou J, Tybjaerg-Hansen A, Moller HJ, Nordestgaard BG, Frikke-Schmidt R. ABC transporter genes and risk of type 2 diabetes: a study of 40,000 individuals from the general population. Diabetes Care 2012; 35:2600–6; PMID:23139370; http://dx.doi.org/10.2337/dc12-0082
- Edgel KA, McMillen TS, Wei H, Pamir N, Houston BA, Caldwell MT, Mai PO, Oram JF, Tang C, Leboeuf RC. Obesity and weight loss result in increased adipose tissue ABCG1 expression in db/db mice. Biochim Biophys Acta 2012; 1821:425–34; PMID:22179025; http://dx.doi.org/10.1016/j.bbalip.2011.11.012
- Campbell KL, Foster-Schubert KE, Makar KW, Kratz M, Hagman D, Schur EA, Habermann N, Horton M, Abbenhardt C, Kuan LY, et al. Gene expression changes in adipose tissue with diet- and/or exercise-induced weight loss. Cancer Prev Res (Phila) 2013; 6:217–31; PMID:23341572; http://dx.doi.org/10.1158/1940-6207.CAPR-12-0212
- Nookaew I, Svensson PA, Jacobson P, Jernas M, Taube M, Larsson I, Andersson-Assarsson JC, Sjöström L, Froguel P, Walley A, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab 2013; 98:E370–8; PMID:23264395; http://dx.doi.org/10.1210/jc.2012-2764
- Rizkalla SW, Prifti E, Cotillard A, Pelloux V, Rouault C, Allouche R, Laromiguière M, Kong L, Darakhshan F, Massiera F, et al. Differential effects of macronutrient content in 2 energy-restricted diets on cardiovascular risk factors and adipose tissue cell size in moderately obese individuals: a randomized controlled trial. Am J Clin Nutr 2012; 95:49–63; PMID:22170375; http://dx.doi.org/10.3945/ajcn.111.017277
- Mutch DM, Pers TH, Temanni MR, Pelloux V, Marquez-Quinones A, Holst C, Martinez JA, Babalis D, van Baak MA, Handjieva-Darlenska T, et al. A distinct adipose tissue gene expression response to caloric restriction predicts 6-mo weight maintenance in obese subjects. Am J Clin Nutr 2011; 94:1399–409; PMID:22030226; http://dx.doi.org/10.3945/ajcn.110.006858
- Hoggard N, Cruickshank M, Moar KM, Bashir S, Mayer CD. Using gene expression to predict differences in the secretome of human omental vs. subcutaneous adipose tissue. Obesity (Silver Spring) 2012; 20:1158–67; PMID:22286531; http://dx.doi.org/10.1038/oby.2012.14
- Johansson LE, Danielsson AP, Parikh H, Klintenberg M, Norstrom F, Groop L, Ridderstråle M. Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. Am J Clin Nutr 2012; 96:196–207; PMID:22648723; http://dx.doi.org/10.3945/ajcn.111.020578
- Capel F, Klimcakova E, Viguerie N, Roussel B, Vitkova M, Kovacikova M, Polák J, Kovácová Z, Galitzky J, Maoret JJ, et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 2009; 58:1558–67; PMID:19401422; http://dx.doi.org/10.2337/db09-0033
