ABSTRACT
Several studies have demonstrated that oncogenic BRAF(V600E) promotes T-cell suppression in melanoma by upregulating the transcription of a multitude of immunomodulatory chemokine and cytokine genes. BRAF(V600E) has now been shown to act even more directly to evade cytotoxic T-cell recognition, by driving rapid internalization of human leukocyte antigen (HLA) class I from the tumor-cell surface and its intracellular sequestration.
Major histocompatibility complex class I (MHC-I) antigen presentation by tumor cells plays a central role in cytotoxic T lymphocyte (CTL)-mediated recognition and elimination of cancer. Accordingly, tumor cells often downregulate MHC-I gene expression or antigen-processing components of the MHC-I pathway to facilitate escape from immune surveillance. Reduction in HLA class I expression has been noted in a wide spectrum of tumor types, with the highest rates of loss having been reported in cervical, breast, esophageal, prostate, and non-small cell lung cancer. Melanoma tumors, by contrast, show significantly lower rates of total HLA loss. However, this tumor type frequently demonstrates upregulation of the MAPK signaling pathway (most commonly initiated by BRAF(V600E) mutation), which several studies have now shown plays a key role in promoting immune suppression.
Constitutively active BRAF(V600E) leads to the upregulation of a plethora of genes associated with the suppression of the T-cell-mediated immune response, including a host of immunomodulatory chemokines and cytokines within the tumor microenvironment that both recruit and activate suppressive immune cell subsets (, right-hand panel). Previous work showed that BRAF(V600E) can induce expression of (IL)-1α/β, IL-8, and CCL2 in melanoma cells, which are all known to act as chemokines enabling the recruitment of monocytes and myeloid suppressor cell subsets into the tumor microenvironment.Citation1,2 IL-1α/β was also shown to upregulate the expression of the immunosuppressive programmed death (PD)-1 ligands PD-L1 and PD-L2 on melanoma tumor-associated fibroblasts, in addition to promoting COX-2 expression. Furthermore, BRAF (V600E) induced the expression of IL-6 and VEGF, which can each inhibit antitumor immunity through a number of previously described mechanisms.Citation3 Other studies have demonstrated that BRAF(V600E) downregulates the expression of melanoma differentiation antigens such as MART-1 and gp100, further promoting decreased tumor-cell recognition by melanoma antigen-specific CTL.Citation4
Figure 1. BRAF (V600E) mediates immune suppression and evasion through multiple mechanisms in melanoma. The acquisition of a somatic BRAF(V600E) mutation is an early event during melanomagenesis and leads to constitutive activation of the MAPK signaling pathway. This promotes: (1) Increased internalization and endosomal sequestration of HLA class I molecules, directly reducing surface expression and tumor-cell recognition by cytotoxic T cells; (2) Upregulation of chemokines CXCL8, CCL2, and IL-1, which can attract myeloid cell subsets including monocytes and tumor-associated macrophages, as well as tumor-associated fibroblasts (TAFs) into the tumor microenvironment (TME); (3) Transcription and expression of IL-1α/β, IL-6, and VEGF, which can condition the cells of the TME. IL-1α/β production by tumor cells can promote T-cell suppression by inducing the expression of programmed death (PD)-1 ligands PD-L1 and PD-L2 on TAFs, in addition to increasing COX-2 transcription and PGE2 upregulation. VEGF can inhibit myeloid cell maturation, in addition to directly promoting the functional inhibition of T cells through VEGFR2.
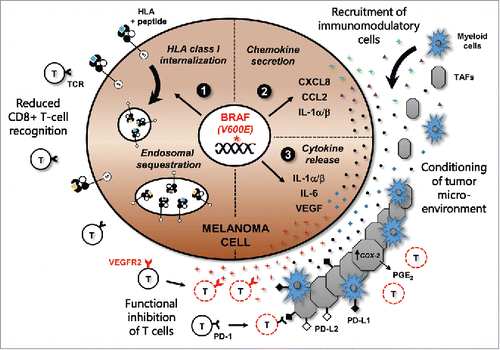
With such an impressive array of mechanisms actively promoting T-cell suppression in melanoma, it might be expected that directly targeting MHC-I would be unnecessary. However, we recently reported that BRAF(V600E) can also promote reduced CTL recognition by inducing cellular redistribution of MHC-I molecules in tumor cells (, left-hand panel). Oncogenic BRAF specifically drove rapid internalization of HLA-A*0201 molecules from the melanoma cell surface and their sequestration within endocytic compartments.Citation5 This process was shown to depend on a highly conserved phosphorylation site (Serine-335) within the MHC-I cytoplasmic tail, a region that has been previously implicated in mediating the rapid internalization and MHC-I recycling that occurs in immune cells upon activation.Citation6 This observation suggests that melanoma tumors with constitutive MAPK pathway activation co-opt a conserved MHC-I internalization pathway in order to evade CTL recognition. Notably, treatment of melanoma cells with BRAF(V600E) or MEK inhibitors reversed this redistribution of HLA-A*0201, restoring surface expression and increasing recognition and cytokine release by melanoma antigen-specific CTL.Citation5 These results are in accordance with other studies in both humans and mouse models demonstrating that BRAF inhibition leads to a more favorable tumor microenvironment with enhanced T-cell infiltration and tumor regression.Citation7,8 More importantly, these collective studies highlight the important role that oncogene-targeted therapies can potentially play in reducing the burden of immune suppression in human cancer.
BRAF (V600E) inhibitors for the treatment of BRAF mutant melanoma demonstrate a remarkable response rate in patients but resistance develops rapidly, limiting progression-free survival to only 6–7 months. The findings summarized in strongly suggest that oncogene-targeted therapies could synergize very well with immunotherapies, not only by directly inducing tumor-cell death but also by decreasing the daunting level of immune suppression present within the tumor microenvironment. Which types of immunotherapies might benefit from combination with oncogene-targeted inhibitors? Mouse models of adoptive CD8+ T cell transfer (ACT) have shown an enhanced antitumor benefit of combining BRAF inhibition with ACT, which increased tumor infiltration of transferred CTL through blocking VEGF production by tumors.Citation8 Human melanoma patients treated with BRAF inhibitors also demonstrate a remarkable increase in tumor-infiltrating lymphocytes (TIL) during tumor regression that retract during development of resistance, progression, and concurrent reactivation of the MAPK pathway.Citation7,9 These results strongly suggest that oncogene-targeted therapy will synergize well with T-cell-mediated immunotherapies. In this context, checkpoint blockade (anti-CTLA-4, anti-PDL1, and anti-PD1), adoptive TIL transfer, and vaccines designed to elicit T-cell responses all stand to potentially benefit from combination with targeted agents.Citation10 Most of these trials are either ongoing or will be initiated within the coming months.
It has become clear that oncogenic BRAF (V600E) plays a master role in melanoma tumor progression, not only by upregulating factors involved in cell survival, metastasis, and proliferation, but also by orchestrating the suppression of antitumor immunity. It will be interesting to determine how many of these attributes are shared with other oncogenes in melanoma such as NRAS, GNAQ, GNA11, or RAC1. Moreover, how well does the connection between oncogene activation and immune suppression extend to other cancers, for example those harboring EGFR, KIT, or KRAS mutations? The sheer complexity of the immunosuppressive pathways induced by BRAF(V600E) has only just begun to be explored, with a very long list of potentially immunomodulatory signature genes upregulated and the downstream effects of molecular cross-talk between multiple cell subsets within the TME yet to be fully elucidated. However, the striking immediacy of MHC-I surface re-localization in melanoma cells following BRAF(V600E) inhibition suggests that the relationship between oncogene activation and immune evasion may be even more intimate than previously thought.
References
- Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT, Li Y et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res 2012; 18:5329-40; PMID:22850568; http://dx.doi.org/10.1158/1078-0432.CCR-12-1632
- Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, Haynes NM, Kinross K, Yagita H, Koya RC et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest 2013; 123:1371-81; PMID:23454771; http://dx.doi.org/10.1172/JCI66236
- Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 2006; 203:1651-6; PMID:16801397; http://dx.doi.org/10.1084/jem.20051848
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213-9; PMID:20551059; http://dx.doi.org/10.1158/0008-5472.CAN-10-0118
- Bradley SD, Chen Z, Melendez B, Talukder A, Khalili JS, Rodriguez-Cruz T, Liu S, Whittington M, Deng W, Li F et al. BRAFV600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8+ T-cell Recognition of Melanoma. Cancer Immunol Res 2015; 3:602-9; PMID:25795007; http://dx.doi.org/10.1158/2326-6066.CIR-15-0030
- Lizee G, Basha G, Jefferies WA. Tails of wonder: endocytic-sorting motifs key for exogenous antigen presentation. Trends Immunol 2005; 26:141-9; PMID:15745856; http://dx.doi.org/10.1016/j.it.2005.01.005
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013; 19:1225-31; PMID:23307859; http://dx.doi.org/10.1158/1078-0432.CCR-12-1630
- Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, Chen JQ, Li HS, Watowich SS, Yang Y et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013; 19:393-403; PMID:23204132; http://dx.doi.org/10.1158/1078-0432.CCR-12-1626
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18:1386-94; PMID:22156613; http://dx.doi.org/10.1158/1078-0432.CCR-11-2479
- Lizee G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med 2013; 64:71-90; PMID:23092383; http://dx.doi.org/10.1146/annurev-med-112311-083918
