Abstract
Immune tolerance induced by regulatory mechanisms is an integral and fundamental part of immunity. In therapeutic settings, however, tolerance may significantly limit efficacy. Here, we summarize possible strategies to enhance therapeutic antibody dependent cellular cytotoxicity by overcoming NK cell tolerance.
Along with their potential for strong cytotoxic responses without previous priming, the common expression of the low-affinity immunoglobulin gamma Fc region receptor III-A (FcγR3A) makes natural killer (NK) cells potent effectors of therapeutic antibody dependent cellular cytotoxicity (ADCC). Nevertheless, ADCC efficacy is limited by NK cell intrinsic and extrinsic regulatory mechanisms. In particular, the interaction between inhibitory killer-cell immunoglobulin-like receptors (KIR) and their HLA Class I ligands is fundamental to NK cell development and tolerance. In a process called “licensing”, only NK cells expressing inhibitory receptors able to interact with self-HLA molecules gain full functional capacity. Potentially autoreactive NK cells carrying KIR that lack a cognate HLA ligand stay hypo-functional.
Rituximab – a chimeric anti-CD20 antibody – is a standard component of regimens used to treat B-cell lymphoma. How NK cell function in rituximab-induced ADCC is affected by regulatory mechanisms has not yet been completely defined.Citation1 We recently addressed the question of how KIR/HLA interactions influence rituximab-induced ADCC, and showed that the advantage of the full functional potential of licensed NK cells is compensated by the inhibitory KIR signal, if target cells express cognate HLA ().Citation2 In line with the concept of unlicensed NK cells being the strongest ADCC effector cells, we observed that killing efficiency correlated positively with the percentage of unlicensed cells. Considering that in Caucasians approximately 30 percent of individuals carry all HLA ligands to the 3 relevant inhibitory KIR receptors (KIR2DL1, KIR2DL2/3, and KIR3DL1), the benefit of anti-CD20 therapy in such patients may be strongly limited by NK cell tolerance. Our in vitro data were recently confirmed by the analysis of follicular lymphoma patients treated with rituximab, which showed that progression-free survival (PFS) decreases with the number of viable KIR/HLA interactions.Citation3 These data indicate a need for strategies to overcome the negative impact of KIR/HLA interactions in order to enhance the efficacy of therapeutic antibodies.
Figure 1. Strategies to induce NK-cell-driven antibody dependent cell cytotoxicity (ADCC) against lymphoma. CD20+ malignant B cells in an HLA-C2+, Bw4− individual are treated with anti-CD20-antibodies. The presence of the HLA-C2 and the lack of HLA-Bw4 defines the KIR2DL1+ natural killer (NK) cell as licensed and KIR3DL1+ as unlicensed, respectively. (A) The rituximab-induced activating signal is inhibited by the KIR2DL1/HLA-C2 interaction in the licensed NK cell. The activation in the KIR3DL1+ NK cell cannot be inhibited because of the lack of the HLA-Bw4 on the malignant cell. However, its activity is compromised by its unlicensed status. (B) The stronger activation induced by obinutuzumab overrides the inhibitory signal in the licensed KIR2DL1+ cell. In addition, the activation is superior to the unlicensed status of the KIR3DL1+ cell. (C) Due to the block of the KIR2DL1/HLA-C2 interaction by lirilumab, the licensed KIR2DL1+ cell can be activated with rituximab. Lirilumab does not react with the KIR3DL1. Therefore, the activity of the unlicensed KIR3DL1+ cell is not influenced.
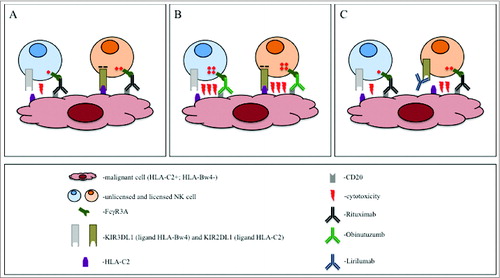
The observation that rituximab had greater clinical efficacy in patients carrying the high-affinity FcγR3ACitation4 led to the development of new anti-CD20 antibodies with modified Fc regions aiming to strengthen Fc/FcR interactions. One such antibody is obinutuzumab carrying an afucosylated glycoengineered Fc part, which increases the affinity to the FcγR3A receptor, and thereby enhances NK cell activation and killing efficiency.Citation5,6 Based on increased response rates and prolonged PFS as compared to rituximab when given in combination with chlorambucil to patients with chronic lymphocytic leukemia, obinutuzumab was recently approved for this indication and is undergoing evaluation in other types of B-cell lymphoma.Citation7
In vitro, obinutuzumab recruited more NK cells for ADCC and activated them more strongly than rituximab. Most notably, licensed and unlicensed cells practically did not differ in their level of activation and the activation was hardly influenced by the presence of cognate HLA KIR ligand on target cells. Importantly, multiple KIR/HLA interactions were necessary to decrease obinutuzumab-induced ADCC to the level achieved by rituximab.Citation2 In line with these observations, target depletion was unaffected by both the percentage of unlicensed effector cells in the repertoire, and by the number of KIR ligands expressed on target cells. In summary, obinutuzumab can induce comparable percentage and quality of activation in all NK cells subpopulations, independent from the licensing status and the KIR/HLA interactions (). These data suggest that in contrast to rituximab, obinutuzumab efficacy may not correlate with either the KIR or HLA genotypes, a hypothesis still awaiting clinical testing.
Another strategy to overcome the suppressive KIR/HLA interaction is based on antibody blockade. To this end, an antagonistic, non-depleting anti-KIRD2 antibody (lirilumab) has been developed. Lirilumab can block the KIR/HLA interaction in approximately half of NK cells (those expressing KIR2D receptors), increasing the number of activated immune effector cells in vitro as well as in vivo.Citation8 However, anti-KIR antibody treatment does not augment the strength of activation, and will therefore not increase the level of activation per cell (). The global blockage of the KIR function may also carry a potential risk of autoimmunity. However, clinical trials in patients with multiple myeloma report no evidence of autoimmune reactions.Citation9 Preclinically, use of rituximab in combination with lirilumab increased ADCC efficacy both in vitro and in mouse models.Citation10 While no clinical data are available on this combination so far, a phase-one studies is currently testing lirilumab in combination with elotuzumab (anti-CS1) in patients with multiple myeloma (NCT02252263).
The differences between the 2 presented strategies are too great to determine which one could be more beneficial. The advantage of the strategy represented by obinutuzumab is maximal percentage and level of activation of all FcγR3A-positive NK cells independently from their licensing status and the KIR/HLA interaction mediated by a single agent therapy. Fc enhancement can easily be incorporated into the development of new therapeutic antibodies. In contrast, lirilumab can be added to existing therapies in which the KIR/HLA interaction limits therapeutic benefits.
Disclosure of Potential Conflicts of Interest
LS is an employee of the University Hospital Basel. CK and MS are employees of Roche.
Funding
GT and MS received grant support from the Roche/University Hospital Basel Translational Medicine Hub.
References
- Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 2011; 118:3347-9; PMID:21768303; http://dx.doi.org/10.1182/blood-2011-05-351411
- Terszowski G, Klein C, Stern M. KIR/HLA interactions negatively affect rituximab- but not GA101 (obinutuzumab)-induced antibody-dependent cellular cytotoxicity. J Immunol 2014; 192:5618-24; PMID:24795454; http://dx.doi.org/10.4049/jimmunol.1400288
- Du J, Lopez-Verges S, Pitcher BN, Johnson J, Jung SH, Zhou L, Hsu K, Czuczman MS, Cheson B, Kaplan L, et al. CALGB 150905 (Alliance): rituximab broadens the antilymphoma response by activating unlicensed NK cells. Cancer Immunol Res 2014; 2:878-89; PMID:24958280; http://dx.doi.org/10.1158/2326-6066.CIR-13-0158
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-8; PMID:11806974; http://dx.doi.org/10.1182/blood.V99.3.754
- Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115:4393-402; PMID:20194898; http://dx.doi.org/10.1182/blood-2009-06-225979
- Liu SD, Chalouni C, Young JC, Junttila TT, Sliwkowski MX, Lowe JB. Afucosylated antibodies increase activation of FcgammaRIIIa-dependent signaling components to intensify processes promoting ADCC. Cancer Immunol Res 2015; 3:173-83
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370:1101-10; PMID:24401022; http://dx.doi.org/10.1056/NEJMoa1313984
- Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM Jr, Blaser BW, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009; 114:2667-77; PMID:19553639; http://dx.doi.org/10.1182/blood-2009-02-206532
- Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 2012; 120:4317-23; PMID:23002117; http://dx.doi.org/10.1182/blood-2012-06-437558
- Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014; 123:678-86; PMID:24326534; http://dx.doi.org/10.1182/blood-2013-08-519199
