A surprise source of adiponectin
pp. 251–269
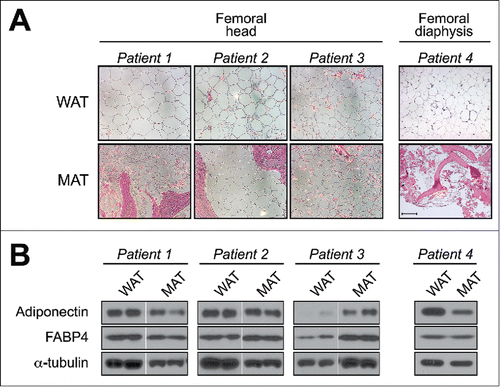
The hormone adiponectin facilitates beneficial cardiovascular and metabolic effects, and circulatory levels of this adipocyte-derived hormone are low in obesity as well as insulin-resistance due to decreased expression from white adipose tissue (WAT). On the other hand, high levels of adiponectin are observed in lean and calorie-restricted (CR) states, surprisingly, without increased production from WAT. In this review, Authors Scheller, Burr, MacDougald, and Cawthorn discuss their research into this phenomenon, with the theory that bone marrow adipose tissue (MAT) is the actual source of CR-associated hyperadiponectinemia ().
Peri-pancreatic adipocyte inflammation and type 1 diabetes mellitus (T1DM)
pp. 270–274
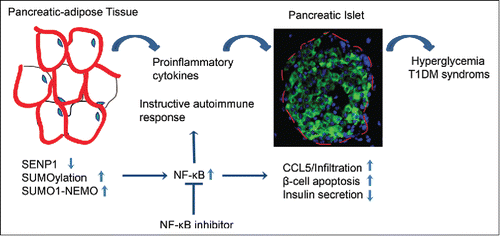
In this review, authors Shao et al. present their recent studies into the increases in proinflammatory cytokine production in peri-pancreatic adipocytes (PATs) of small ubiquitin-like modifier (SUMO)-specific protease SENP-deficient mice. These cytokines are not only cytotoxic to pancreatic islets, but, also increases islet CCL5 expression inducing a state of chronic inflammation which contributes to T1DM.
The review further discusses how the biological functions of cellular proteins can be modulated by SUMO, known as SUMOylation, and the various aspects of this processes that may be associated with T1DM susceptibility ().
Adipocytes, adipose stem cells and chronic inflammation
pp. 275–282
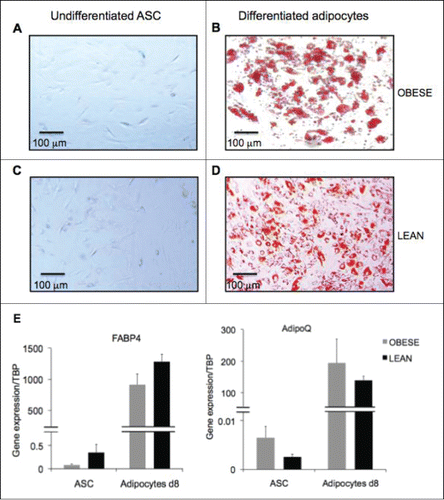
Having recently shown that a pro-inflammatory environment can be induced by co-culturing peripheral blood mononuclear cells (MNC) with adipose tissue-derived stem cells (ASC) from obese donors, authors Chehimi et al. present new findings in this research paper related to inflammatory potential of differentiated adipocytes from adipose tissues of obese subjects. Through an analysis of blood MNC activation in co-culture assays with differentiated adipocytes, the authors demonstrate a pattern of proinflammatory activation similar to that of ASCs, with, increased secretion of IL-17A, IL-1β, and IL-6. These findings support the idea that both ASC and adipocytes contribute to the state of obesity-associated low-grade chronic inflammation via induction of Th-17 cells and activation of monocytes ().
A more detailed look into MicroRNA's adipogenesis regulation
pp. 283–297
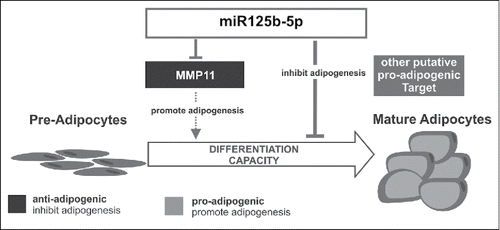
The non-coding RNA's known as MicroRNAs may play a part in controlling adipogenesis and are able to regulate gene expression. However, few investigations have identified and expanded on microRNA's role on adipogenesis. Here, authors Rockstroh et al. used microarray-analysis in order to pinpoint exactly what microRNA was involved in human adipogenesis regulation. Their research led them to miR125b-5p, which during adipocyte differentiation was upregulated, and which in turn downregulated matrix metalloproteinase 11, an anti-adipogenic mediator (MMP11). Combined with the findings that miR125b-5P can directly inhibit adipogenesis, there is evidence that miR125b-5P may be used through MMP11 and other targets to affect human adipogenesis ().
Cortisone's regulatory properties on HSD1 and AQP7
pp. 298–305
Glucocorticoids (GC) regulate adipose tissue function and contribute to obesity-associated metabolic disease. Tissue activation of GCs occurs via 11 β-Hydroxysteroid dehydrogenase (Hsd1), and, overexpression of Hsd1 results in obesity whereas its deficiency protects against high fat diet-induced obesity and hyperglycemia. Adipocyte membrane-localized Aquaporin 7 (Aqp7), mobilizes H2O and glycerol, and, competes with Hsd1 in adipocyte triglyceride metabolism. The synthetic steroid dexamethasone (DXM) downregulates Aqp7 and induces differentiation of 3T3-L1 adipocytes. This brief report by Quesada-López, González-Dávalos, Piña, and Mora compares the action of cortisone on Hsd1 and Aqp7 expression in 3T3-L1 adipocytes. The authors observed immediate and parallel mRNA expression for both Hsd1 and Aqp7 in response to cortisone, as opposed to their hypothesis that cortisone reduces Aqp7 expression while favoring Hsd1.