Abstract
Exosomes are a kind of nanometric membrane vesicles and can be released by almost all kinds of cells, including cancer cells. As the important mediators in intercellular communications, exosomes mediate exchange of protein and genetic material derived from parental cells. Emerging evidences show that exosomes secreted by either host cells or cancer cells are involved in tumor initiation, growth, invasion and metastasis. Moreover, communications between immune cells and cancer cells via exosomes play dual roles in modulating tumor immunity. In this review, we focus on exosome-mediated immunosuppression via inhibition of antitumor responses elicited by immune cells (DCs, NK cells, CD4+ and CD8+ T cells, etc.) and induction of immunosuppressive or regulatory cell populations (MDSCs, Tregs and Bregs). Transfer of cytokines, microRNAs (miRNAs) and functional mRNAs by tumor-derived exosomes (TEXs) is crucial in the immune escape. Furthermore, exosomes secreted from several kinds of immune cells (DCs, CD4+ and CD8+ Tregs) also participate in immunosuppression. On the other hand, we summarize the current application of DC-derived and modified tumor-derived exosomes as tumor vaccines. The potential challenges about exosome-based vaccines for clinical application are also discussed.
Abbreviations:
- APC, antigen presenting cell
- Breg, regulatory B cell
- CTL, cytotoxic lymphocyte
- DC, dendritic cell
- DEX, dendritic cell-derived exosome
- EGFR, epidermal growth factor receptor
- EV, extracellular vesicle
- HSP, heat shock protein
- IFN, interferon
- IL, interleukin
- miRNA, microRNA
- MDSC, myeloid-derived suppressor cell
- MHC, major histocompatibility complex
- NK cell, natural killer cell
- NKG2D, natural killer group 2 member D
- PRR, pattern recognition receptor
- TEX, tumor-derived exosome
- TGF-β1, transforming growth factor β 1
- Th, helper T cell
- TLR, toll-like receptor
- Treg, regulatory T cell
Introduction
Extracellular vesicle (EV)-mediated exchanges of proteins, RNAs and lipids are emerging as an important aspect in cell–cell communications.Citation1 As the generic term for secreted membrane vesicles, EVs include different types of vesicles such as microvesicles (MVs) and exosomes.Citation2 They are distinguished by intracellular site of origin, physical properties (size and morphology) and methods for collection. Accordingly, exosomes are often described as endosomal origin and 30 to 100 nm in diameter. However, previous studies had conflicting and ambiguous definitions of different EVs. In this review, we still use the term “exosomes” as the cited articles indicated. Exosomes can be released by different types of cell including cancer cells, fibroblast cells, immune cells and mesenchymal cells.Citation3-5 When shuttled from a donor cell, they can transfer a broad array of biological contents including functional mRNAs, miRNAs, DNA fragments, lipids and proteins to recipient cells. Wrapped in bilayered membranes of exosomes, these contents are much stable even after being transferred to a distant site. Therefore, exosomes are an effective mode to affect surrounding or distant cells to induce systemic responses.Citation6 Evidences have shown that this kind of intercellular communication by exosomes is involved in multiple physiological and pathological processes, especially cancers.Citation7
Both cancer and non-cancer cells can produce large amounts of exosomes. They are widely distributed in plasma, ascites and pleural effusions from cancer patients and tumor-modeling animal.Citation8-10 Through secreting exosomes, cancer cells communicate with host cells and exchange different cellular components, like a kind of “infection”. This subtle and sophisticated system can manipulate the local and distant environment to facilitate tumor progression, including tumor growth, invasion, metastasis and even tumorigenesis.Citation11 In terms of immune system, exosomes can mediate immune activation or immunosuppression, thus dictating the outcomes of tumor progression. Numerous studies have indicated the roles of exosomes in tumor immunity.Citation12 Yet, crosstalk between cancer cells and immune cells via exosomes remains not fully illustrated. The aim of this review is to gather the most recent data regarding the “yin and yang” of exosomes in the regulation of tumor immunity and immunotherapy, hoping to guide the current diagnostic and therapeutic regimens of cancers.
Overview of Exosomes in Cancer Progression
Exosomes have emerged as a new mode of intercellular communication during cancer via transferring of oncogenes and proteins between different cells.Citation1 Recent studies have uncovered their roles in tumorigenesis. Melo et al. indicated that TEXs promoted tumorigenesis by modulating cell-independent miRNAs biogenesis.Citation13 Exosomes from breast cancer cells contained miRNAs associated with the RNA-induced silencing complex (RISC), Dicer, TAR RNA-binding protein 2 (TRBP) and Argonaute-2 (AGO2). When transferred to recipient cells, the exosomes could efficiently silence mRNA expression and thereby instigate non-tumorigenic epithelial cells to form tumors in a Dicer-dependent manner.
More attention is paid to investigate the roles and the underlying mechanisms of exosomes in promoting tumor growth and aggressiveness. These exosomes contain large amount of tumor-promoting RNAs (mRNAs, miRNAs and other non-coding RNAs) and proteins (EGFR, HSPs, KIT, etc.).Citation14-16 Exosome-mediated exchange of mRNAs and miRNAs (named as exosomal shuttle RNA, esRNA) is believed to be a novel way for genetic intervention between cells.Citation17 Plenty of miRNAs had been detected in exosomes. A subset of highly expressed miRNAs (miR-584, miR-517c, miR-378, etc.) extracted from hepatocellular carcinoma (HCC)-derived exosomes had been identified.Citation18 These miRNAs could target transforming growth factor β activated kinase-1 (TAK1). As a result, these miRNAs mediated TAK1 downregulation and then enhanced transformed cell growth in recipient cells. Zhang et al. reported that miR-150 packaged in human acute monocytic leukemia cell (THP-1)-secreted exosomes could be delivered into HMEC-1 cells, a human microvascular endothelial cell line.Citation19 The miR-150 reduced c-Myb expression and enhanced migration of HMEC-1 cells. Additionally, exosomes from metastatic gastric cancer cells, but not non-metastatic ones, were enriched in the let-7 family of miRNAs.Citation20 The enrichment of the miRNAs might display their oncogenic properties, especially metastasis. Direct exchange of proteins, especially oncoproteins, is also investigated in depth. Clinical aggressive human gliomas often expressed the oncogenic EGFR variant 3, known as EGFRvIII, which could be transferred from EGFRvIII positive glioma cells to negative ones via exosomes.Citation21 This process resulted in increased anti-apoptotic gene expression and tumor growth. In melanoma cancer, exosomes derived from highly metastatic melanomas educated bone marrow (BM) progenitors by the receptor tyrosine kinase MET, leading to the “pre-metastatic” niche formation and subsequent tumor metastasis.Citation22 Taking these together, transfer of RNAs and proteins by exosomes is becoming a novel mechanism contributing to initiation, growth, invasion and metastasis of cancers.
Exosome-Mediated Immunosuppression in Tumor-Bearing Host
Impairment of dendritic cell differentiation and maturation
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) with unique ability of antigen presentation and initiation of primary T cell responses, including antitumor responses. Although DC-based tumor vaccines are now one of the most prominent strategies for cancer therapy, DCs without artificial intervention are usually dysfunctional and unable to trigger effective T cell responses.Citation23 Under tumor conditions, exosomes released from tumor cells can inhibit both differentiation and maturation of DCs.
Myeloid precursors in the BM give rise to DCs, which is greatly obstructed by TEXs. In turn, the myeloid precursors will differentiate into myeloid-derived suppressor cells (MDSCs), as a cluster of immature myeloid cells with the ability to facilitate tumor progression.Citation24 When added to the classical in vitro culture system of mouse DCs, TEXs inhibited the differentiation of BM myeloid precursors into DCs via induction of IL-6.Citation25 In addition, exosomes from human cancers also induced CD14+ monocytes to differentiate into CD14+ HLA-DR−/low cells, which suppressed T cell proliferation and cytolytic functions.Citation26 Detailed mechanisms of the inhibition have focused on protein contents in exosomes, such as TGF-β, IL-6, PGE2 and so on.Citation25-27 Moreover, mice pretreated with TEXs also showed an accumulation of MDSCs in spleen, peripheral blood and lung.Citation28 Interestingly, heat shock protein 72 (HSP72) expressed at the surface of TEXs could induce activation of Stat3 and production of IL-6 in a TLR2/MyD88-dependent manner, thus promoting suppressive functions of MDSCs.Citation29
Furthermore, TEXs could be uptaken by immature DCs and then block DC maturation. In a mouse model of delayed-type hypersensitivity (DTH), TEXs loaded with ovalbumin (OVA) failed to induce DTH responses by inhibiting DC maturation via TGF-β1.Citation30 TEXs can also impair the antigen recognition of DCs via affecting their expression of pattern recognition receptors (PRRs). A typical study indicated that exosomes from pancreatic cancers regulated toll-like receptor 4 (TLR4) expression in DCs via miRNA-203, which was highly detected in exosomes derived from pancreatic cancer cells.Citation31 When uptaken by DCs, these exosomes downregulated the expression of TLR4 and production of the related cytokines including TNF-α and IL-12 in DCs. As a result, exosomes inhibited DCs-mediated antitumor responses triggered by TLR4. In summary, TEXs mediate host immunosuppression by modulating the differentiation, maturation and function of DCs.
Polarization of tumor-promoting macrophages
Macrophages display remarkable plasticity and change their physiology according to environmental cues, especially tumor microenvironment.Citation32 It has been reported that macrophages could be activated by TEXs, but different in cytokine profiles from that by LPS and IL-4.Citation33 After stimulated by exosomes, macrophages showed reduced levels of TIMP1, IFNγ, IL-16 and a marked increase in the levels of IL-8, CCL2, MIP2 and IL-1Ra, which were closely related with tumor invasion and metastasis. Direct communication between macrophages and cancer cells also plays crucial roles in the invasion of breast cancer.Citation34 TEXs but not particle-free supernatants or exosomes from benign cells induced Wnt5a expression in macrophages. Wnt5a could be transferred from macrophages to cancer cells via exosomes, resulting in the activation of β-Catenin-independent Wnt signaling pathway. This interesting feedback loop provided a new mechanism for macrophage-induced tumor invasion.
Meanwhile, macrophages can recognize protein and RNA compounds in exosomes via PRRs to induce inflammatory responses and promote subsequent tumor progression. Recently, TEXs have been described as a ligand of TLR2. These exosomes stimulated TLR2 to activate NF-κB pathway in macrophages, resulting in the secretion of pro-inflammatory cytokines such as IL-6, TNF-α, and CCL2.Citation35 Additionally, exosomes contain large amounts of small non-coding RNAs, especially miRNAs, which can function as agonists of RNA-binding TLRs. TLR7 and TLR8 were found to recognize exosome-derived miRNAs and stimulate downstream NF-κB pathway and inflammatory cytokine secretion in macrophage.Citation36 Therefore, induction of tumor-associated chronic inflammation by TEXs promoted tumor growth, invasion and metastasis. Besides TLRs, a recent study mentioned above showed that exosomes from stromal cells contained 5-Triphosphate RNAs, which could activate RIG-I in breast cancer cells and promote resistance to radiation therapy.Citation37
Decrease of NK cell cytotoxicity
Natural killer (NK) cells are a typical cytotoxic lymphocyte in innate immunity and take a variety of strategies to kill cancer cells directly.Citation38 It has been reported that exosomes derived from anticancer drug-treated human HCC cells were rich in heat shock proteins, which could act as endogenous danger signals to stimulate NK cell-elicited antitumor responses in vitro.Citation39 However, more studies indicated that the cytotoxicity of NK cells was greatly impaired in tumor conditions.
Activating receptors such as NKG2D, NKP30, NKP46 and NKG2C play an important role in NK cell cytotoxicity. However, TEXs restrained the expression of these receptors, among which NKG2D was the most profound one. Clinical researches showed reduced surface expression of NKG2D on circulating NK cells in cancer patients compared to healthy individuals.Citation40 Human prostate cancer cells-derived exosomes were found to express ligands for NKG2D, resulting in downregulation of NKG2D on NK cells and impaired NK cell cytotoxic function,Citation41 which might be due to TGF-β1 presented by TEX.Citation42,43 Neutralizing TGF-β1 antibody strongly abrogated NKG2D reduction on NK cells, suggesting TGF-β1 may be an effective mediator. This was consistent with the report that TGF-β1 derived from exosomes in AML patients' sera could induce Smad phosphorylation in NK cells and reduce NKG2D expression.Citation42
Impairment of CTL response and induction of regulatory T cells
Effective CD4+ and CD8+ T cell responses are critical for antitumor immunity. However, TEXs can affect proliferation, activation and apoptosis of these T cells. In human nasopharyngeal carcinoma (NPC), miRNAs from TEXs inhibited T cell proliferation and differentiation into Th1 and Th17 cells, while promoting regulatory T cell (Treg) generation.Citation44 Five over-expressed miRNAs were identified in the exosomes from patient sera and NPC cell lines: hsa-miR-24-3p, hsa-miR-891a, hsa-miR-106a-5p, hsa-miR-20a-5p and hsa-miR-1908. These miRNA clusters could downregulate the MARK1 signaling pathway through decreasing phosphorylation of ERK, STAT1 and STAT3. In a mouse model of glioblastoma, transfusion of exosomes derived from glioblastoma cell line GL26 reduced the percentages of CD8+ T cells and inhibited the activation of CD8+ T cells, including decreased expression of IFNγ and granzyme B.Citation45 CD3-ζ chain, as an integral component of T-cell receptor (TCR) complex, is important for competent signaling after TCR–MHC–peptide interactions and T cell activation.Citation46 Exosomal TNF-α from cancer cells affected TCR–CD3 complex by a reactive oxygen species way. As a result, signals to activate CD4+ and CD8+ T cells were disrupted.Citation47 TEXs also induce apoptosis of T cells.Citation48-50 Fas ligand (FasL)-positive membranous exosomes isolated from sera of oral cancer patients could induce apoptosis of activated T lymphocytes.Citation51 TEXs could also mediate Fas/FasL-associated apoptosis of CD8+ T cells and downregulate expression of CD3ζ and Janus kinase 3 (JAK3) in activated T cells.
Apart from TEXs, our works revealed that exosomes from gene-modified DCs inhibited Th1 and Th17 activation but maintained the regulatory capacity of T cells via TGF-β1.Citation52 Interestingly, these exosomes expressing membrane-associated TGF-β1 (mTGF-β1) demonstrated more potent immunosuppressive activity.Citation53 We also found that FasL-expressed exosomes derived from activated CD8+ T cells could promote the invasion of murine melanoma cell line B16 and Lewis lung cancer cell line 3LL. However, they had little effect on apoptosis induction and proliferation of these two indicated cancer cell lines.Citation54
Tregs use a variety of strategies to help cancer cells escape from immune attack by secreting immunosuppressive cytokines (IL-10, TGF-β1, etc.).Citation55 In addition to impairment of cytotoxic lymphocyte (CTL) responses, TEXs could promote the generation and function of Tregs.Citation49,56 When co-incubated with exosomes purified from supernatants of tumor cells, CD4+ CD25− T cells were converted into Tregs. These Tregs displayed elevated expression of IL-10, TGF-β1 and CTLA4.Citation49 Once secreted, tumor-derived miRNA-214 was transferred into T cells through exosomes, and then downregulated phosphatase and tensin homolog (PTEN) in T cells so as to promote Treg expansion.Citation57 Another study revealed that exosomes from lung cancer were rich in the EGFR. These exosomes induced tolerogenic DCs, which further facilitated Treg generation.Citation56
Tregs can also act as immune suppressors via exosomes. Okoye et al. found that Tregs could suppress effecter T cells by delivering miRNAs via exosomes.Citation58 Treg-derived exosomes contained premature and mature miRNAs, particularly with pro-apoptotic or anti-proliferative functions. These miRNAs could inhibit Ptgs2, thus limiting Th1 cell-associated responses. In a mouse model of B16 melanoma, exosomes secreted by natural CD8+ CD25+ Tregs were capable of suppressing CTL responses.Citation59 Both in vitro and in vivo experiments indicated that exosomes derived from CD8+ Tregs inhibited DC-activated CD8+ T cell responses. Therefore, exosomes derived from Treg cells may be a potential target for the design of cancer immunotherapy.
Induction of regulatory B cells
Synthesis and release of exosomes from activated B cells can stimulate effective T cell responses against cancer cells and elicit antitumor immune responses.Citation60 However, a few studies focused on the roles of TEXs in the induction of regulatory B cells. Yang et al. found that exosomes from mycoplasma-infected tumor cells carried mycoplasma components, and these components could promote the generation of regulatory B cells and then inhibit T cell activity. This interesting finding provides a new mechanism for mycoplasmas-infected tumor cells to modulate B cells with inhibitory property by exosomal pathway.Citation61
Exosome-Mediated Activation of Immune Response Against Tumors
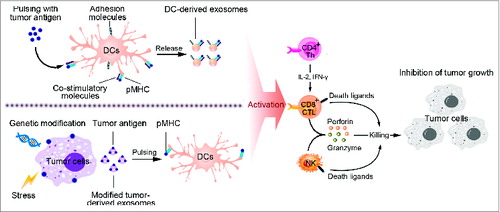
Dendritic cell-derived exosomes (DEXs) as tumor vaccine
Through intercellular communication, exosomes may trigger the immune system to elicit antitumor responses, in which APCs take the central place.Citation62 At early time in 1996, B lymphocytes were found to secrete extracellular antigen-presenting vesicles.Citation60 This kind of exosomes released by B cells carried Major Histocompatibility Complex (MHC) class II, co-stimulatory and adhesion molecules. As a result, B cell-derived exosomes could directly stimulate effective CD4+ T cell responses against cancer cells. Two years later, the groups of Dr Zitvogel, Dr Raposo and Dr Amigorena described that DCs produced antigen-presenting exosomes, which contained functional MHC class I and class II, and co-stimulatory molecules.Citation63 Accordingly, DEXs primed specific CTL response and promoted antitumor responses in a T cell-dependent manner. Since then, DEXs as cell-free tumor vaccines have aroused widespread concerns.
How DEXs can stimulate effective antitumor responses has been investigated intensively. These exosomes are enriched in membrane proteins involved in antigen presentation, including MHC Class I and II molecules, MHC Class I-like molecule (CD1), co-stimulatory molecules (CD80 and CD86), adhesion molecule (ICAM-1, MFG-E8).Citation64-67 Proteomic analysis of DEXs also identified new exosomal proteins, which were mainly cytoskeleton-related molecules (cofilin and profilin I) and membrane transport and signaling factors (annexins, rab 7 and 11, rap1B, and syntenin), suggesting a role of exosomes in transmembrane transport.Citation64 Furthermore, HSP73 was another exosomal content presented in endocytic compartments of DCs, which could elicit antitumor responses through T cell-dependent or -independent way.Citation68
As indicated above, DEXs present peptide-loaded MHC Class I and II complexes to stimulate both CD8+ CTL and CD4+ T cell responses, collaborating with co-stimulatory and adhesion molecules.Citation69 In addition, MHC class I-restricted, peptide-specific CTL priming is critical for effective antitumor responses.Citation70 Interestingly, CTL responses mediated by DEXs were not only dependent on CD4+ T cells but also on B cells. Mice deficient in B cells showed lower responses to protein-loaded DEXs because of impaired complement activation and antigen shuttling by B cells.Citation71 However, DEXs are less efficient in stimulating naïve CD4+ T cells than stimulating activated and memory T cells. DEXs also needed DCs to efficiently stimulate specific T cells.Citation66,72,73 In coculture system with T cells in vitro, DEXs could not induce antigen-dependent T cell activation unless DEXs were incubated with mature DCs in the cultures. Accordingly, exosomes mediated exchange of functional peptide-MHC complexes with bystander DCs, thus amplifying adaptive immune responses by increasing the number of DCs bearing antigen peptides.Citation66
Besides T cells, DEXs can promote NK cell proliferation and activation, resulting in NK cell-dependent tumor rejection.Citation74-76 A series of membrane stimulators were found in DEXs. They expressed functional IL-15R and promoted proliferation and IFNγ secretion by NK cells.Citation75 DEXs also harbored multiple TNF superfamily ligands (TNFSFLs) on their surface and activated NK cells through interaction of DEX-expressing TNF with TNF receptors on NK cells.Citation74 Activating receptors expressed on NK cells are critical for direct cytotoxicity against tumor cells. Exosomal expression of NKp30 ligand BAT3 (HLA-B-associated transcription 3) and NKG2D ligand had been implicated in direct activation of NK cells.Citation75,76
On the basis of these clues, DEXs have been used as a cell-free vaccine to trigger host antitumor immune response to suppress tumor growth. Vaccination of patients with DEXs from metastatic melanoma and advanced non-small cell lung cancer resulted in activation of immune responses and prolonged stability of diseases, as demonstrated in phase I clinical trials.Citation77,78 However, how to break down the obstacles of host immunosuppression and rescue insufficient T cell responses when using DEXs as tumor vaccines calls for further studies.
Modified tumor cell-derived exosomes (TEXs)
TEXs carry tumor antigens and can also trigger efficient antigen presentation of APCs. Because of easy and non-traumatic acquisition, these exosomes are taken as ideal resources of antigens for DC education.Citation12 Early reports showed that antigens in TEXs could be transferred to DCs and induce specific CTL activation.Citation10 Whole native tumor antigens were found in exosomes, e.g. HSP70-80, Her2/Neu, Mart1, TRP and gp100 in melanoma, P1A (intracisternal A particle protein) and HSP70 in plasmacytoma cells.Citation79-81 However, these antitumor immune responses induced by TEXs are relatively weak and prone to induce tolerance. Therefore, these strategies are limited to in vitro observations and mouse model studies.
Accordingly, researches have made some effects to develop modified exosome-based tumor vaccines artificially. One of the common strategies is to make genetic modification of original cells to improve immunogenicity of exosomes. For example, we extracted exosomes from tumor cells genetically modified with cytokine genes (IL-2 and IL-18).Citation82,83 These exosomes were found to induce DC maturation and CTL responses efficiently. Exosomes from Rab27a-overexpressing lung cancer cells and OVA-expressing EG7 elicited efficient antitumor immune responses.Citation81,84 Other successful examples based on this strategy included CD40L-modified lung cancer cells, CIITA-transfected CT26 cells and TNF-engineered J558 tumor cell.Citation85-87
Apart from genetic modification, external stimulus is added to drive tumor cell release of exosomes. We have extracted exosomes from a series of stress-induced tumor cells such as lymphoma cells, CEA-positive tumor cells, Lewis lung carcinoma and melanoma cells. These exosomes contained more immunogenic substances (MHC-I, CD40, CD86, RANTES, CEA, HSP70, etc) as well as chemokines.Citation88-90 Resistant anticancer drugs were found to enhance release of exosomes coated with heat shock proteins (HSP60, HSP70, and HSP90) from human hepatocellular carcinoma cells. The HSP-bearing exosomes efficiently stimulated NK cell cytotoxicity against tumors.Citation39
Other strategy involves direct fusion of TEXs with antigens and combined therapies. As GM-CSF and IL-12 anchoring tumor cells induced tumor-specific T cell responses, we modified TEXs via surface anchorage of superantigen SEA, resulting in enhanced antitumor immune responses.Citation91 Exosomes in combination with cyclophosphamide (CTX) and polyinosinic-polycytidylic acid (poly I:C) were shown to promote the cytotoxic effect of T cells and suppress tumor growth.Citation92,93
Taking together, application of TEXs as tumor vaccines is dependent on types of cancers and immunogenicity of their antigens. Modification of TEXs is developed to improve their immunogenicity, aiming to make full use of TEXs as tumor vaccines.
Conclusions and perspectives
Exosomes are well-known as mediators of intercellular communication and participate in multitude biological processes. In the past decade, numerous studies have indicated roles of exosomes in multiple aspects of tumor processes as well as in interactions with the immune system. Now, much more researches reveal that TEXs suppress host immune responses and induce immune tolerance via their contents including miRNAs, cytokines, etc (). However, exosomes can be recognized by APCs to induce antitumor responses as sources of natural tumor antigens, and accordingly, exosome-based vaccines have been developed for cancer therapeutics (). Therefore, several fundamental issues must be fully addressed before their applications in clinic. First, the mechanism for exosome release from original cells has not been fully understood. Cancer cells may secret abundant exosomes under stress, such as surgery, radiotherapy and chemotherapy. Controlling the release of immunosuppressive exosomes may benefit these treatments. Secondly, the different effects mediated by exosomes are dependent on their proteins and genetic contents. So composition analysis of these contents is important for substantial application. RNA composition between original cells and exosomes was found differently, making this issue to be more interesting and challenging. Lastly but not least, how can we adjust the balance between tumor-promoting effects and antitumor effects of exosomes, the key point which needs further investigation. In sum, exosomes have paramount functions in tumor immunity. How to make best use of the advantages while bypass the disadvantages of exosomes in modulating tumor immunity is attracting increasing attention. Further researches may finally make the diagnostic and therapeutic potential of exosomes to be a reality in the clinics.
Figure 1. Exosome-mediated immunosuppression of tumor immunity. Exosomes derived from cancer cells have been shown to be involved in the modulation of tumor immunity in various ways: (A) Inhibition of proliferation and differentiation of CD4+ T cells into Th1 and Th17, inhibition of cytotoxicity of CTLs, NK cells and macrophages, inhibition of differentiation and maturation of DCs. (B) Promotion of CD4+, CD8+ Tregs and Bregs generation, induction of myeloid precursor differentiation into MDSCs, alternative activation of macrophages.
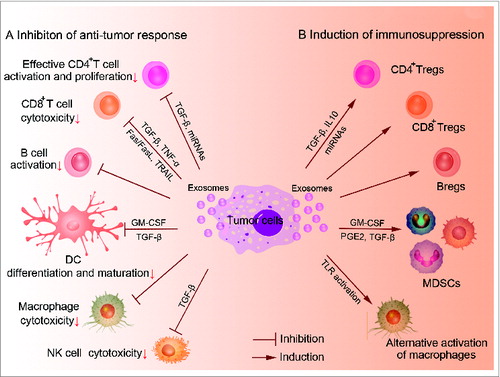
Figure 2. Strategies for exosome-based tumor immunotherapy. This figure presents two main strategies for application of exosomes in tumor therapeutics. Exosomes secreted from antigen-loaded DCs carry functional peptide-MHC complexes, co-stimulatory and adhesion molecules and induce activation of CD4+ T cells, CD8+ T cells and NK cells, thus mediating cytotoxicity to tumor cells and inhibition of tumor growth. These molecules can also be exchanged between DCs to induce antitumor immune response indirectly. Additionally, tumor-derived exosomes (TEXs) carry tumor antigens and can trigger efficient antigen presentation of APCs, thus being used as resources of tumor antigens to prepare tumor vaccines. Moreover, modification of TEXs is developed to improve their immunogenicity, such as genetic engineering stress and protein loading.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Yanmei Han, Dr Taoyong Chen and Dr Sheng Xu for helpful discussion. The figures were generated by software “epath3d” (http://www.epath3d.com/).
References
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9:581-93; PMID:19498381; http://dx.doi.org/10.1038/nri2567
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2013; 2:20389; PMID:24009890; http://dx.doi.org/10.3402/jev.v2i0.20389
- Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol 2004; 164:1807-15; PMID:15111327; http://dx.doi.org/10.1016/S0002-9440(10)63739-X
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 2010; 70:9621-30; PMID:21098712; http://dx.doi.org/10.1158/0008-5472.CAN-10-1722
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012; 30:1556-64; PMID:22605481; http://dx.doi.org/10.1002/stem.1129
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30:255-89; PMID:25288114; http://dx.doi.org/10.1146/annurev-cellbio-101512-122326
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012; 1820:940-8; PMID:22503788; http://dx.doi.org/10.1016/j.bbagen.2012.03.017
- Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol 2007; 179:2235-41; PMID:17675484; http://dx.doi.org/10.4049/jimmunol.179.4.2235
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 2008; 16:782-90; PMID:18362931; http://dx.doi.org/10.1038/mt.2008.1
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002; 360:295-305; PMID:12147373; http://dx.doi.org/10.1016/S0140-6736(02)09552-1
- Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013; 91:431-7; PMID:23519402; http://dx.doi.org/10.1007/s00109-013-1020-6
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14:195-208; PMID:24566916; http://dx.doi.org/10.1038/nri3622
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell 2014; 26:707-21; PMID:25446899; http://dx.doi.org/10.1016/j.ccell.2014.09.005
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008; 110:13-21; PMID:18589210; http://dx.doi.org/10.1016/j.ygyno.2008.04.033
- Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 2009; 106:3794-9; PMID:19234131; http://dx.doi.org/10.1073/pnas.0804543106
- Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A 2014; 111:711-6; PMID:24379393; http://dx.doi.org/10.1073/pnas.1310501111
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 2011; 54:1237-48; PMID:21721029; http://dx.doi.org/10.1002/hep.24504
- Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010; 39:133-44; PMID:20603081; http://dx.doi.org/10.1016/j.molcel.2010.06.010
- Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One 2010; 5:e13247; PMID:20949044; http://dx.doi.org/10.1371/journal.pone.0013247
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10:619-24; PMID:18425114; http://dx.doi.org/10.1038/ncb1725
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18:883-91; PMID:22635005; http://dx.doi.org/10.1038/nm.2753
- Xu H, Cao X. Dendritic cell vaccines in cancer immunotherapy: from biology to translational medicine. Front Med 2011; 5:323-32; PMID:22198743; http://dx.doi.org/10.1007/s11684-011-0172-4
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol 2011; 32:19-25; PMID:21067974; http://dx.doi.org/10.1016/j.it.2010.10.002
- Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, Michalek S, Grizzle W, Zhang HG. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol 2010; 176:2490-9; PMID:20348242; http://dx.doi.org/10.2353/ajpath.2010.090777
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 2006; 66:9290-8; PMID:16982774; http://dx.doi.org/10.1158/0008-5472.CAN-06-1819
- Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 2007; 178:6867-75; PMID:17513735; http://dx.doi.org/10.4049/jimmunol.178.11.6867
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009; 124:2621-33; PMID:19235923; http://dx.doi.org/10.1002/ijc.24249
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010; 120:457-71; PMID:20093776; http://dx.doi.org/10.1172/JCI40483
- Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One 2011; 6:e22517; PMID:21829629; http://dx.doi.org/10.1371/journal.pone.0022517
- Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol 2014; 292:65-9; PMID:25290620; http://dx.doi.org/10.1016/j.cellimm.2014.09.004
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496:445-55; PMID:23619691; http://dx.doi.org/10.1038/nature12034
- Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy K, Szegletes Z, Varo G, Siklos L, Katona RL et al. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett 2012; 148:34-8; PMID:22898052; http://dx.doi.org/10.1016/j.imlet.2012.07.006
- Menck K, Klemm F, Gross JC, Pukrop T, Wenzel D, Binder C. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget 2013; 4:2057-66; PMID:24185202
- Xiang X, Liu Y, Zhuang X, Zhang S, Michalek S, Taylor DD, Grizzle W, Zhang HG. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol 2010; 177:1606-10; PMID:20802178; http://dx.doi.org/10.2353/ajpath.2010.100245
- Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep 2014; 4:5750; PMID:25034888; http://dx.doi.org/10.1038/srep05750
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014; 159:499-513; PMID:25417103; http://dx.doi.org/10.1016/j.cell.2014.09.051
- Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012; 12:239-52; PMID:22437937; http://dx.doi.org/10.1038/nri3174
- Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012; 287:15874-85; PMID:22396543; http://dx.doi.org/10.1074/jbc.M112.340588
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419:734-8; PMID:12384702; http://dx.doi.org/10.1038/nature01112
- Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol 2008; 180:7249-58; PMID:18490724; http://dx.doi.org/10.4049/jimmunol.180.11.7249
- Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 2011; 96:1302-9; PMID:21606166; http://dx.doi.org/10.3324/haematol.2010.039743
- Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One 2011; 6:e16899; PMID:21364924; http://dx.doi.org/10.1371/journal.pone.0016899
- Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009; 113:1957-66; PMID:19005181; http://dx.doi.org/10.1182/blood-2008-02-142596
- Liu ZM, Wang YB, Yuan XH. Exosomes from murine-derived GL26 cells promote glioblastoma tumor growth by reducing number and function of CD8+T cells. Asian Pac J Cancer Prev 2013; 14:309-14; PMID:23534743; http://dx.doi.org/10.7314/APJCP.2013.14.1.309
- Kim HR, Jeon BH, Lee HS, Im SH, Araki M, Araki K, Yamamura K, Choi SC, Park DS, Jun CD. IGSF4 is a novel TCR zeta-chain-interacting protein that enhances TCR-mediated signaling. J Exp Med 2011; 208:2545-60; PMID:22084409; http://dx.doi.org/10.1084/jem.20110853
- Soderberg A, Barral AM, Soderstrom M, Sander B, Rosen A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med 2007; 43:90-9; PMID:17561097; http://dx.doi.org/10.1016/j.freeradbiomed.2007.03.026
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 2005; 35:169-73; PMID:16081306; http://dx.doi.org/10.1016/j.bcmd.2005.07.001
- Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol 2009; 183:3720-30; PMID:19692638; http://dx.doi.org/10.4049/jimmunol.0900970
- Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med 2002; 195:1303-16; PMID:12021310; http://dx.doi.org/10.1084/jem.20011624
- Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 2005; 92:305-11; PMID:15655551
- Cai Z, Zhang W, Yang F, Yu L, Yu Z, Pan J, Wang L, Cao X, Wang J. Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res 2012; 22:607-10; PMID:22157651; http://dx.doi.org/10.1038/cr.2011.196
- Yu L, Yang F, Jiang L, Chen Y, Wang K, Xu F, Wei Y, Cao X, Wang J, Cai Z. Exosomes with membrane-associated TGF-beta1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction. Eur J Immunol 2013; 43:2461-72; PMID:23716181; http://dx.doi.org/10.1002/eji.201243295
- Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol 2012; 188:5954-61; PMID:22573809; http://dx.doi.org/10.4049/jimmunol.1103466
- Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol 2009; 182:111-20; PMID:19109141; http://dx.doi.org/10.4049/jimmunol.182.1.111
- Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One 2010; 5:e11469; PMID:20661468; http://dx.doi.org/10.1371/journal.pone.0011469
- Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J, Wang Z, Chen X, Zhang W, Yokoyama S et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res 2014; 24:1164-80; PMID:25223704; http://dx.doi.org/10.1038/cr.2014.121
- Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014; 41:89-103; PMID:25035954; http://dx.doi.org/10.1016/j.immuni.2014.05.019
- Xie Y, Zhang X, Zhao T, Li W, Xiang J. Natural CD8(+)25(+) regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma. Biochem Biophys Res Commun 2013; 438:152-5; PMID:23876314; http://dx.doi.org/10.1016/j.bbrc.2013.07.044
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183:1161-72; PMID:8642258; http://dx.doi.org/10.1084/jem.183.3.1161
- Yang C, Chalasani G, Ng YH, Robbins PD. Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PLoS One 2012; 7:e36138; PMID:22558358; http://dx.doi.org/10.1371/journal.pone.0036138
- Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology 2012; 1:1111-34; PMID:23170259; http://dx.doi.org/10.4161/onci.21494
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4:594-600; PMID:9585234; http://dx.doi.org/10.1038/nm0598-594
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001; 166:7309-18; PMID:11390481; http://dx.doi.org/10.4049/jimmunol.166.12.7309
- Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods 2001; 247:163-74; PMID:11150547; http://dx.doi.org/10.1016/S0022-1759(00)00321-5
- Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol 2004; 172:2126-36; PMID:14764678; http://dx.doi.org/10.4049/jimmunol.172.4.2126
- Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Théry C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005; 106:216-23; PMID:15790784; http://dx.doi.org/10.1182/blood-2005-01-0220
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999; 147:599-610; PMID:10545503; http://dx.doi.org/10.1083/jcb.147.3.599
- Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, Zitvogel L. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol 2014; 193:1006-11; PMID:25049431; http://dx.doi.org/10.4049/jimmunol.1400703
- Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol 2006; 3:205-11; PMID:16893501
- Naslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol 2013; 190:2712-9; PMID:23418627; http://dx.doi.org/10.4049/jimmunol.1203082
- Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol 2002; 14:713-22; PMID:12096030; http://dx.doi.org/10.1093/intimm/dxf048
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 2002; 3:1156-62; PMID:12426563; http://dx.doi.org/10.1038/ni854
- Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 2012; 1:1074-83; PMID:23170255; http://dx.doi.org/10.4161/onci.20897
- Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 2009; 4:e4942; PMID:19319200; http://dx.doi.org/10.1371/journal.pone.0004942
- Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 2008; 3:e3377; PMID:18852879; http://dx.doi.org/10.1371/journal.pone.0003377
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 2005; 3:10; PMID:15740633; http://dx.doi.org/10.1186/1479-5876-3-10
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005; 3:9; PMID:15723705; http://dx.doi.org/10.1186/1479-5876-3-9
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7:297-303; PMID:11231627; http://dx.doi.org/10.1038/85438
- Altieri SL, Khan AN, Tomasi TB. Exosomes from plasmacytoma cells as a tumor vaccine. J Immunother 2004; 27:282-8; PMID:15235389; http://dx.doi.org/10.1097/00002371-200407000-00004
- Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X. Exosomes derived from Rab27a-overexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep 2013; 8:1876-82; PMID:24146068; http://dx.doi.org/10.3892/mmr.2013.1738
- Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y, Cao X. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol 2007; 133:389-99; PMID:17219198; http://dx.doi.org/10.1007/s00432-006-0184-7
- Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, Wan T, Yu Y, Cao X. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med (Berl) 2006; 84:1067-76; PMID:17016692; http://dx.doi.org/10.1007/s00109-006-0102-0
- Yao Y, Chen L, Wei W, Deng X, Ma L, Hao S. Tumor cell-derived exosome-targeted dendritic cells stimulate stronger CD8+ CTL responses and antitumor immunities. Biochem Biophys Res Commun 2013; 436:60-5; PMID:23707941; http://dx.doi.org/10.1016/j.bbrc.2013.05.058
- Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol Med Rep 2014; 9:125-31; PMID:24173626; http://dx.doi.org/10.3892/mmr.2013.1759
- Fan W, Tian XD, Huang E, Zhang JJ. Exosomes from CIITA-transfected CT26 cells enhance anti- tumor effects. Asian Pac J Cancer Prev 2013; 14:987-91; PMID:23621273; http://dx.doi.org/10.7314/APJCP.2013.14.2.987
- Xie Y, Bai O, Zhang H, Li W, Xiang J. Tumor necrosis factor gene-engineered J558 tumor cell-released exosomes stimulate tumor antigen P1A-specific CD8+ CTL responses and antitumor immunity. Cancer Biother Radiopharm 2010; 25:21-8; PMID:20187793; http://dx.doi.org/10.1089/cbr.2009.0714
- Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 2006; 36:1598-607; PMID:16708399; http://dx.doi.org/10.1002/eji.200535501
- Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res 2005; 11:7554-63; PMID:16243831; http://dx.doi.org/10.1158/1078-0432.CCR-05-0810
- Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol 2011; 186:2219-28; PMID:21242526; http://dx.doi.org/10.4049/jimmunol.1002991
- Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med (Berl) 2007; 85:511-21; PMID:17219095; http://dx.doi.org/10.1007/s00109-006-0154-1
- Guo F, Chang CK, Fan HH, Nie XX, Ren YN, Liu YY, Zhao LH. Anti-tumour effects of exosomes in combination with cyclophosphamide and polyinosinic-polycytidylic acid. J Int Med Res 2008; 36:1342-53; PMID:19094445; http://dx.doi.org/10.1177/147323000803600623
- Ren WN, Chang CK, Fan HH, Guo F, Ren YN, Yang J, Guo J, Li X. A combination of exosomes carrying TSA derived from HLA-A2-positive human white buffy coat and polyI:C for use as a subcellular antitumor vaccination. J Immunoassay Immunochem 2011; 32:207-18; PMID:21574092; http://dx.doi.org/10.1080/15321819.2011.559295
