ABSTRACT
Objective: In tumor patients, IL-6 appears to be one component of a consistent cancer-associated cytokine network resulting in both a systemic immune stimulation and a microenvironment of cancer-induced immune suppression that ultimately protects the cancer cells. IL-6 has been associated with prognosis in cancer patients, but so far a systemical analysis has not been carried out. Methods: The present meta-analysis studies the relation between IL-6 serum levels and the prognosis of cancer patients in the available clinical literature of 100 articles published between 1993 and 2013 comprising 11,583 patients. Results: The IL-6 serum level was described as significantly correlating with survival in 82/101 series comprising 85.6% of patients (9917/11,583) with 23 different cancer types. A total of 64 studies dichotomized patient cohorts according to various cut-off IL-6 serum levels: in 59/64 of these series corresponding to 94.5% of the reported patients (7694/8142) significant correlations between IL-6 serum level and survival were seen. The median survival of cancer patients had been determined above various cut-off levels of serum IL-6 in 24 dichotomized studies (26 cohorts). There was a highly significant inverse correlation between median survival of the cohorts with IL-6 serum level above cut-off (1272 patients) and their corresponding IL-6 cut-off values (Spearman R -0,48 p= < 0.001) following a linear regression when both parameters were log-transformed (p < 0.001). A significant correlation between increasing serum IL-6 and tumor stage or metastases was described in 39/44 studies and 91% of published patients (4221/4636) where clinical parameters had been specified. Conclusions: Closely associated with the patient's clinical condition and independent of the cancer histology, the increased IL-6 serum level uniformly appears to correlate with survival as paraneoplastic condition in later cancer stages independent of the cancer type. Modifications of this paraneoplastic immune reaction may offer new therapeutic options in cancer.
Introduction
Independent of the original tumor pathology, patients with advanced stage cancer appear to experience a simultaneous immunostimulation and immunosuppression with increased concentrations of cytokines including MIF, TNF-a, IL-18, IL-8, IL-6, TGF-β, and IL-10.(Reviewed in.Citation1) The result is a local inflammatory environment that appears to be a consistent component of malignant tumors.Citation2,3 The simultaneous cancer-induced activation of the immunosuppressive cytokines IL-10 and TGF-β, however, results in a dysfunction of antigen-presenting cells (APCs) and the conversion of conventional T cells into Tregs in the tumor microenvironment.Citation4 The functional result is an impaired antigen detection and dysfunction of effector cells of both the innate and adaptive immune systems despite a general environment of immune stimulation. The described cytokine pattern interferes with immunological effector mechanisms and could prevent both physiological tumor immunodetection and tumor destruction. This dysfunctional immunostimulation could be one of the reasons why macrophages are locally attracted but inactivated and even incorporated into neoplasms: tumor-associated macrophages (TAM) with a predominantly immunosppressive (IL-12low/IL-10high; M2) phenotype represent the major inflammatory component of the stroma of many tumors.Citation5
This consistent paraneoplastic cytokine pattern appears to be independent of the initial tumor histology and a phenomenon of late-stage cancer patients.Citation1 The cytokine IL-6 is an essential component in this functional circuit: IL-6 and its soluble receptor (sIL-6Ra) appear to have a key role in the transition from an acute toward a sustained or even chronic inflammation by decreasing neutrophil and favoring mononuclear-cell accumulations.Citation6-8 IL-6 induces the final maturation of B cells into antibody producing cells.Citation9,10 and, together with TGF-b it induces a key regulatory signal in the generation of Th17 cells,Citation11-15 while concomitantly blocking the differentiation of CD4+ cells into regulatory T cells.Citation15-20 IL-6 stimulates proliferation and migration of circulating endothelial progenitor cells.Citation20,21 and has a pivotal physiological role in wound healing, possibly by regulating leukocyte infiltration and collagen deposition.Citation22 Cytokine interactions comprise sophisticated interdependent positive and negative feedback mechanisms that provide homeostasis and control. Cytokines interact in functional circuits with impact on both agonistic and compensatory antagonistic effects. For example, IL-6 can regulate the production of IL-10 from carcinoma cells,Citation23,24 which in turn can have a downregulating effect on IL-6.Citation25
Several clinical series have independently reported a correlation between IL-6 and patient prognosis in various cancer types. In general, clinical research is focused on analysis of specific tumors types and the search for common traits in different clinical cancer types is almost obsolete with respect to cancer heterogeneity. Despite multiple independent recordings, the correlation between IL-6 and prognosis has so far not been comprehensively cross-referenced or systematically analyzed and has thus been widely un-noticed. So far, there is no oncological concept for the role of IL-6 in clinical cancer.
The current review analyses the available clinical literature of the recent two decades and identified studies that describe the prognostic and clinical impact of IL-6 in cancer patients. An attempt is made to quantify the cumulative results in a meta-analysis.
Materials and methods
Article selection and analysis
A systematic search of IL-6 in cancer patients was conducted using the PubMed database. The search was filtered according to ‘prognostic impact of IL-6 in cancer patients’ with the publication interval 1993–2013 (the search algorithm is added as an attachment). Only human clinical studies were included. No limits were set on cancer type or duration of follow-up. The studies were determined eligible for inclusion if they were original research studies that reported on IL-6 in cancer patients, and reviews were excluded. Corresponding authors were contacted to clarify missing data. The inclusion of duplicated or overlapping data was avoided by comparison of authors and institutions. A thorough review of all referenced sources was performed. Among 100 articles, 101 published series were identified in which the serum level of IL-6 was analyzed with respect to survival, with 92 articles being identified via direct searches and 8 articles via cross-references. (One study differentiated between the outcome of 159 patients in a gemcitabine/placebo group and 169 patients in the gemcitabine/bevacizumab group with different results with respect to IL-6, and these were considered as two separate series.Citation26) Correlations between IL-6 serum level and overall survival or tumor stage were the primary and secondary outcomes of interest.
Quantitative analysis of survival of cancer patients at specific cut-off values for serum IL-6
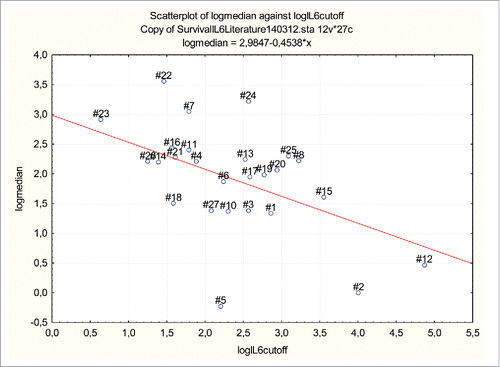
Among the selected series, those studies were identified that had applied various cut-off values for serum IL-6 levels and had reported the median survival of the cancer patients with IL-6 serum levels above the cut-off. In one study, the median survival was not mentioned in the text and was communicated personally.Citation27 A total of 24 series, each comprising more than 50 patients were analyzed quantitatively using the Statistica 12 software (Citation28,29 excluded due to small sample size). Two studies determined survival using two different cut-off values for serum IL-6, resulting in a total of 26 pairs of variables. The median survival of cohorts of patients with serum IL-6 above the reported cut-off level (in 1272 patients) was plotted against the applied cut-off level of serum IL-6 of each series (). The non-parametric Spearman's Rank order Correlation was applied in a non-weighted and weighted mode. Both parameters were log-transformed and a linear regression model was applied for the determination of correlations. In one version, the result was weighted according to the number of patients in each study and in the second version results were not weighted. Fisher Test was applied to test the regression model for significance.
Figure 1. Median survival of 1272 cancer patients with IL-6 serum levels above various chosen cut-off values in 24 dichotomized studies (26 cohorts). Both x and y parameters have been have log-transformed in the graph. Sample size was used to weigh the studies. The median survival at chosen cut-off points was inversely correlated to the minimum IL-6 serum level at this cut-off, both when sample size was used to weigh the studies (p < 0.001) and also when the cohorts were not weighted (p < 0.005). Numbers indicate series as listed in .
Results
IL-6 serum level in cancer and controls
The median serum level of IL-6 in cancer patients was documented in 72 studies. The median of the reported median IL-6 serum level in cancer patients was 6.95 pg/mL (range: 0.23–78.5 pg/mL). The median of the reported median IL-6 serum level in control cohorts (available in 40 series) was 1.31 pg/mL (range 0–37 pg/mL). In 57 studies, serum IL-6 was compared against a healthy control group: IL-6 was increased in cancer patients in 53/57 studies comprising 94.02% of patients (5985/6366 patients).
Serum IL-6 and survival
The serum level of IL-6 was studied with regard to survival in 101 series in 100 articles encompassing 11,583 patients. Overall survival was reported in 100 series and failure-free survival was reported in one article.Citation30 The serum level of IL-6 was described as significantly associated with survival in 82/101 series (100 articles) in 9917/11,583 patients (85.6%) in 23 different cancer types (). Seven series analyzed multiple cancer types comprising patients with hepatobiliary system cancer, gastric cancer, head-and-neck cancer, melanoma, liver cancer, lymphomas, squamous cell cancers, sarcoma, leukemias, renal cancers, unknown adenocarcinoma, ovarial cancer, colorectal cancer, lung cancer, breast cancer and cervical cancer.Citation27,31-36 In all of these seven non-selected cohorts of cancer patients,Citation27,31-36 IL-6 was significantly associated with survival. In 32/57 studies comprising 4441/7362 patients, multivariate analyses had been applied revealing a prognostic impact of serum IL-6, while in 25/57 studies comprising 2921 patients the correlation was only apparent using univariate analysis. The serum level of IL- 6 had no significant prognostic relevance in one series of glioblastoma.Citation37 and in both series of patients with acute myelogenous leukemia.Citation38,39
Table 1. Summary of clinical series with cancer patients that reported the serum level of IL-6 and its potential association with survival.
Table 2. In 24 dichotomized studies (26 cohorts) comprising 1272 patients, the median survival was reported for patients in whom the IL-6 serum level was higher than the chosen cut-off value. References, primary cancer type, number of patients, cut-off for IL-6 serum level (pg/mL) and median survival are given in the table.
Dichotomized studies
Patients were dichotomized according to various IL-6 serum cut-off levels in 64 studies comprising 8142 patients. The overall median of the reported median cut-offs of serum IL-6 was 10 pg/mL (range: 1.9–130 pg/mL) in cancer patients. Among the dichotomized studies a significant correlation between IL-6 serum level and survival was found in 59/64 series (92.2% of studies) comprising 94.5% (7694/8142) of the reported patients.
Quantitative analysis of survival of cancer patients at specific cut-off values for serum IL-6
The median survival of cancer patients had been determined below and above various cut-off levels of serum IL-6 in 24 studies each containing more than 50 patients and thus comprising a total of 2830 cancer patients. In a total of 1272 patients in 26 cohorts, the median survival was reported for patients in whom the IL-6 serum level was higher than the chosen cut-off value (). The cut-off value hence represented the minimum serum IL-6 level of the cohort. When sample size was used to weigh the studies, the median survival at the chosen cut-off points was inversely correlated to the minimum IL-6 serum level at this cut-off and this inverse correlation between median survival and chosen IL-6 serum level was highly significant (Spearman R -0.48, p = < 0.001). The correlation was similarly significant, when the median survival above serum IL-6 cut-off was determined without respect to sample size (Spearman R -0,402 p = < 0.04). The median survival of all 26 cohorts was plotted against this minimum IL-6 serum level and both parameters were log-transformed. On linear regression analysis, the median survival at the chosen cut-off value was inversely correlated to the corresponding minimum IL-6 serum level, both when sample size was used to weigh the studies (p < 0.001) () and also when the cohorts were not weighted (p < 0.005).
Serum IL-6 and tumor stage or metastases
Clinical parameters had been recorded and correlated with IL-6 serum levels in 44 series. A significant correlation between increasing serum IL-6 and increasing tumor stage or metastases was found in 39/44 studies comprising 91.04% (4221/4636) of the studied patients.
Discussion
Cancer induces both directly tumor-related symptoms and a systemic paraneoplastic condition that is not directly associated with the neoplasm. Besides highly heterogeneous aspects of various tumor types, there are common clinical denominators in patients with late-stages cancer. Corresponding to these clinical symptoms, there is evidence for a common paraneoplastic phenomenon in patients with advanced cancer that is expressed through a uniform cytokine pattern that appears to be independent of the cancer type.Citation1 The resulting condition can be summarized as cancer-associated dysfunctional immunostimulation, where the inflammatory microenvironment has a significant impact in the development of cancer.Citation2 IL-6 is an essential component in the cytokine cascade that is involved in the generation and regulation of inflammation. It has been previously discussed that IL-6 could be associated with survival in individual cancer patients, but the available clinical data have so far been fragmented and inconclusive. The present comprehensive literature review studied IL-6 with regard to survival in in 100 articles comprising a total of 11,583 cancer patients. The goal was to describe the clinical impact of IL-6 in cancer patients and to integrate the existing experimental immunological data into the clinical context in a translational analysis.
The fact that a majority of studies (39/44) reported a correlation between increasing IL-6 and increasing tumor stage or metastases demonstrates that the increase of the IL-6 serum level appears to be a late-stage phenomenon in cancer patients.
The current review demonstrates that the serum level of IL-6 was increased in the vast majority of clinical cancer studies with a significant correlation between serum IL-6 and survival being documented in 86% of reported patients in 23 different cancer types (). Hence, the increase of serum IL-6 appears to reflect a systemic phenomenon that is independent of the initial tumor histology.
The median survival of cancer patients had been determined below and above various cut-off levels of serum IL-6 in 24 studies and 26 cohorts with the cut-off value hence representing the minimum serum IL-6 level of a specific published cohort. The analysis showed a highly significant inverse correlation between median survival of the cohorts and their corresponding IL-6 cut-off values (Spearman R -0.48, p = < 0.001) following a linear regression when both parameters were log-transformed (p < 0.001) (). The result is surprising since each variable represents the median survival of a specific patient cohort with different cancer types, where an independent predictor of survival would be counterintuitive. This form of graphic representation and resulting analysis was chosen to allow an initial meta-analysis of the potential impact of IL-6 in a larger number of published patients.
The finding that IL-6 correlates with overall survival in the majority of studies in 23 different cancer types supports the concept of a uniform and paraneoplastic immune reaction in late-stage cancer patients. Although this finding clearly requires confirmation in specific prospective studies, the significant correlation between the serum IL-6 level and the survival in late-stage cancer could provide a novel and simple way of monitoring the systemic involvement of the immune system in cancer patients and the resulting clinical deterioration.
An essential question is if the cancer-associated immunological involvement and consistent cytokine pattern in late-stage patients.Citation1 is instrumental in the clinical deterioration of cancer patients or if the increasing dysfunction of the immune system is a mere neoplastic epiphenomenon.
In concordance with the concept of IL-6 as a prognostic indicator, several studies documented reduced IL-6 serum levels in cancer patients who had been successfully treated through either chemotherapy or surgery. In lung cancer patients, serum IL-6 levels on the first post-operative day were significant independent predictors for early recurrence,Citation40 and others showed that positive response to chemotherapy correlated with lower IL-6 levels.Citation41 In ovarian cancer patients, the serum level of IL-6 (and of CA-125, IL-7, IL-8, IL-10) decreased following chemotherapyCitation42 or surgery.Citation43,44 and early changes in serum IL-6 levels predicted the clinical outcome in patients with non-Hodgkin's lymphoma, where IL-6 levels decreased significantly in responding patients.Citation45
It is interesting that a similar relation between IL-6 serum level and prognosis has also been described in patients with septic shock, where elevated IL-6 plasma concentrations have been defined as an important biochemical indicator for early prediction of a lethal outcome,Citation46-53 even in large prospective randomized series.Citation54
Inflammation appears to be a hallmark of cancer. Despite being initially postulated by Virchow more than a century ago, for a long time the correlation between inflammation and cancer has received very little attention. IL-6 is an essential component of the systemic inflammatory immune reaction. The widespread histology-independent presence of IL-6 in late-stage cancer patients could indicate that the immune response mechanism is initiated by the tumor, but ultimately results in a paraneoplastic systemic reaction that is independent of the cancer form.
The widespread and uniform impact of IL-6 on clinical parameters including overall prognosis of cancer patients is counterintuitive with regard to the diversity of cancer types. It could be argued that a potential uniform immune reaction pattern in cancer would not reflect the differences in malignant phenotypes and variability in biological neoplastic behaviors. Conversely, the process of immune-editing is an increasingly established principle stating that a neoplasm is formed as the result of an immunological selection process ultimately producing tumor cells which are resistant to potential immune attacks.Citation55 In this scenario, the malignant cellular selection process is uniformly shaped by the homogenous physiological conditions of the human immune response. These consistent conditions could explain a uniform paraneoplastic cytokine pattern that is associated with cancer.
Exceptions
A number of publications did not determine a correlation between overall survival and IL-6. Several of these studies can be categorized: some reported atypically high serum IL-6 levels in control cohorts or patientsCitation56,57.(20.41 pg/ml Citation56 37 pg/mLCitation57) whereas the median level of all the published medians of IL-6 in control groups and cancer patients were 1.31 pg/mL (40 series: range 0–37 pg/mL) and 6.95 pg/mL (72 series: range 0.23–78.5 pg/mL), respectively. Other series without prognostic impact of IL-6 had not determined a difference between IL-6 in patients and controls,Citation57-59 which is an unusual finding since median IL-6 serum level in cancer patients was otherwise increased in 53/57 studies comprising 94.02% (5985/6366) of patients. In some studies of patients in a very early stage of cancerCitation60 or with a very long survival time (median survival 9 y)Citation61 IL-6 did not have an impact on survival. For example, in a study of indolent lymphoma, where IL-6 was unrelated to prognosis, only very few patients had died after the observation period, thus precluding conclusions.Citation30 In two studies with colorectal and gastric cancer patients, serum IL-6 was not predictive for survival but nevertheless the levels of IL-6 increased with tumor stage, and concentrations of IL-6 in the patients who died from cancer were significantly higher than in those who survived.Citation62,63 Five studies did not show a correlation between survival and serum IL-6 levels without falling under the described categories.Citation26,37,64-66 One of these studies analyzed patients with glioblastoma,Citation37 where the local tumor growth in the brain could become the determining factor for survival rather than the systemic immune response.
Functions of IL-6
A physiological immune reaction is based on multiple positive and negative feedback mechanisms involving multiple cell types that require a coordinated choreography for their efficacy and homeostasis. Cytokines have important roles in the coordination of events. The following simplified sequence of events has been proposed by Kaplanski and colleagues.Citation6 and clarifies some aspects of IL-6 in the interactive process of the immune reaction: during acute inflammation macrophages respond to inflammatory stimuli by immediate production of TNF-α, IL-1 and chemokines. TNF-a induces IL-8, which induces early neutrophil recruitment into the inflammatory site.Citation6,67 Activation of neutrophils by IL-8 promotes cleavage and shedding of IL-6R from the surface of neutrophils.Citation6,68 thus promoting ‘trans-signaling’ of IL-6 to cells that do not express the IL-6 receptor but that express gp130.Citation69-72 In addition, TNF-α induces IL-6 gene expression in monocytes and macrophages (Reviewed in Citation20) and IL-6 expression triggers the hepatic acute phase reaction.Citation20,73 IL-6 supports the inflammation since it provides a signal in the development of Th17 cells while blocking the differentiation of CD4+ cells into Treg cells.Citation11 Th17 cells are inducers of tissue inflammation and Treg cells have immunosuppressive functions.Citation74
Initially neutrophils infiltrate the tissue during acute inflammation but are later replaced by a more sustained population of mononuclear cells. It has been said that IL-6 “orchestrates this temporal switch” during inflammation.Citation7 This step appears to be controlled by IL-6 and its soluble receptor (sIL-6R),Citation7 which activate endothelial cells to produce monocyte chemotactic protein-1, thus stimulating monocyte recruitment,Citation6 while inducing polymorphonuclear-cell apoptosis.Citation6,75 Macrophages are hence attracted toward the tumor where they are integrated and where they represent the major inflammatory component of the stroma as TAMs.Citation5 These appear to predominantly comprise an M2 population, thus promoting angiogenesis, tissue remodeling and repair.Citation5,76 IL-6 together with CCL2 induces such an M2-type macrophage polarization.Citation77,78
In the physiological regulation of inflammation, IL-6, along with the other major inflammatory cytokines IL-1, IL-12, and TNF-α, is downregulated by IL-10,Citation25 which is supposed to induce the termination of the inflammatory response. Studies of co-cultures of macrophages and colon cancer cells indicated that tumor cells first stimulated macrophages to produce IL-6, which was then followed by IL-6-induced IL-10 production by the tumor cells.Citation24 In cancer patients, this physiological negative feedback between these cytokines appears to be dysfunctional since a concomitant hyperactivation of both IL-6 and IL-10 is often apparent (Reviewed inCitation1)
In summary, IL-6 appears to be part of a cytokine network that regulates inflammation by triggering a consolidated phase through monocyte recruitment with a direct effect on the organization and integration of TAMs, which are essential components of the tumor structure. Solid tumors contain substantial amounts of non-malignant stromal cells, predominantly macrophages, lymphocytes, endothelial cells and fibroblasts.Citation79,80 In this context, IL-6 appears to have a formative role in the process of the chronic cancer-related inflammation, which becomes a genuine component of the tumor and the associated systemic reaction.
Source of IL-6
IL-6 is produced in a variety of cells, including fibroblasts, endothelial cells, keratinocytes, macrophages, T cells and mast cells.(Reviewed inCitation81) Although IL-6 can also be produced by cancer tissueCitation82,83 and cancer cell linesCitation65,84-86 and IL-6 mRNA was detected in tumor cells,Citation87,86 the serum expression of IL-6 does not necessarily correlate with the tissue expression of IL-6.Citation88 High levels of IL-6 in the ascitic fluid of ovarian cancer patients was measured while IL-6 mRNA was not detected in tumor cells.Citation44 IL-6 was predictive for survival in elderly patients with chronic lymphocytic leukemia (CLL), while a corresponding IL-6 secretion by CLL cells was not found.Citation89 Similarly, the tumor cell expression of IL-6R was measured as a surrogate marker for IL-6 in a study of renal cell carcinoma, and was not significantly associated with circulating levels of CRP. This was interpreted as an indication that the main source of IL-6 responsible for an elevated CRP level in these patients was unlikely to be the tumor itself.Citation90
Hence, an increased serum IL-6 is not necessarily related to an increased tumor cytokine production. As a potential alternative source, monocytes were shown to produce significantly higher levels of IL-6 in in cachectic cancer patients compared with healthy controls,Citation91 and pancreatic cancer cells were able to stimulate IL-6 production in peripheral blood mononuclear cells by 14-fold.Citation92 Similarly, monocytes in head-and-neck cancer patients have been reported to secrete higher levels of IL-6 than monocytes from control individuals.Citation93 As a consequence, it is so far unsolved if the increased IL-6 serum level in cancer patients is a function of an increased tumor cytokine production or the result of a systemic immune reaction. However, independent of the source, the resulting increase of IL-6 has a systemic impact.
Throughout the variety of cancer types, IL-6 appears to be linked to the patients' prognosis as a common denominator of the clinical deterioration. This allows a novel perspective on the ultimately lethal systemic paraneoplastic process, and new treatment alternatives could be directed against the resulting systemic paraneoplastic syndrome.
Clinical options?
Being part of an intricate network of cytokine interactions, it is highly unlikely that IL-6 alone is responsible for the clinical deterioration in cancer patients. IL-6 rather reflects a cascade of interdependent cytokines.Citation1 Hence, it is so far unknown if IL-6 has a causative role in the clinical deterioration in cancer patients or if it represents a mere epiphenomenon. The consist relation between the IL-6 serum level and clinical condition could possibly provide a non-invasive instrument in the long-term follow-up of cancer patients and could potentially be used for an initial assessment of treatment effects. Provided these retrospective and unselected data from the literature can be confirmed in future prespective analyses, IL-6 could be an indicator for a clinical cascade of events that may be relevant both in a diagnostic and a therapeutic environment.
Since IL-6 could be the ‘end-product’ of a cancer-induced chain of events and may not have an impact of its own, it is unclear if the pharmaceutical downregulation of IL-6 would be promising for cancer patients. However, several options exist already: in keratinocytes, nicotinamide appears to downregulate the gene expression of IL-6 (and IL-10).Citation94 Furthermore, drugs such as adalimumab and methotrexate suppress the serum IL-6 level (in rheumatoid arthritis patients).Citation95 Tocilizumab is a humanized monoclonal antibody acting as an IL-6 receptor antagonist and is used in the treatment of patients with rheumatoid arthritis. Tocilizumab inhibits both classic signaling and trans-signaling of IL-6. Pooled and meta-analyses demonstrated efficacy and tolerability even during long-term therapy,Citation96 but unexpectedly these improvements were accompanied by increases in serum levels of IL-6 and IL-6R, possibly due to changed bioavailability.Citation97 The potential impact of IL-6 modifying medications on the paraneoplastic symptoms in cancer patients is yet to be established.
Conclusion
IL-6 is one component of a complex cancer-induced cytokine cascade. In the vast majority of clinical studies in cancer patients, the IL-6 serum level is increased. The increase of the IL-6 serum level appears to be a late-stage phenomenon in cancer patients and reflects a tumor-histology-independent systemic phenomenon. The available literature suggests a correlation between IL-6 serum level and the survival of cancer patients. Future studies will have to analyze correlations between individual IL-6 serum levels and prognosis on a larger scale in order to determine if IL-6 has a causative role in the clinical deterioration of cancer patients or if the IL-6 serum level is a mere epiphenomenon.
Article selection and analysis
To identify eligible studies, systematic searches of IL-6 in cancer patients were conducted using the PubMed database. Specifically, the search was filtered according to “prognostic impact of IL-6 in cancer patients” with the publication year after 1993 (until 2013) using the following algorithm:
((((((((Il-6[Title/Abstract]) AND Cancer[Title/Abstract]) AND survival[Title/Abstract]) AND patients[Title/Abstract]) AND (“1993”[Date - Publication] : “3000” [Date - Publication]))) OR (((((interleukin-6[Title/Abstract]) AND Cancer[Title/Abstract]) AND survival[Title/Abstract]) AND patients[Title/Abstract]) AND (“1993” [Date - Publication] : “3000” [Date - Publication]))) OR (((((Il-6[Title/Abstract]) AND lymphoma[Title/Abstract]) AND survival[Title/Abstract]) AND patients[Title/Abstract]) AND (“1993” [Date - Publication] : “3000” [Date - Publication]))) OR (((((interleukin-6[Title/Abstract]) AND lymphoma[Title/Abstract]) AND survival[Title/Abstract]) AND patients[Title/Abstract]) AND (“1993” [Date - Publication] : “3000” [Date - Publication])).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We would like to thank Magnus Backheden from the Department of Learning, Informatics, Management and Ethics (LIME) at the Karolinska Institute, Stockholm for his assistance in the statistical analysis.
References
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013; 14:e218-28; PMID:23639322; http://dx.doi.org/10.1016/S1470-2045(12)70582-X
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/10.1038/nature07205
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/10.1016/j.cell.2010.01.025
- Zitvogel L, Tanchot C, Granier C, Tartour E. Following up tumor-specific regulatory T cells in cancer patients. Oncoimmunology 2013; 2:e25444; PMID:24073383; http://dx.doi.org/10.4161/onci.25444
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 2006; 25:315-22; PMID:16967326; http://dx.doi.org/10.1007/s10555-006-9001-7
- Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 2003; 24:25-9; PMID:12495721; http://dx.doi.org/10.1016/S1471-4906(02)00013-3
- Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001; 14:705-14; PMID:11420041; http://dx.doi.org/10.1016/S1074-7613(01)00151-0
- Marin V, Montero-Julian FA, Gres S, Boulay V, Bongrand P, Farnarier C, Kaplanski G. The IL-6-soluble IL-6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J Immunol 2001; 167:3435-42; PMID:11544336; http://dx.doi.org/10.4049/jimmunol.167.6.3435
- Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood 1995; 86:1243-54; PMID:7632928
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986; 324:73-6; PMID:3491322; http://dx.doi.org/10.1038/324073a0
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235-8; PMID:16648838; http://dx.doi.org/10.1038/nature04753
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the T(H)17 lineage. Nature 2006; 441:231-4; PMID:16648837; http://dx.doi.org/10.1038/nature04754
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006; 24:179-89; PMID:16473830; http://dx.doi.org/10.1016/j.immuni.2006.01.001
- Kimura A, Kishimoto T. Th17 cells in inflammation. Int Immunopharmacol 2011; 11:319-22; PMID:21035432; http://dx.doi.org/10.1016/j.intimp.2010.10.004
- Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 2011; 22:83-9; PMID:21377916; http://dx.doi.org/10.1016/j.cytogfr.2011.02.003
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875-86; PMID:14676299; http://dx.doi.org/10.1084/jem.20030152
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and downregulation of Smad7. J Immunol 2004; 172:5149-53; PMID:15100250; http://dx.doi.org/10.4049/jimmunol.172.9.5149
- Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, Ripley B, Serada S, Nishikawa T, Kimura A et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol 2011; 186:32-40; PMID:21106853; http://dx.doi.org/10.4049/jimmunol.0903314
- Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol 2007; 179:2041-5; PMID:17675459; http://dx.doi.org/10.4049/jimmunol.179.4.2041
- Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost 2009; 102:215-22; PMID:19652871; http://dx.doi.org/10.1160/TH09-05-0297
- Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, Chen Y, Lawton MT, Young WL, Yang GY. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab 2008; 28:90-8; PMID:17519976; http://dx.doi.org/10.1038/sj.jcbfm.9600509
- Ghazizadeh M. Essential role of IL-6 signaling pathway in keloid pathogenesis. J Nippon Med Sch 2007; 74:11-22; PMID:17384473; http://dx.doi.org/10.1272/jnms.74.11
- Suzuki S, Mita S, Kamohara H, Sakamoto K, Ishiko T, Ogawa M. IL-6 and IFN-gamma regulation of IL-10 production by human colon carcinoma cells. Int J Oncol 2001; 18:581-6; PMID:11179490
- Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol 2004; 172:4630-6; PMID:15034082; http://dx.doi.org/10.4049/jimmunol.172.7.4630
- Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 2008; 226:205-18; PMID:19161426; http://dx.doi.org/10.1111/j.1600-065X.2008.00706.x
- Nixon AB, Pang H, Starr MD, Friedman PN, Bertagnolli MM, Kindler HL, Goldberg RM, Venook AP, Hurwitz HI. Prognostic and Predictive Blood-Based Biomarkers in Patients with Advanced Pancreatic Cancer: Results from CALGB80303 (Alliance). Clin Cancer Res 2013; 19:6957-66; PMID:24097873; http://dx.doi.org/10.1158/1078-0432.CCR-13-0926
- Tredan O, Ray-Coquard I, Chvetzoff G, Rebattu P, Bajard A, Chabaud S, Perol D, Saba C, Quiblier F, Blay JY et al. Validation of prognostic scores for survival in cancer patients beyond first-line therapy. BMC Cancer 2011; 11:95; PMID:21406082; http://dx.doi.org/10.1186/1471-2407-11-95
- Ramsey S, Aitchison M, Graham J, McMillan DC. The longitudinal relationship between the systemic inflammatory response, circulating T-lymphocytes, interleukin-6 and -10 in patients undergoing immunotherapy for metastatic renal cancer. BJU Int 2008; 102:125-9; PMID:18336617; http://dx.doi.org/10.1111/j.1464-410X.2008.07466.x
- Duletic-Nacinovic A, Stifter S, Marijic B, Lucin K, Valkovic T, Petranovic D, Jonjic N. Serum IL-6, IL-8, IL-10 and beta2-microglobulin in association with International Prognostic Index in diffuse large B cell lymphoma. Tumori 2008; 94:511-7; PMID:18822687
- Fayad L, Cabanillas F, Talpaz M, McLaughlin P, Kurzrock R. High serum interleukin-6 levels correlate with a shorter failure-free survival in indolent lymphoma. Leuk Lymphoma 1998; 30:563-71; PMID:9711918
- Suh SY, Choi YS, Yeom CH, Kwak SM, Yoon HM, Kim DG, Koh SJ, Park J, Lee MA, Lee YJ et al. Interleukin-6 but not tumour necrosis factor-α predicts survival in patients with advanced cancer. Support Care Cancer 2013; 21:3071-7; PMID:23828393; http://dx.doi.org/10.1007/s00520-013-1878-4
- Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle 2012; 3:245-51; PMID:22648739; http://dx.doi.org/10.1007/s13539-012-0075-5
- Grim-Stieger M, Keilani M, Mader RM, Marosi C, Schmidinger M, Zielinski CC, Fialka-Moser V, Crevenna R. Serum levels of tumour necrosis factor-α and interleukin-6 and their correlation with body mass index, weight loss, appetite and survival rate–preliminary data of Viennese outpatients with metastatic cancer during palliative chemotherapy. Eur J Cancer Care (Engl) 2008; 17:454-62; PMID:18637115; http://dx.doi.org/10.1111/j.1365-2354.2007.00874.x
- Heikkila K, Ebrahim S, Rumley A, Lowe G, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with survival in women with and without cancer: findings from the British Women's Heart and Health Study. Cancer Epidemiol Biomarkers Prev 2007; 16:1155-9; PMID:17548678; http://dx.doi.org/10.1158/1055-9965.EPI-07-0093
- Di Nisio M, Niers TM, Reitsma PH, Buller HR. Plasma cytokine and P-selectin levels in advanced malignancy: prognostic value and impact of low-molecular weight heparin administration. Cancer 2005; 104:2275-81; PMID:16216004; http://dx.doi.org/10.1002/cncr.21485
- Mantovani G, Maccio A, Madeddu C, Mura L, Massa E, Mudu MC, Mulas C, Lusso MR, Gramignano G, Piras MB. Serum values of proinflammatory cytokines are inversely correlated with serum leptin levels in patients with advanced stage cancer at different sites. J Mol Med (Berl) 2001; 79:406-14; PMID:11466563; http://dx.doi.org/10.1007/s001090100234
- Reynes G, Vila V, Martin M, Parada A, Fleitas T, Reganon E, Martinez-Sales V. Circulating markers of angiogenesis, inflammation, and coagulation in patients with glioblastoma. J Neurooncol 2011; 102:35-41; PMID:20607353; http://dx.doi.org/10.1007/s11060-010-0290-x
- Tsimberidou AM, Estey E, Wen S, Pierce S, Kantarjian H, Albitar M, Kurzrock R. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer 2008; 113:1605-13; PMID:18683214; http://dx.doi.org/10.1002/cncr.23785
- Thomas X, Hirschauer C, Troncy J, Assouline D, Joly MO, Fiere D, Archimbaud E. Serum interleukin-6 levels in adult acute myelogenous leukemia: relationship with disease characteristics and outcome. Leuk Lymphoma 1997; 24:291-300; PMID:9156658
- Kita H, Shiraishi Y, Watanabe K, Suda K, Ohtsuka K, Koshiishi Y, Goya T. Does postoperative serum interleukin-6 influence early recurrence after curative pulmonary resection of lung cancer? Ann Thorac Cardiovasc Surg 2011; 17:454-60; PMID:21881374; http://dx.doi.org/10.5761/atcs.oa.10.01627
- De Vita F, Orditura M, Auriemma A, Infusino S, Roscigno A, Catalano G. Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep 1998; 5:649-52; PMID:9538169
- Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, ten Hoor KA, Braid M, van der Zee AG, Daemen T, Nijman HW, Kast WM. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res 2007; 13:2385-91; PMID:17438097; http://dx.doi.org/10.1158/1078-0432.CCR-06-1828
- Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol 1997; 66:27-30; PMID:9234916; http://dx.doi.org/10.1006/gyno.1997.4726
- Scambia G, Testa U, Panici PB, Martucci R, Foti E, Petrini M, Amoroso M, Masciullo V, Peschle C, Mancuso S. Interleukin-6 serum levels in patients with gynecological tumors. Int J Cancer 1994; 57:318-23; PMID:8168990; http://dx.doi.org/10.1002/ijc.2910570305
- Pedersen LM, Klausen TW, Davidsen UH, Johnsen HE. Early changes in serum IL-6 and VEGF levels predict clinical outcome following first-line therapy in aggressive non-Hodgkin's lymphoma. Ann Hematol 2005; 84:510-6; PMID:15834569; http://dx.doi.org/10.1007/s00277-005-1020-x
- Novotny A, Emmanuel K, Bartels H, Siewert JR, Holzmann B. [Indicators for early prediction of outcome in sepsis]. Chirurg 2005; 76:837-44; PMID:16094522; http://dx.doi.org/10.1007/s00104-005-1077-z
- Rodriguez-Gaspar M, Santolaria F, Jarque-Lopez A, Gonzalez-Reimers E, Milena A, de la Vega MJ, Rodriguez-Rodriguez E, Gomez-Sirvent JL. Prognostic value of cytokines in SIRS general medical patients. Cytokine 2001; 15:232-6; PMID:11563884; http://dx.doi.org/10.1006/cyto.2001.0932
- Viallon A, Guyomarc'h S, Marjollet O, Berger C, Carricajo A, Robert F, Laporte S, Lambert C, Page Y, Zeni F et al. Can emergency physicians identify a high mortality subgroup of patients with sepsis: role of procalcitonin. Eur J Emerg Med 2008; 15:26-33; PMID:18180663; http://dx.doi.org/10.1097/MEJ.0b013e3280ec539b
- Wu HP, Chen CK, Chung K, Tseng JC, Hua CC, Liu YC, Chuang DY, Yang CH. Serial cytokine levels in patients with severe sepsis. Inflamm Res 2009; 58(7):385-93; PMID:19262987; http://dx.doi.org/10.1007/s00011-009-0003-0
- Ghani RA, Zainudin S, Ctkong N, Rahman AF, Wafa SR, Mohamad M, Manaf MR, Ismail R. Serum IL-6 and IL-1-ra with sequential organ failure assessment scores in septic patients receiving high-volume haemofiltration and continuous venovenous haemofiltration. Nephrology (Carlton) 2006; 11:386-93; PMID:17014550; http://dx.doi.org/10.1111/j.1440-1797.2006.00600.x
- Groeneveld AB, Tacx AN, Bossink AW, van Mierlo GJ, Hack CE. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin Immunol 2003; 106:106-15; PMID:12672401; http://dx.doi.org/10.1016/S1521-6616(02)00025-6
- Patel RT, Deen KI, Youngs D, Warwick J, Keighley MR. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg 1994; 81:1306-8; PMID:7953393; http://dx.doi.org/10.1002/bjs.1800810914
- Bencosme A, Warner A, Healy D, Verme C. Prognostic potential of cytokines, nitrates, and APACHE II score in sepsis. Ann Clin Lab Sci 1996; 26:426-32; PMID:8879360
- Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock 2005; 23:488-93; PMID:15897799; http://dx.doi.org/10.1097/01.shk.0000163802.46355.59
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137-48; PMID:15308095; http://dx.doi.org/10.1016/j.immuni.2004.07.017
- Vizio B, Novarino A, Giacobino A, Cristiano C, Prati A, Ciuffreda L, Montrucchio G, Bellone G. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med 2012; 4:70-8; PMID:23060925; http://dx.doi.org/10.3892%2Fetm.2012.553
- Su C, Zhou C, Zhou S, Xu J. Serum cytokine levels in patients with advanced non-small cell lung cancer: correlation with treatment response and survival. Med Oncol 2011; 28:1453-7; PMID:20714944; http://dx.doi.org/10.1007/s12032-010-9645-6
- Candido EB, Silva LM, Carvalho AT, Lamaita RM, Filho RM, Cota BD, da Silva-Filho AL. Immune response evaluation through determination of type 1, type 2, and type 17 patterns in patients with epithelial ovarian cancer. Reprod Sci 2013; 20:828-37; PMID:23239818; http://dx.doi.org/10.1177/1933719112466299
- Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim KH, Goh RY, Choi HJ, Park KJ, Roh MS et al. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer 2010; 10:203; PMID:20465852; http://dx.doi.org/10.1186/1471-2407-10-203
- Svobodova S, Topolcan O, Holubec L, Jr., Levy M, Pecen L, Svacina S. Parameters of biological activity in colorectal cancer. Anticancer Res 2011; 31:373-8; PMID:21273626
- Labidi SI, Menetrier-Caux C, Chabaud S, Chassagne C, Sebban C, Gargi T, Biron P, Blay JY, Ghesquieres H. Serum cytokines in follicular lymphoma. Correlation of TGF-β and VEGF with survival. Ann Hematol 2010; 89:25-33; PMID:19582455; http://dx.doi.org/10.1007/s00277-009-0777-8
- Groblewska M, Mroczko B, Wereszczynska-Siemiatkowska U, Kedra B, Lukaszewicz M, Baniukiewicz A, Szmitkowski M. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patients. Clin Chem Lab Med 2008; 46:1423-8; PMID:18844497; http://dx.doi.org/10.1515/CCLM.2008.278
- Lukaszewicz-Zajac M, Mroczko B, Gryko M, Kedra B, Szmitkowski M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med 2011; 11:89-96; PMID:20938721; http://dx.doi.org/10.1007/s10238-010-0114-5
- Ozdemir F, Aydin F, Yilmaz M, Kavgaci H, Bektas O, Yavuz MN, Yavuz AA. The effects of IL-2, IL-6 and IL-10 levels on prognosis in patients with aggressive Non-Hodgkin's Lymphoma (NHL). J Exp Clin Cancer Res 2004; 23:485-8; PMID:15595640
- Walther MM, Johnson B, Culley D, Shah R, Weber J, Venzon D, Yang JC, Linehan WM, Rosenberg SA. Serum interleukin-6 levels in metastatic renal cell carcinoma before treatment with interleukin-2 correlates with paraneoplastic syndromes but not patient survival. J Urol 1998; 159:718-22; PMID:9474133; http://dx.doi.org/10.1016/S0022-5347(01)63709-1
- Vuoristo MS, Kellokumpu-Lehtinen P, Laine S, Parvinen LM, Hahka-Kemppinen M, Korpela M, Kumpulainen E. The value of serum S-100beta and interleukins as tumour markers in advanced melanoma. Melanoma Res 2000; 10:237-41; PMID:10890377; http://dx.doi.org/10.1097/00008390-200010030-00005
- Ren Y, Tsui HT, Poon RT, Ng IO, Li Z, Chen Y, Jiang G, Lau C, Yu WC, Bacher M et al. Macrophage migration inhibitory factor: roles in regulating tumor cell migration and expression of angiogenic factors in hepatocellular carcinoma. Int J Cancer J Int Du Cancer 2003; 107:22-9; PMID:12925952; http://dx.doi.org/10.1002/ijc.11287
- Marin V, Montero-Julian F, Gres S, Bongrand P, Farnarier C, Kaplanski G. Chemotactic agents induce IL-6Ralpha shedding from polymorphonuclear cells: involvement of a metalloproteinase of the TNF-α-converting enzyme (TACE) type. Eur J Immunol 2002; 32:2965-70; PMID:12355450; http://dx.doi.org/10.1002/1521-4141(2002010)32:10%3c2965::AID-IMMU2965%3e3.0.CO;2-V
- Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 2006; 63:321-9; PMID:16640655; http://dx.doi.org/10.1111/j.1365-3083.2006.01750.x
- Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol 2005; 28:187-96; PMID:16129903; http://dx.doi.org/10.1385/CRIAI:28:3:187
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer 2007; 110:1911-28; PMID:17849470; http://dx.doi.org/10.1002/cncr.22999
- O'Reilly S, Ciechomska M, Cant R, Hugle T, van Laar JM. Interleukin-6, its role in fibrosing conditions. Cytokine Growth Factor Rev 2012; 23:99-107; PMID:22561547; http://dx.doi.org/10.1016/j.cytogfr.2012.04.003
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon β 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A 1987; 84:7251-5; PMID:2444978; http://dx.doi.org/10.1073/pnas.84.20.7251
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Ann Rev Immunol 2009; 27:485-517; PMID:19132915; http://dx.doi.org/10.1146/annurev.immunol.021908.132710
- Afford SC, Pongracz J, Stockley RA, Crocker J, Burnett D. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J Biol Chem 1992; 267:21612-6; PMID:1400472
- Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011; 41:2522-5; PMID:21952810; http://dx.doi.org/10.1002/eji.201141894
- Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 2009; 284:34342-54; PMID:19833726; http://dx.doi.org/10.1074/jbc.M109.042671
- Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci 2010; 95:31-53; PMID:21075328; http://dx.doi.org/10.1016/B978-0-12-385071-3.00003-4
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004; 4:540-50; PMID:15229479; http://dx.doi.org/10.1038/nrc1388
- Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol 1997; 150:1723-34; PMID:9137096
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol 1990; 8:253-78; PMID:2188664; http://dx.doi.org/10.1146/annurev.iy.08.040190.001345
- Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer 1999; 85:2526-31; PMID:10375098; http://dx.doi.org/10.1002/(SICI)1097-0142(19990615)85:12%3c2526::AID-CNCR6%3e3.0.CO;2-3
- Chen MF, Chen PT, Lu MS, Lin PY, Chen WC, Lee KD. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer 2013; 12:26; PMID:23561329; http://dx.doi.org/10.1186/1476-4598-12-26
- Wu CW, Wang SR, Chao MF, Wu TC, Lui WY, P'Eng FK, Chi CW. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol 1996; 91:1417-22; PMID:8678006
- Fukuyama T, Ichiki Y, Yamada S, Shigematsu Y, Baba T, Nagata Y, Mizukami M, Sugaya M, Takenoyama M, Hanagiri T et al. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci 2007; 98:1048-54.; PMID:17511773; http://dx.doi.org/10.1111/j.1349-7006.2007.00507.x
- Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother 2006; 55:684-98; PMID:16094523; http://dx.doi.org/10.1007/s00262-005-0047-0
- Chow KC, Chiou SH, Ho SP, Tsai MH, Chen CL, Wang LS, Chi KH. The elevated serum interleukin-6 correlates with the increased serum butyrate level in patients with nasopharyngeal carcinoma. Oncol Rep 2003; 10:813-9; PMID:12792728
- Chung YC, Chaen YL, Hsu CP. Clinical significance of tissue expression of interleukin-6 in colorectal carcinoma. Anticancer Res 2006; 26:3905-11; PMID:17094421
- Yoon JY, Lafarge S, Dawe D, Lakhi S, Kumar R, Morales C, Marshall A, Gibson SB, Johnston JB. Association of interleukin-6 and interleukin-8 with poor prognosis in elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 2012; 53:1735-42; PMID:22475215; http://dx.doi.org/10.3109/10428194.2012.666662
- Lamb GW, McArdle PA, Ramsey S, McNichol AM, Edwards J, Aitchison M, McMillan DC. The relationship between the local and systemic inflammatory responses and survival in patients undergoing resection for localized renal cancer. BJU Int 2008; 102:756-61; PMID:18384626; http://dx.doi.org/10.1111/j.1464-410X.2008.07666.x
- Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep 2009; 21:1091-5; PMID:19288013; http://dx.doi.org/10.3892/or_00000328
- Martignoni ME, Kunze P, Hildebrandt W, Kunzli B, Berberat P, Giese T, Kloters O, Hammer J, Buchler MW, Giese NA et al. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res 2005; 11:5802-8; PMID:16115919; http://dx.doi.org/10.1158/1078-0432.CCR-05-0185
- Gallo O, Gori AM, Attanasio M, Martini F, Giusti B, Boddi M, Gallina E, Fini O, Abbate R. Interleukin-1 β and interleukin-6 release by peripheral blood monocytes in head and neck cancer. Br J Cancer 1993; 68:465-8; PMID:8353036; http://dx.doi.org/10.1038/bjc.1993.371
- Monfrecola G, Gaudiello F, Cirillo T, Fabbrocini G, Balato A, Lembo S. Nicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor-α gene expression in HaCaT keratinocytes after ultraviolet B irradiation. Clin Exp Dermatol 2013; 38:185-8; PMID:23397947; http://dx.doi.org/10.1111/ced.12018
- Yue C, You X, Zhao L, Wang H, Tang F, Zhang F, Zhang X, He W. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int 2010; 30:1553-7; PMID:19847432; http://dx.doi.org/10.1007/s00296-009-1179-x
- Dhillon S. Intravenous tocilizumab: a review of its use in adults with rheumatoid arthritis. BioDrugs 2014; 28:75-106; PMID:24255004; http://dx.doi.org/10.1007/s40259-013-0076-8
- Assier E, Boissier MC, Dayer JM. Interleukin-6: from identification of the cytokine to development of targeted treatments. Joint Bone Spine 2010; 77:532-6; PMID:20869898; http://dx.doi.org/10.1016/j.jbspin.2010.07.007
- Schultz NA, Christensen IJ, Werner J, Giese N, Jensen BV, Larsen O, Bjerregaard JK, Pfeiffer P, Calatayud D, Nielsen SE et al. Diagnostic and Prognostic Impact of Circulating YKL-40, IL-6, and CA 19.9 in Patients with Pancreatic Cancer. PLoS One 2013; 8:e67059; PMID:23840582; http://dx.doi.org/10.1371/journal.pone.0067059
- Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, Higashi S, Kato H, Terao K, Ochiai A. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 2013; 108:2063-9; PMID:23591198; http://dx.doi.org/10.1038/bjc.2013.174
- Arshad A, Chung WY, Steward W, Metcalfe MS, Dennison AR. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB (Oxford) 2013; 15:428-32; PMID:23458624; http://dx.doi.org/10.1111/hpb.12002
- Mroczko B, Groblewska M, Gryko M, Kedra B, Szmitkowski M. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal 2010; 24:256-61; PMID:20626020; http://dx.doi.org/10.1002/jcla.20395
- Ravishankaran P, Karunanithi R. Clinical significance of preoperative serum interleukin-6 and C-reactive protein level in breast cancer patients. World J Surg Oncol 2011; 9:18; PMID:21294915; http://dx.doi.org/10.1186/1477-7819-9-18
- Fuksiewicz M, Kowalska M, Kotowicz B, Rubach M, Chechlinska M, Pienkowski T, Kaminska J. Serum soluble tumour necrosis factor receptor type I concentrations independently predict prognosis in patients with breast cancer. Clin Chem Lab Med 2010; 48:1481-6; PMID:20578967; http://dx.doi.org/10.1515/CCLM.2010.278
- Bozcuk H, Uslu G, Samur M, Yildiz M, Ozben T, Ozdogan M, Artac M, Altunbas H, Akan I, Savas B. Tumour necrosis factor-α, interleukin-6, and fasting serum insulin correlate with clinical outcome in metastatic breast cancer patients treated with chemotherapy. Cytokine 2004; 27:58-65; PMID:15242694; http://dx.doi.org/10.1016/j.cyto.2004.04.002
- Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer 2003; 88:1721-6; PMID:12771987; http://dx.doi.org/10.1038/sj.bjc.6600956
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer J Int Du Cancer 2003; 103:642-6; PMID:12494472; http://dx.doi.org/10.1002/ijc.10833
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 1999; 19:1427-32; PMID:10365118
- Dean-Colomb W, Hess KR, Young E, Gornet TG, Handy BC, Moulder SL, Ibrahim N, Pusztai L, Booser D, Valero V et al. Elevated serum P1NP predicts development of bone metastasis and survival in early-stage breast cancer. Breast Cancer Res Treat 2013; 137:631-6; PMID:23242617; http://dx.doi.org/10.1007/s10549-012-2374-0
- Shimazaki J, Goto Y, Nishida K, Tabuchi T, Motohashi G, Ubukata H. In patients with colorectal cancer, preoperative serum interleukin-6 level and granulocyte/lymphocyte ratio are clinically relevant biomarkers of long-term cancer progression. Oncology 2013; 84:356-61; PMID:23689116; http://dx.doi.org/10.1159/000350836
- Yeh KY, Li YY, Hsieh LL, Lu CH, Chou WC, Liaw CC, Tang RP, Liao SK. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Jpn J Clin Oncol 2010; 40:580-7; PMID:20194250; http://dx.doi.org/10.1093/jjco/hyq010
- Nikiteas NI, Tzanakis N, Gazouli M, Rallis G, Daniilidis K, Theodoropoulos G, Kostakis A, Peros G. Serum IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients: prognostic implications. World J Gastroenterol 2005; 11:1639-43; PMID:15786541; http://dx.doi.org/10.3748/wjg.v11.i11.1639
- Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol 2003; 83:222-6; PMID:12884234; http://dx.doi.org/10.1002/jso.10269
- Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Lise M, Jessup JM. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol 2000; 7:133-8; PMID:10761792; http://dx.doi.org/10.1007/s10434-000-0133-7
- Liu Y, Starr MD, Bulusu A, Pang H, Wong NS, Honeycutt W, Amara A, Hurwitz HI, Nixon AB. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med 2013; 2:234-42; PMID:23634291; http://dx.doi.org/10.1002/cam4.71
- Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer 2008; 7:331-7; PMID:18794066; http://dx.doi.org/10.3816/CCC.2008.n.044
- Lukaszewicz-Zajac M, Mroczko B, Kozlowski M, Niklinski J, Laudanski J, Szmitkowski M. Higher importance of interleukin 6 than classic tumor markers (carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis Esophagus 2012; 25:242-9; PMID:21895853; http://dx.doi.org/10.1111/j.1442-2050.2011.01242.x
- Hao W, Zhu Y, Zhou H. Prognostic value of interleukin-6 and interleukin-8 in laryngeal squamous cell cancer. Med Oncol 2013; 30:333; PMID:23269580; http://dx.doi.org/10.1007/s12032-012-0333-6
- Scambia G, Testa U, Benedetti Panici P, Foti E, Martucci R, Gadducci A, Perillo A, Facchini V, Peschle C, Mancuso S. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer 1995; 71:354-6; PMID:7841052; http://dx.doi.org/10.1038/bjc.1995.71
- Wang YS, Miao LY, Liu L, Cai HR, Ding JJ, Ren SX, Zhou CC, Schmid-Bindert G. Serum cytokine levels in patients with advanced non-small cell lung cancer: correlation with clinical outcome of erlotinib treatment. Chin Med J (Engl) 2013; 126:3931-5; PMID:24157160; http://dx.doi.org/10.3760/cma.j.issn.0366-6999.20130578
- Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH, Chen YM, Huang MS, Chen HL, Li YJ, Yang PC et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer 2013; 132:1977-85; PMID:23034889; http://dx.doi.org/10.1002/ijc.27892
- Ujiie H, Tomida M, Akiyama H, Nakajima Y, Okada D, Yoshino N, Takiguchi Y, Tanzawa H. Serum hepatocyte growth factor and interleukin-6 are effective prognostic markers for non-small cell lung cancer. Anticancer Res 2012; 32:3251-8; PMID:22843899
- Sanchez-Lara K, Turcott JG, Juarez E, Guevara P, Nunez-Valencia C, Onate-Ocana LF, Flores D, Arrieta O. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer 2012; 64:526-34; PMID:22489794; http://dx.doi.org/10.1080/01635581.2012.668744
- Koh E, Iizasa T, Yamaji H, Sekine Y, Hiroshima K, Yoshino I, Fujisawa T. Significance of the correlation between the expression of interleukin 6 and clinical features in patients with non-small cell lung cancer. Int J Surg Pathol 2012; 20:233-9; PMID:22334615; http://dx.doi.org/10.1177/1066896911436274
- Dehing-Oberije C, Aerts H, Yu S, De Ruysscher D, Menheere P, Hilvo M, van der Weide H, Rao B, Lambin P. Development and validation of a prognostic model using blood biomarker information for prediction of survival of non-small-cell lung cancer patients treated with combined chemotherapy and radiation or radiotherapy alone (NCT00181519, NCT00573040, and NCT00572325). Int J Radiat Oncol Biol Phys 2011; 81:360-8; PMID:20888135; http://dx.doi.org/10.1016/j.ijrobp.2010.06.011
- Songur N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 2004; 90:196-200; PMID:15237582
- Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, Alberg AJ, Harris CC. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 2009; 18:215-22; PMID:19124500; http://dx.doi.org/10.1158/1055-9965.EPI-08-0705
- Martin F, Santolaria F, Batista N, Milena A, Gonzalez-Reimers E, Brito MJ, Oramas J. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 1999; 11:80-6; PMID:10080883; http://dx.doi.org/10.1006/cyto.1998.0398
- Katsumata N, Eguchi K, Fukuda M, Yamamoto N, Ohe Y, Oshita F, Tamura T, Shinkai T, Saijo N. Serum levels of cytokines in patients with untreated primary lung cancer. Clin Cancer Res 1996; 2:553-9; PMID:9816203
- Kaminska J, Kowalska M, Kotowicz B, Fuksiewicz M, Glogowski M, Wojcik E, Chechlinska M, Steffen J. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF - an independent prognostic factor. Oncology 2006; 70:115-25; PMID:16645324; http://dx.doi.org/10.1159/000093002
- Wojcik E, Jakubowicz J, Skotnicki P, Sas-Korczynska B, Kulpa JK. IL-6 and VEGF in small cell lung cancer patients. Anticancer Res 2010; 30:1773-8; PMID:20592377
- Necula LG, Chivu-Economescu M, Stanciulescu EL, Bleotu C, Dima SO, Alexiu I, Dumitru A, Constantinescu G, Popescu I, Diaconu CC. IL-6 and IL-11 as markers for tumor aggressiveness and prognosis in gastric adenocarcinoma patients without mutations in Gp130 subunits. J Gastrointestin Liver Dis 2012; 21:23-9; PMID:22457856
- Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, Kim SG, Kim SH, Jang JS, Kim MC et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer 2009; 9:155; PMID:19457231; http://dx.doi.org/10.1186/1471-2407-9-155
- Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer 2009; 12:95-100; PMID:19562463; http://dx.doi.org/10.1007/s10120-009-0509-8
- Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer 2005; 8:124-31; PMID:15864720; http://dx.doi.org/10.1007/s10120-005-0315-x
- De Vita F, Romano C, Orditura M, Galizia G, Martinelli E, Lieto E, Catalano G. Interleukin-6 serum level correlates with survival in advanced gastrointestinal cancer patients but is not an independent prognostic indicator. J Interferon Cytokine Res 2001; 21:45-52; PMID:11177580; http://dx.doi.org/10.1089/107999001459150
- Yoshitomi M, Yutani S, Matsueda S, Ioji T, Komatsu N, Shichijo S, Yamada A, Itoh K, Sasada T, Kinoshita H. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med 2012; 3:463-9; PMID:22969912; http://dx.doi.org/10.3892/etm.2011.424
- Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM, Figlin RA, Hutson TE, Sternberg CN, Amado RG et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012; 13:827-37; PMID:22759480; http://dx.doi.org/10.1016/S1470-2045(12)70241-3
- Montero AJ, Diaz-Montero CM, Millikan RE, Liu J, Do KA, Hodges S, Jonasch E, McIntyre BW, Hwu P, Tannir N. Cytokines and angiogenic factors in patients with metastatic renal cell carcinoma treated with interferon-α: association of pretreatment serum levels with survival. Ann Oncol 2009; 20:1682-7; PMID:19541791; http://dx.doi.org/10.1093/annonc/mdp054
- Guida M, Casamassima A, Monticelli G, Quaranta M, Colucci G. Basal cytokines profile in metastatic renal cell carcinoma patients treated with subcutaneous IL-2-based therapy compared with that of healthy donors. J Transl Med 2007; 5:51; PMID:17953739; http://dx.doi.org/10.1186/1479-5876-5-51
- Negrier S, Perol D, Menetrier-Caux C, Escudier B, Pallardy M, Ravaud A, Douillard JY, Chevreau C, Lasset C, Blay JY. Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6–from the Groupe Francais d'Immunotherapie. J Clin Oncol 2004; 22:2371-8; PMID:15197198; http://dx.doi.org/10.1200/JCO.2004.06.121
- Kallio JP, Tammela TL, Marttinen AT, Kellokumpu-Lehtinen PL. Soluble immunological parameters and early prognosis of renal cell cancer patients. J Exp Clin Cancer Res 2001; 20:523-8; PMID:11876546
- Favaro D, Santarosa M, Quaia M, Galligioni E. Interleukin-6 and soluble intercellular adhesion molecule-1 in renal cancer patients and cultured renal cancer cells. Urol Oncol 1997; 3:51-8; PMID:21227060; http://dx.doi.org/10.1016/S1078-1439(97)00036-7
- Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008; 113:750-7; PMID:18536030; http://dx.doi.org/10.1002/cncr.23615
- George DJ, Halabi S, Shepard TF, Sanford B, Vogelzang NJ, Small EJ, Kantoff PW. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res 2005; 11:1815-20; PMID:15756004; http://dx.doi.org/10.1158/1078-0432.CCR-04-1560
- Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-α correlate with clinicopathological features and patient survival in patients with prostate cancer. British J Cancer 2004; 90:2312-6; PMID:15150588; http://dx.doi.org/10.1038/sj.bjc.6601814
- Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res 2000; 6:2702-6; PMID:10914713
- Andrews B, Shariat SF, Kim JH, Wheeler TM, Slawin KM, Lerner SP. Preoperative plasma levels of interleukin-6 and its soluble receptor predict disease recurrence and survival of patients with bladder cancer. J Urol 2002; 167:1475-81; PMID:11832773; http://dx.doi.org/10.1016/S0022-5347(05)65348-7
- Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol 2003; 84:151-9; PMID:14598359; http://dx.doi.org/10.1002/jso.10305
- Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J. Cytokine serum levels in soft tissue sarcoma patients: correlations with clinico-pathological features and prognosis. Int J Cancer J Int Du Cancer 2002; 100:463-71; PMID:12115531; http://dx.doi.org/10.1002/ijc.10496
- Hoejberg L, Bastholt L, Johansen JS, Christensen IJ, Gehl J, Schmidt H. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res 2012; 22:287-93; PMID:22617301; http://dx.doi.org/10.1097/CMR.0b013e3283550aa5
- Guida M, Riccobon A, Biasco G, Ravaioli A, Casamassima A, Freschi A, Palma MD, Galligioni E, Nortilli R, Chiarion-Sileni V et al. Basal level and behaviour of cytokines in a randomized outpatient trial comparing chemotherapy and biochemotherapy in metastatic melanoma. Melanoma Res 2006; 16:317-23; PMID:16845327; http://dx.doi.org/10.1097/01.cmr.0000200491.00841.5f
- Mouawad R, Soubrane C, Rixe O, Khayat D, Spano JP. An unexpected inverse correlation between soluble epidermal growth factor receptor and interleukin-6 in metastatic malignant melanoma patients. Melanoma Res 2006; 16:335-40; PMID:16845329
- Tas F, Oguz H, Argon A, Duranyildiz D, Camlica H, Yasasever V, Topuz E. The value of serum levels of IL-6, TNF-α, and erythropoietin in metastatic malignant melanoma: serum IL-6 level is a valuable prognostic factor at least as serum LDH in advanced melanoma. Medical Oncol 2005; 22:241-6; PMID:16110135; http://dx.doi.org/10.1385/MO:22:3:241
- Soubrane C, Rixe O, Meric JB, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res 2005; 15:199-204; PMID:15917702; http://dx.doi.org/10.1097/00008390-200506000-00009
- Casasnovas RO, Mounier N, Brice P, Divine M, Morschhauser F, Gabarre J, Blay JY, Voillat L, Lederlin P, Stamatoullas A et al. Plasma cytokine and soluble receptor signature predicts outcome of patients with classical Hodgkin's lymphoma: a study from the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2007; 25:1732-40; PMID:17389336; http://dx.doi.org/10.1200/JCO.2006.08.1331
- Preti HA, Cabanillas F, Talpaz M, Tucker SL, Seymour JF, Kurzrock R. Prognostic value of serum interleukin-6 in diffuse large-cell lymphoma. Ann Intern Med 1997; 127:186-94; PMID:9245223; http://dx.doi.org/10.7326/0003-4819-127-3-199708010-00002
- Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol 1995; 13:575-82; PMID:7884418
- Giachelia M, Voso MT, Tisi MC, Martini M, Bozzoli V, Massini G, D'Alo F, Larocca LM, Leone G, Hohaus S. Interleukin-6 plasma levels are modulated by a polymorphism in the NF-kappaB1 gene and are associated with outcome following rituximab-combined chemotherapy in diffuse large B-cell non-Hodgkin lymphoma. Leuk Lymphoma 2012; 53:411-6; PMID:21902578; http://dx.doi.org/10.3109/10428194.2011.621566
- Fabre-Guillevin E, Tabrizi R, Coulon V, Monnereau A, Eghbali H, Soubeyran I, Soubeyran P. Aggressive non-Hodgkin's lymphoma: concomitant evaluation of interleukin-2, soluble interleukin-2 receptor, interleukin-4, interleukin-6, interleukin-10 and correlation with outcome. Leuk Lymphoma 2006; 47:603-11; PMID:16690518; http://dx.doi.org/10.1080/10428190500361029
- el-Far M, Fouda M, Yahya R, el-Baz H. Serum IL-10 and IL-6 levels at diagnosis as independent predictors of outcome in non-Hodgkin's lymphoma. J Physiol Biochem 2004; 60:253-8; PMID:15957243; http://dx.doi.org/10.1007/BF03167070
- Niitsu N, Okamato M, Nakamine H, Yoshino T, Tamaru J, Nakamura S, Higashihara M, Hirano M. Simultaneous elevation of the serum concentrations of vascular endothelial growth factor and interleukin-6 as independent predictors of prognosis in aggressive non-Hodgkin's lymphoma. Eur J Haematol 2002; 68:91-100; PMID:12038454; http://dx.doi.org/10.1034/j.1600-0609.2002.01609.x
- Kurzrock R, Redman J, Cabanillas F, Jones D, Rothberg J, Talpaz M. Serum interleukin 6 levels are elevated in lymphoma patients and correlate with survival in advanced Hodgkin's disease and with B symptoms. Cancer Res 1993; 53:2118-22; PMID:8481913
- Inagaki A, Ishida T, Ishii T, Komatsu H, Iida S, Ding J, Yonekura K, Takeuchi S, Takatsuka Y, Utsunomiya A et al. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: high interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int J Cancer 2006; 118:3054-61; PMID:16425276; http://dx.doi.org/10.1002/ijc.21688
- Yamamura M, Yamada Y, Momita S, Kamihira S, Tomonaga M. Circulating interleukin-6 levels are elevated in adult T-cell leukaemia/lymphoma patients and correlate with adverse clinical features and survival. Br J Haematol 1998; 100:129-34; PMID:9450801; http://dx.doi.org/10.1046/j.1365-2141.1998.00538.x
- Egler RA, Burlingame SM, Nuchtern JG, Russell HV. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin Cancer Res 2008; 14:7028-34; PMID:18980999; http://dx.doi.org/10.1158/1078-0432.CCR-07-5017
