ABSTRACT
Secreted frizzled-related protein (SFRP) 4 is an extracellular antagonist of Wnt signalling that regulates adipogenesis, and is highly in the visceral adipose tissue of obese individuals. However, it is still unclear how exactly SFRP4 regulates the secretion of adipokines in the adipose tissue in vivo, an event that is closely related to the pathogenesis of obesity and insulin resistance. In this study, we generated transgenic (Tg) mice overexpressing SFRP4 in the liver and investigated SFRP4 role in adipokine secretion in mice on a regular normal diet. In Tg mice, SFRP4 protein was overexpressed in the liver, as compared to wild-type littermates (non-Tg), and released into the blood. Moreover, the size of adipocytes was smaller in the visceral adipose tissue of Tg mice compared to controls. Additionally, SFRP4 overexpression affected the expression of genes related to adipocyte differentiation, causing the upregulation of adiponectin and glucose transporter 4, and the downregulation of CCAAT/enhancer-binding protein-β, in both visceral and subcutaneous adipose tissue. However, there was no difference in body weight or body composition between Tg and non-Tg mice. In summary, our data showed that SFRP4 overexpression altered adipocyte size and adipokine secretion, possibly affecting adipocyte differentiation, obesity, and glucose metabolism.
Introduction
Obesity is a health risk factor, closely associated with chronic non-communicable diseases such as metabolic syndrome, type 2 diabetes, and hypertension [Citation1]. With the increase in obesity rate, the incidence of type 2 diabetes, impaired glucose tolerance, and other obesity-related complications has escalated [Citation2–4]. The adipose tissue secretes a wide variety of adipokines that participate in various physiological processes, including food intake, insulin sensitivity, immunity, and inflammation [Citation5–7].
Secreted frizzled-related protein 4 (SFRP4) belongs to a family of secreted proteins containing a frizzled-like cysteine-rich domain, which binds to Wnt ligands or frizzled receptors extracellularly, thus modulating the Wnt cascade [Citation8,Citation9]. Wnt family proteins regulate adipocyte differentiation and lipid metabolism by paracrine or autocrine mechanisms [Citation10–12]. SFRP4 is expressed in adipocytes, as well as in various tissues including pancreas, heart, bone, and uterus, and is involved in multiple pathophysiological processes including fibrosis, angiogenesis, apoptosis, malignancy, adipogenic differentiation, and bone metabolism [Citation13–19]. In recent years, SFRP4 was reported to play a critical role in glucose and lipid metabolism [Citation20,Citation21]. First, SFRP4 is overexpressed in obese individuals and patients with type 2 diabetes [Citation22,Citation23]. Second, circulating SFRP4 significantly correlates with body fat percentage, body mass index, and insulin sensitivity [Citation23]. Third, SFRP4 is overexpressed in the islets of patients with type 2 diabetes and is considered a marker of early beta cell dysfunction [Citation24]. In addition, SFRP4 promotes the differentiation of human adipose tissue-derived mesenchymal stem cells into mature adipocytes [Citation19].
Our previous study reported that SFRP4 knockdown inhibits the differentiation of preadipocytes derived from visceral adipose tissue, while promoting the differentiation of precursor cells isolated from subcutaneous adipose tissue, indicating that SFRP4 may have distinct functions in different adipose depots [Citation25]. In addition, we discovered that SFRP4 knockdown in adipocytes downregulates the expression of adipokines, including adiponectin and glucose transporter 4 (GLUT4) [Citation25]. In order to investigate the effect of SFRP4 on the expression of adipokines from adipose tissue in vivo, we generated transgenic mice with liver-specific SFRP4 overexpression to increase the level of circulating SFRP4. Our results indicated that SFRP4 overexpression affected the expression of adipokines, as well as the size of adipocytes.
Materials and methods
Animals
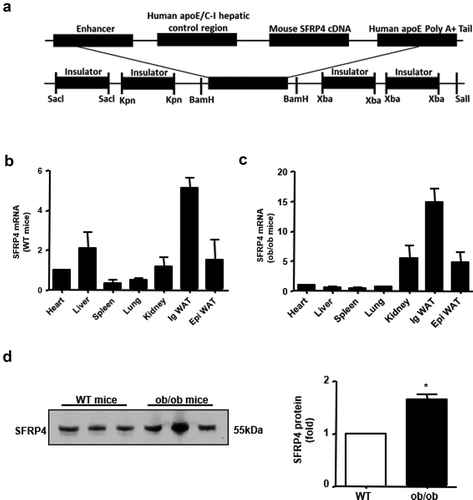
Transgenic (Tg) mice of C57BL/6 N background were generated in our laboratory as previously reported [Citation26,Citation27]. The DNA construct used for microinjection contained mouse SFRP4 cDNA under the control of the liver-specific sequences containing partial apoE and liver-specific region of the apoE/apoCI gene locus [Citation28], along with four copies of the chicken β globin insulator ()), to prevent position effects of transgene insulators [Citation29]. The transgene was confirmed by PCR. F1 descendants from three of the five founders carried the gene insert, while the other two were chimeras. One of the three Tg founders, exhibiting the highest SFRP4 mRNA expression level, was selected for progeny generation. Leptin-null (ob/ob) mice of C57BL/6 N background were purchased from Vital River Laboratory (Beijing Vital River Laboratory Animal Technologies Co. Ltd, Beijing, China). All mice were maintained in 12-h light/dark cycle, fed a standard rodent diet, and provided with water ad libitum. All animal procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of Xi’an Jiaotong University.
Figure 1. SFRP4 is highly expressed in ob/ob mice. (a) Construction of transgenic expression vector. (b) Expression of SFRP4 in various tissues of wild type (WT) mice, as determined by qPCR. (c) Expression of SFRP4 in various tissues of ob/ob mice, as determined by qPCR. (d) Western blotting analysis of SFRP4 expression in the plasma of WT and ob/ob mice. The data are expressed as the mean ± SEM, n = 6 for each group. *P < 0.05, vs. WT mice. ig WAT, inguinal white adipose tissue (subcutaneous adipose tissue); epi WAT, epididymal white adipose tissue (visceral adipose tissue)
DNA extraction
About 0.5 cm of mouse tail was cut, then placed in a mixture of proteinase K and lysis buffer, digested overnight at 55°C, extracted with an organic solvent (saturated phenol: chloroform: isoamyl alcohol = 25:24:1), washed with 75% absolute ethanol, and finally dissolved in double-distilled water. DNA purity and concentration were measured by Nano Drop 2000 (ThermoFisher, Waltham, MA, USA).
Polymerase chain reaction (PCR)
Genomic DNA samples were subjected to PCR analysis using a pair of primers specific for Tg mice (forward: 5ʹ- CCTGGCAACATACCTGAGCA-3ʹ and reverse: 5ʹ- CACTCCTCTGGACGGCTTTT-3ʹ). The 20-µL reactions included 8.2 µL of ddH2O, 10 µL of Taq DNA Polymerase mix, 1 µL of genomic DNA sample, 0.4 µL of 25 µmol/L Forward primer, and 0.4 µL of 25 µmol/L Reverse primer. The PCR conditions were as follows: 95°C 3 min (1 X), 94°C 30 s, 55°C 30 min, 72°C 45 s, 30 cycles, 72°C 10 min, 4°C 10 min. Equal amounts of PCR products were analysed by 1% agarose gels.
RNA extraction and quantitative real-time PCR (qPCR)
Total RNA of adipose tissues was extracted by using RNAiso Plus (TaKaRa Biology Inc., Shiga, Japan). Reverse transcription was performed from 1 µg of total RNA by using the PrimeScript RT reagent kit (TaKaRa Biology Inc., Shiga, Japan). As we previously described [Citation30], qPCR analysis was performed in triplicates, and the values were normalized to β-actin. Each 20-µL PCR reaction included 0.8 µL (10 µM) of forward and reverse primers and 10 µL of 2× SYBR Green PCR Master Mix. The PCR reactions were performed by using a Thermal Cycler Dice Real Time System TP-800 (TaKaRa Biology Inc., Shiga, Japan). The generation of specific PCR products was confirmed by melting curve analysis and gene expression levels were calculated by the comparative Ct method, X = 2−ΔΔCt. The primer sequences are shown in .
Table 1. The sequences of primers used in this study
Protein extraction and western blotting analysis
The adipose tissue and liver of mice were homogenized in ice-cold lysis buffer containing 1% protease inhibitors and phosphorylase inhibitors, then clarified by centrifugation (12, 000 g, 5 min). The protein content was determined by using a BCA protein concentration assay kit (Thermo Fisher, Waltham, MA, USA). After protein denaturation, the expression of target protein was evaluated by western blotting. Briefly, 30 µg of protein sample was subjected to 10% SDS-PAGE, transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA), and immunoblotted by using primary antibodies (Abs) against SFRP4 (1:1000; Cat. No.15328, Proteintech), adiponectin (1:1000; Cat. No.AF1119, R&D), fatty acid binding protein 4 (FABP4) (1:1000; Cat. No. AF1443, R&D), β-Actin (1:1000; Cat.No.SC69879, Santa Cruz) or β-Tubulin (1:2000; Cat. No. ab15246, Abcam) at 4°C overnight, as per the manufacturer’s instructions. After washing with tris-buffered saline-Tween-20, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature (20°C – 25°C) for 1 h. After washing with tris-buffered saline, the antigen-antibody complexes were visualized by using an enhanced chemiluminescence detection kit (Millipore, Billerica, MA, USA). β-Actin or β-Tubulin was used as a loading control.
Morphological analysis of adipose tissue
Adipose tissues were excised and fixed in 10% formalin buffer. The fixed specimens were processed to paraffin blocks, sectioned, and stained with haematoxylin-eosin (H&E) according to standard procedures [Citation31]. The sections for microscopic quantification were photographed under a microscope equipped with a digital camera (Nikon, Tokyo, Japan). The diameter and area of adipocytes were measured by using Image J software (https://imagej.nih.gov/ij/).
Statistical analysis
All data were expressed as the mean ± SEM. For statistical analyses, the Student’s t-test or the Welch’s t test were applied in case of equal or non-equal F values, respectively. P values < 0.05 were considered statistically significant. All analyses were performed by using GraphPad PRISM 5.0 (GraphPad Software, Inc, San Diego, CA, USA).
Results
SFRP4 was highly expressed in ob/ob mice
To determine the tissue distribution of SFRP4 in wild-type (WT) and ob/ob mice, we isolated the heart, liver, spleen, lung, kidney, inguinal white adipose tissue (ig WAT), and epididymal white adipose tissue (epi WAT) from WT and ob/ob mice to analyse SFRP4 mRNA expression. The results showed that in WT mice, SFRP4 expression was highest in the ig WAT, followed by the liver and epi WAT ()). Similarly, in ob/ob mice, SFRP4 showed the highest expression in ig WAT, followed by kidney and epi WAT ()). Plasma was collected from the tail vein and western blotting analysis was performed to measure SFRP4 protein expression. The results showed that the plasma level of SFRP4 protein was significantly higher in ob/ob mice than in WT mice (P < 0.05) ()). The observed differences in SFRP4 expression between visceral and subcutaneous adipose tissue, as well as in plasma SFRP4 level between WT and ob/ob mice, suggested that SFRP4 played an important role in adipogenesis and adipocyte differentiation.
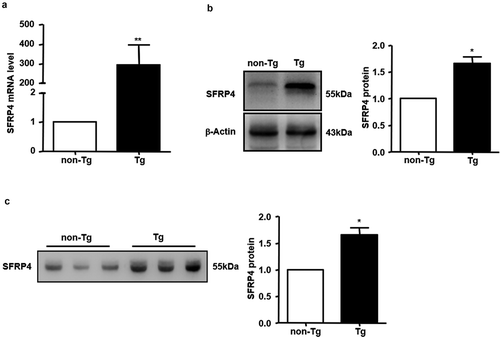
The expression of SFRP4 was increased in Tg mice
We successfully created Tg mice with liver-specific SFRP4 overexpression. The results showed that the hepatic levels of SFRP4 mRNA and protein were significantly higher in Tg compared to non-Tg mice (P < 0.05 or P < 0.01) (,)). The plasma level of SFRP4 protein was also compared by western blotting in the two groups of mice. Tg mice had significantly higher levels of plasma SFRP4 compared to non-Tg mice (P < 0.05) ()), because of protein secretion by the liver.
Figure 2. The expression of SFRP4 was increased in Tg mice. (a) SFRP4 mRNA expression was determined in the liver of Tg mice and WT littermates. (b) and (c) SFRP4 expression in the liver (B) and plasma (C) was determined by western blotting (β-actin was used as a loading control). A representative blot and data quantification from three independent experiments are shown. Data are expressed as the mean ± SEM, n = 6 for each group. *P < 0.05, **P < 0.01 vs. non-Tg
SFRP4 overexpression was associated with decreased size of visceral adipocytes
Eight-week-old male Tg mice and control littermates were fed with chow diet for 12 weeks. There were no significant differences in body weight, fat mass and liver weight between Tg and non-Tg mice (). H&E staining was used to explore the morphological characteristics of adipocytes in Tg and non-Tg mice. We found that the size of epi WAT adipocytes was smaller in Tg mice compared to control littermates. However, no significant differences were observed in the size of ig WAT adipocytes between the two groups ().
Figure 3. Body mass, fat mass, and liver weight in Tg mice. (a) Body weight changes in Tg mice and non-Tg littermates. (b) The ig WAT weight, epi WAT weight, and liver weight of non-Tg and Tg-SFRP4 mice. Data are expressed as the mean ± SEM, n = 8 for each group. **P < 0.01 vs. non-Tg
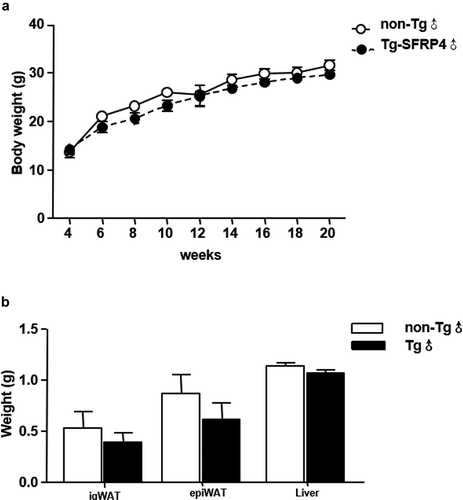
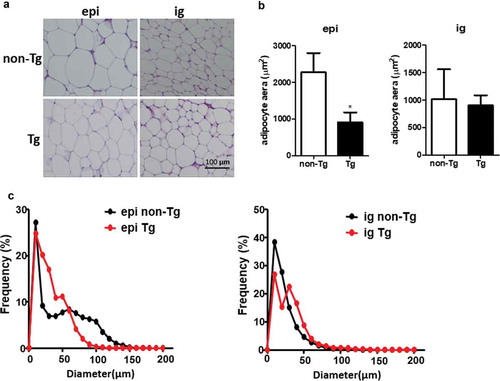
Figure 4. Adipocyte size was smaller in the epi WAT of Tg mice compared to WT littermates. (a) Representative image of adipocyte staining with haematoxylin & eosin. (b) The area of adipocytes was measured in Tg mice and non-Tg littermates. (c) A frequency distribution plot of cell diameters was calculated to determine the mean diameter of fat cells. Data are expressed as the mean ± SEM, n = 8 for each group. *P < 0.05 vs. non-Tg
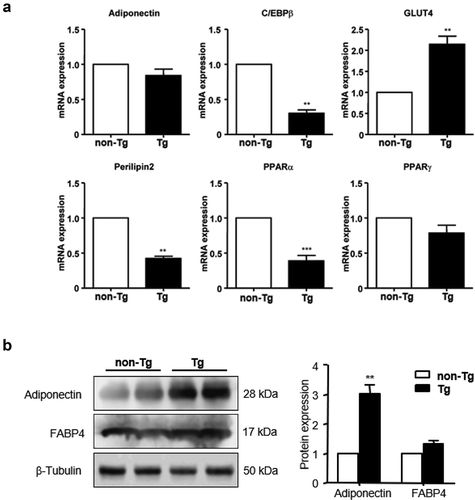
SFRP4 regulated the expression of genes involved in the differentiation of adipocytes from visceral adipose tissue
The epi WAT of non-Tg and Tg male mice was isolated, total RNA and protein were extracted, and the expression of adipocytokines was analysed by qPCR and western blotting. The results showed that the mRNA expression of CCAAT/enhancer-binding protein-β (C/EBPβ), perilipin2, and peroxisome proliferator-activated receptor alpha (PPARα) was significantly decreased (P < 0.01), while that of GLUT4 was significantly increased (P < 0.01), in Tg mice compared to controls. No significant differences were observed in the expression of adiponectin and PPARγ ()). Additionally, adiponectin protein expression was significantly higher in Tg compared to non-Tg mice, while FABP4 expression level was comparable in the two groups ()).
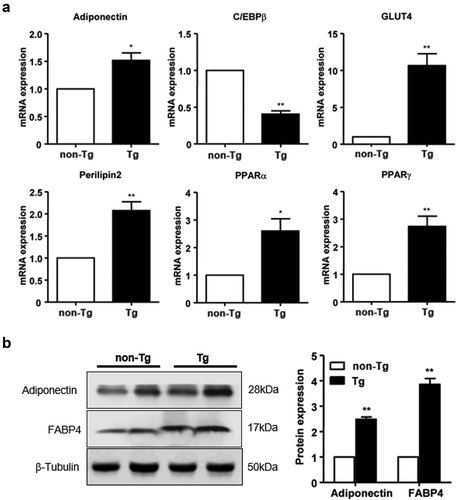
Effect of SFRP4 expression on subcutaneous adipose formation
To determine the effect of SFRP4 overexpression on subcutaneous adipose tissue, the ig WAT was collected from Tg mice and control littermates, and total RNA and protein were extracted. The mRNA expression of C/EBPβ was significantly lower in Tg mice compared to controls, while that of adiponectin, GLUT4, perilipin2, PPARα, and PPARγ was significantly increased in SFRP4-overexpressing mice (P < 0.05 or P < 0.01) ()). Moreover, adiponectin and FABP4 protein levels were significantly higher in Tg mice than in control littermates (P < 0.01) ()).
Discussion
The main purpose of the current study was to investigate the impact of increased levels of circulating SFRP4 on adipose tissue deposition and glucose metabolism. In order to obtain an increase in the level of circulating SFRP4, we generated Tg mice overexpressing SFRP4 under the human apoE/C-I hepatic control region, thus inducing preferential expression in the liver. In this preliminary study, we investigated the effects of systemic SFRP4 overexpression on adipose tissue.
SFRP4 was highly expressed in the adipose tissue of C57BL/6 and ob/ob mice, in line with previous studies reporting high SFRP4 expression in the human subcutaneous adipose tissue and in obese individuals [Citation23,Citation32]. However, we also found that SFRP4 did not contribute to fat deposition, since the Tg mice had no significant differences in body weight, and body composition (epi WAT, ig WAT, liver) compared with non-Tg mice, which was complemented by the related research in SFRP4 knock out mice [Citation33].
We also found that SFRP4 overexpression was associated with a reduction in the size of epididymal adipocytes, which may reflect substantial changes in metabolism [Citation34,Citation35]. Previous studies demonstrated that the size of adipocytes in the abdominal depot is a reliable predictor of type 2 diabetes [Citation36–38]. Adipocyte hypertrophy contributes to insulin resistance, and SFRP4 is overexpressed in type 2 diabetes mellitus [Citation15,Citation39,Citation40]. It is worth mentioning that adiponectin expression was increased in the adipose tissue of Tg mice. Adiponectin, an adipokine, is the most abundant peptide secreted by adipocytes. Its level is decreased in obese individuals, and inversely correlated with insulin resistance and glucose intolerance [Citation41,Citation42]. Adiponectin-overexpressing mice show improved insulin sensitivity [Citation43]. In contrast, adiponectin-deficient mice exhibit insulin resistance and glucose intolerance [Citation44,Citation45]. We found increased expression of GLUT4 in SFRP4 Tg mice. GLUT4 is an insulin-regulated glucose transporter, primarily expressed in the adipose tissue and striated muscle [Citation46], which mediates glucose uptake, thus alleviating insulin resistance [Citation47]. Our results suggested that SFRP4 overexpression in mice might affect glucose metabolism. Of course, additional work is required to in-depth characterize the relationship between SFRP4 and insulin resistance.
Our previous in vitro experiments demonstrated that SFRP4 might play different roles in subcutaneous and visceral adipose tissue [Citation25]. On the other hand, in vivo studies are the most suitable approach to investigate the function of SFRP4 in different types of adipose tissue. Due to its role in the storage and mobilization of triglycerides, the adipose tissue is a crucial regulator of energy homoeostasis. Its growth is characterized by an increase in adipocyte size, as well by the formation of new adipocytes from precursor cells (adipocyte proliferation) [Citation48,Citation49]. Major differences were previously observed in adipogenic potential between visceral and subcutaneous adipose tissue [Citation50]. Moreover, adipocyte precursors from subcutaneous adipose tissue exert a protective effect against obesity-associated metabolic complications [Citation51]. Furthermore, the accumulation of visceral adipose tissue is strongly associated with obesity-related complications, including type 2 diabetes and coronary artery disease [Citation52]. Our results demonstrated that the expression patterns of genes related to adipocyte differentiation, including PPARγ, PPARα, perilipin 2, and FABP4, were distinct in visceral and subcutaneous adipose tissue, suggesting that SFRP4 may play different roles in the two tissues in mice. C/EBPs belong to a highly conserved family of leucine zinc finger proteins serving as transcription factors and include ɑ, β, and δ subtypes [Citation53]. The three subtypes of C/EBPs contribute to the regulation of lipogenic gene expression, affect glucose uptake in adipocytes, and play important roles in different stages of adipocyte differentiation [Citation53]. C/EBPβ is an early-stage regulator of adipocyte differentiation. It triggers lipid synthesis by inducing the expression of PPARγ and C/EBPα [Citation54–56]. The development of adipose tissue is impaired in C/EBPβ knockout mice, and the efficiency of adipogenic differentiation is significantly reduced in the precursors of C/EBPβ [Citation57]. Our results indicated that C/EBPβ mRNA levels were significantly decreased in both visceral and subcutaneous adipose tissues of Tg mice, compared to control mice. In the next study, the impact of SFRP4 on glucose metabolism and adipocyte differentiation will be explored in high-fat diet-induced obese Tg mice or, alternatively, by crossing these animals with ob/ob mice.
Conclusion
Our study demonstrated that SFRP4 overexpression in C57BL/6 N mice affected the size of epididymal adipocytes and the expression of adipokines. In particular, the expression of adiponectin and GLUT4 was found increased in Tg mice, suggesting a role of SFRP4 in glucose metabolism and adipocyte differentiation in mice.
Abbreviations
| C/EBP | = | CCAAT/enhancer-binding protein |
| epi WAT | = | epididymal white adipose tissue |
| FABP4 | = | fatty acid binding protein-4 |
| GLUT4 | = | glucose transporter 4 |
| ig WAT | = | inguinal white adipose tissue |
| PPAR | = | peroxisome proliferator activated receptor |
| SFRP4 | = | secreted frizzled-related proteins |
| Tg | = | transgenic |
| WT | = | wild type |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81270348) and the Natural Science Foundation of Shaanxi Province (2019-ZZ-JC034, 2020PT-001).
Disclosure statement
The authors declare that they have no competing interests.
References
- Collaborators GBDO, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The third national health and nutrition examination survey, 1988-1994. Diabetes Care. 1998;21(4):518–524.
- Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–4.
- Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S64–73.
- Nicholson T, Church C, Baker DJ, et al. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflamm (Lond). 2018;15:9.
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355.
- Conde J, Scotece M, Gómez R, et al. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors. 2011;37(6):413–420.
- Pawar NM, Rao P. Secreted frizzled related protein 4 (sFRP4) update: A brief review. Cell Signal. 2018;45:63–70.
- Ehrlund A, Mejhert N, Lorente-Cebrián S, et al. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab. 2013;98(3):E503–8.
- Christodoulides C, Lagathu C, Sethi JK, et al. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20(1):16–24.
- Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953.
- Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19(6):612–617.
- Huang A, Huang Y. Role of Sfrps in cardiovascular disease. Ther Adv Chronic Dis. 2020;11:2040622320901990.
- Zeng W, Cao Y, Jiang W, et al. Knockdown of Sfrp4 attenuates apoptosis to protect against myocardial ischemia/reperfusion injury. J Pharmacol Sci. 2019;140(1):14–19.
- Mahdi T, Hänzelmann S, Salehi A, et al. Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab. 2012;16(5):625–633.
- Muley A, Majumder S, Kolluru GK, et al. Secreted frizzled-related protein 4: an angiogenesis inhibitor. Am J Pathol. 2010;176(3):1505–1516.
- Sandsmark E, Andersen MK, Bofin AM, et al. SFRP4 gene expression is increased in aggressive prostate cancer. Sci Rep. 2017;7(1):14276.
- Nakanishi R, Akiyama H, Kimura H, et al. Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res. 2008;23(2):271–277.
- Park JR, Jung J-W, Lee Y-S, et al. The roles of Wnt antagonists Dkk1 and sFRP4 during adipogenesis of human adipose tissue-derived mesenchymal stem cells. Cell Prolif. 2008;41(6):859–874.
- Bukhari SA, Yasmin A, Zahoor MA, et al. Secreted frizzled-related protein 4 and its implication in obesity and type-2 diabetes. IUBMB Life. 2019;71(11):1701–1710.
- Horbelt T, Knebel B, Fahlbusch P, et al. The adipokine sFRP4 induces insulin resistance and lipogenesis in the liver. Biochim Biophys Acta Mol Basis Dis. 2019;1865(10):2671–2684.
- Anand K, Vidyasagar S, Lasrado I, et al. Secreted Frizzled-Related Protein 4 (SFRP4): A novel biomarker of beta-cell dysfunction and insulin resistance in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2016;39(9):e147–8.
- Garufi G, Seyhan AA, Pasarica M. Elevated secreted frizzled-related protein 4 in obesity: a potential role in adipose tissue dysfunction. Obesity (Silver Spring). 2015;23(1):24–27.
- Bergmann K, Sypniewska G. Secreted frizzled-related protein 4 (SFRP4) and fractalkine (CX3CL1) - Potential new biomarkers for beta-cell dysfunction and diabetes. Clin Biochem. 2014;47(7–8):529–532.
- Guan H, Zhang Y, Gao S, et al. Differential patterns of secreted frizzled-related protein 4 (SFRP4) in adipocyte differentiation: adipose depot specificity. Cell Physiol Biochem. 2018;46(5):2149–2164.
- Gao S, Wang X, Cheng D, et al. Overexpression of cholesteryl ester transfer protein increases macrophage-derived foam cell accumulation in atherosclerotic lesions of transgenic rabbits. Mediators Inflamm. 2017;2017:3824276.
- Zhao S, Li Y, Gao S, et al. Autocrine human urotensin II enhances macrophage-derived foam cell formation in transgenic rabbits. Biomed Res Int. 2015;2015:843959.
- Fan J, Wang J, Bensadoun A, et al. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A. 1994;91(18):8724–8728.
- Chen Y, Waqar AB, Nishijima K, et al. Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J Cell Mol Med. 2020; 24(7):4261–4274.
- Wang W, Chen Y, Bai L, et al. Transcriptomic analysis of the liver of cholesterol-fed rabbits reveals altered hepatic lipid metabolism and inflammatory response. Sci Rep. 2018;8(1):6437.
- Parlee SD,Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93–122.
- Yue F, Cheng Y, Breschi A, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515(7527):355–364.
- Mastaitis J, Eckersdorff M, Min S, et al. Loss of SFRP4 alters body size, food intake, and energy expenditure in diet-induced obese male mice. Endocrinology. 2015;156(12):4502–4510.
- Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res. 1988;19:26–29.
- Despres JP, Fong BS, Julien P, et al. Regional variation in HDL metabolism in human fat cells: effect of cell size. Am J Physiol. 1987;252(5 Pt 1):E654–9.
- Weyer C, Foley JE, Bogardus C, et al. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–1506.
- Fang L, Guo F, Zhou L, et al. The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte. 2015;4(4):273–279.
- Yang J, Eliasson B, Smith U, et al. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity (Silver Spring). 2012;20(5):932–938.
- Hoffmann MM, Werner C, Böhm M, et al. Association of secreted frizzled-related protein 4 (SFRP4) with type 2 diabetes in patients with stable coronary artery disease. Cardiovasc Diabetol. 2014;13:155.
- Verboven K, Wouters K, Gaens K, et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci Rep. 2018;8(1):4677.
- Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321.
- Esteve E, Ricart W, Fernandez-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32(Suppl 2):S362–7.
- Combs TP, Pajvani UB, Berg AH, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145(1):367–383.
- Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281(5):2654–2660.
- Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–737.
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5(4):237–252.
- Stenbit AE, Tsao T-S, Li J, et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med. 1997;3(10):1096–1101.
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783–809.
- Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130(12):3122S–3126S.
- Wang QA, Tao C, Gupta RK, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344.
- Longo M, Zatterale F, Naderi J, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20(9):2358.
- Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2(4):367–373.
- Yeh WC, Cao Z, Classon M, et al. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9(2):168–181.
- Chen M-H, Tong Q. An update on the regulation of adipogenesis. Drug Discov Today Dis Mech. 2013;10(1–2):e15–e19.
- Loftus TM, Lane MD. Modulating the transcriptional control of adipogenesis. Curr Opin Genet Dev. 1997;7(5):603–608.
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5(9):1538–1552.
- Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. Embo J. 1997;16(24):7432–7443.