ABSTRACT
Multiple non-redundant immunosuppressive pathways are active within the microenvironment of cancers to avoid tumor eradication by the immune system. Our results demonstrate that the CD73-adenosine pathway is a major immunosuppressive mechanism co-opted by ovarian tumors to escape antitumor immunity. In ovarian cancer patients, high CD73 expression correlates with poor outcome and impaired CD8+ T cell immunosurveillance.
As the fifth leading cause of cancer-related death among North American women, ovarian cancer still constitutes an important public health issue with unmet medical needs. High-grade serous ovarian cancer (HGSC) is the most common and lethal histological subtype of epithelial ovarian cancer. Despite a generally good responsiveness to first-line chemotherapy, most of HGSC patients will relapse with a more aggressive disease.
Increasing evidence suggests that HGSC is potentially amenable to immunotherapy.Citation1 In support of this, the accumulation of tumor-infiltrating lymphocytes significantly correlate with increased survival in HGSC patients.Citation1 Additionally, recent early phase clinical trials have reported clinical activity (including complete responses) in some patients with HGSC in response to PD-1/PD-L1 blockade. Notwithstanding some encouraging clinical responses following monotherapy with ICI, the presence of multiple non-redundant immunosuppressive mechanisms will likely contribute to resistance or lack of activity for many patients. Hence, there is a strong rational to simultaneously target multiple immune checkpoints or immune-suppressive pathways to increase clinical response rates in HGSC patients.
Among the multiple immunosuppressive pathways co-opted by the tumor microenvironment, the CD73-adenosine pathway is being increasingly recognized as a promising anticancer target.Citation2 CD73 is a plasma membrane, GPI-anchored ecto-enzyme that catalyzes the production of extracellular adenosine. CD73 is expressed on both hematopoietic and non-hematopoietic cells and its expression is further upregulated in hypoxic microenvironment. Notably, CD73 is expressed/induced on immune-regulatory cells and upregulated on tumor cells in response to oncogenic signals. CD73 has pivotal roles in the regulation of immune reactions through the production of extracellular adenosine.Citation3 Adenosine mediates its immunosuppressive actions through four GPCRs adenosine receptors. In particular, the A2A (ADORA2A) and A2B (ADORA2B) adenosine receptors expressed by immune cells are emerging as key regulators of antitumor immunity.
Building on the initial work of M. Sitkovsky and colleagues, we and others established the proof-of-concept the targeted blockade of CD73 can significantly reduce tumor growth and metastasis in murine breast cancer,Citation4 ovarian cancerCitation5 and various other pre-clinical models.Citation6 Moreover, these studies demonstrated that blocking CD73 or A2A adenosine receptor significantly enhanced the antitumor activity of immune-checkpoint therapies.Citation7-9
In cancer patients, high levels of CD73 expression is increasingly reported to be associated with worse outcome in various types of cancers, including triple-negative breast cancer. Until recently, however, the prognostic value of CD73 in ovarian cancer, and in particular in HGSC, was unclear. In our recent study, we investigated the clinical impact of CD73 mRNA and protein expression in HGSC and assessed whether CD73 expression was associated with altered CD8+ T cell-mediated immunosurveillance.Citation10
First, using the Australian Ovarian Cancer Study dataset and a meta-analysis of 13 independent datasets regrouping more than 1,500 patients, we demonstrated that high levels of CD73 gene expression indeed correlated with poor prognosis (disease-free survival [DFS] and overall survival [OS]) in HGSC. When we investigated CD73 gene expression with regard to the different molecular subtypes of HGSC, we noted that CD73 was preferentially expressed in the C1/mesenchymal subtype, which is characterized by a reactive stromal. Interestingly, despite its inflammatory gene signature, the C1 subtype is associated with a poor prognosis, suggesting the presence of immunosuppressive mechanisms. We hypothesize that CD73-dependent and adenosine-mediated immunosuppression constitutes to immune escape in C1 HGSC.
Using quantitative immunofluorescence of CD73 protein expression, we next validated our gene expression results in an independent cohort of 208 cases of HGSC: high levels of CD73 protein expression on tumor cells also significantly correlated with worse disease-free survival and overall survival. To better understand the role of CD73 expression in ovarian cancer cells, we performed shRNA-mediated knocked down experiments and found that CD73 silencing significantly reduced ovarian cancer cell proliferation and survival, and that this was associated with reduced BCL-XL and BCL-2 expression.
In addition to being expressed in the epithelial compartment, our immunofluorescence analysis revealed that CD73 was often highly expressed in the tumor stroma of HGSC, presumably cancer-associated fibroblasts (CAFs). High CD73 expression on CAFs was also associated with worse prognosis. To investigate whether CD73 expression on CAFs regulated antitumor immunity, we established a murine model of ovarian cancer where ID8 tumor cells were co-injected with mouse fibroblasts (MEFs) derived from either wild-type (WT) or CD73-deficient mice. WT MEFs, expressing high levels of CD73, significantly enhanced ID8 tumorigenesis in immunocompetent mice. Interestingly this effect was abrogated in immunodeficient mice. Co-implantation of WT or CD73-deficient MEFs with ovalbumin-expressing ID8 tumor cells further revealed that CD73 expression on fibroblasts significantly reduced antitumor CD8+ T cells in ID8 tumors.
Finally, given the association between the presence of intratumoral CD8+ T cells and improved overall survival in HGS ovarian cancer, we tested the hypothesis that high levels of CD73 could hinder the prognostic value of intratumoral CD8+ T cells. We co-analyzed CD73 protein expression and CD8+ T cell density, and found that for tumors with high levels of CD73, the presence of CD8+ T cells was no longer associated with improved survival. These correlative observations support the notion that CD73 expression and extracellular adenosine impair the tumoricidal functions of CD8+ T cells in human HGSC.
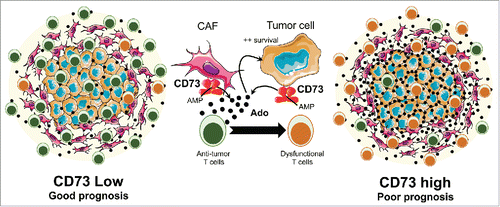
Taken together, our results thus suggest a model whereby CD73 expression on ovarian tumor cells and CAFs drive tumor progression by promoting both cancer cell survival and immune escape (). Our study thus supports previous preclinical studies that the CD73-adensoine pathway is a potential novel therapeutic target for HGSC.
Figure 1. High CD73 expression in high-grade serous ovarian cancers promotes tumor cell survival and immune escape. CD73 expression on both tumor cells and cancer-associated fibroblasts (CAFs) can be induced in the ovarian tumor microenvironment (TME). When CD73 is highly expressed, adenosine accumulates in the TME and mediates immunosuppressive effects on antitumor immune cells, such as CD8+ T cells. Extracellular adenosine also promotes ovarian cancer cell survival in an autocrine or paracrine manner. Hence, HGSC patients with high levels of CD73 in the TME have a worse prognosis and show impaired CD8+ T cell-mediated antitumor immunity. Conversely, patients with low levels of CD73 have a better prognosis associated with functional antitumor CD8+ T cells. Ado: Adenosine. AMP: Adenosine monophosphate.
Disclosure of potential conflict of interest
J. Stagg is a paid consultant, SAB member and owns stocks of Surface Oncology and received sponsored research grants from Medimmune LLC, Palobiofarma, Amorchem and Surface Oncology.
References
- Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009; 4:e6412; PMID:19641607; http://dx.doi.org/10.1371/journal.pone.0006412
- Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin. Ther. Targets 2014; 18:863-81; PMID:24798880; http://dx.doi.org/10.1517/14728222.2014.915315
- Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013; 19:355-67; PMID:23601906; http://dx.doi.org/10.1016/j.molmed.2013.03.005
- Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. 2010; 107:1547-52; PMID:20080644; http://dx.doi.org/10.1073/pnas.0908801107
- Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010; 70:2245-55; PMID:20179192; http://dx.doi.org/10.1158/0008-5472.CAN-09-3109
- Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MWL, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011; 71:2892-900; PMID:21292811; http://dx.doi.org/10.1158/0008-5472.CAN-10-4246
- Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin. Cancer Res. 2013; 19:5626-35; PMID:23983257; http://dx.doi.org/10.1158/1078-0432.CCR-13-0545
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol. Res. 2015; 3:506-17; PMID:25672397; http://dx.doi.org/10.1158/2326-6066.CIR-14-0211
- Mittal D, Young A, Stannard K, Yong M, Teng MWL, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014; 74:3652-8; PMID:24986517; http://dx.doi.org/10.1158/0008-5472.CAN-14-0957
- Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DMA, Haibe-Kains B, Karn T et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015; 75:4494-503; PMID:26363007; http://dx.doi.org/10.1158/0008-5472.CAN-14-3569
