ABSTRACT
NK-cell number and function have been associated with cancer progression. A detailed analysis of phenotypic and functional characteristics of NK-cells in HCC is still lacking. NK-cell function is regulated by activating and inhibitory receptors determined by genetic factors and engagement with cognate ligands on transformed or infected cells. We evaluated phenotypic and functional characteristic of NK-cells in HCC patients undergoing curative treatment in relation to clinical outcome. NK-cells from 70 HCC patients undergoing resection or ablative treatment, 18 healthy volunteers and 12 cirrhotic patients with HCV-infection (controls) were phenotypically characterized. Unsupervised clustering based on the frequency of cells expressing different phenotypic NK-cell markers segregated HCC patients into different cohorts that were compared for outcome. NK-cell cytokine production and cytotoxicity were compared between cohorts with different overall survival (OS) and time to disease recurrence (TTR). By multivariate analysis, age, Child-Pugh class and NK-cell phenotypic clustering could independently identify patients with significantly different OS. NK-cells from patients with better outcome expressed higher levels of cytotoxic granules and CD3ζ and lower levels of natural cytotoxic receptors (NCRs) that were co-expressed with the inhibitory receptor NKG2A known to negatively regulate NCR function. Cytotoxic function and IFNγ production were significantly lower in the cohort of patients with worse outcome compared to controls (p < 0.05). Our results show a role for NK-cells in the control of HCC progression and survival providing the basis for the development of immunotherapeutic strategies to potentiate NK-cell response.
Abbreviations
| BCLC | = | Barcelona Clinic liver cancer |
| HBsAg | = | hepatitis B surface antigen |
| HCC | = | hepatocellular carcinoma |
| HCV | = | hepatitis C virus |
| HLA | = | human leukocyte antigen |
| IFN | = | interferon |
| IL | = | interleukin |
| KIR | = | killer immunoglobulin-like receptor |
| MICA | = | MHC class I chain-related gene A |
| MRI | = | magnetic resonance imaging |
| NCR | = | natural cytotoxicity receptor |
| NK | = | natural killer |
| NKG2A | = | natural-killer group 2, member A |
| NKG2D | = | natural-killer group 2, member D |
| NKp30 | = | natural killer cell p30-related protein |
| NKp46 | = | natural killer cell p46-related protein |
| OS | = | overall survival |
| PBMCs | = | peripheral blood mononuclear cells |
| RTA | = | radiofrequency thermal ablation |
| SR | = | surgical resection |
| TNF | = | tumor necrosis factor |
| TTR | = | time to recurrence |
| ULBP1 | = | UL16 binding protein 1 |
Introduction
Several evidences support the relevance of NK-cell response in tumor development and progression. It has been shown that a high number of NK-cells in the tumor correlates with improved prognosis.Citation1 In most of studies, functionally impaired NK-cells have been isolated from tumors, and their defective function appears to be associated with cancer progression.Citation2 Evidence in HCC showed decreased NK subset in peripheral blood and in tumor compared to non-tumor liver tissue.Citation3,4 In addition, functional defects such as impaired cytokine production and killingCitation3-6 were observed, mainly in NK-cells from patients with advanced stage HCC,Citation4 and were shown to represent a negative prognostic factor after hepatectomy.Citation5 Conversely, accumulation of functional NK-cells in HCC tissues has been shown to predict improved survival of patients.Citation6 However, a detailed analysis of the prognostic correlates of NK-cell phenotype and function in HCC is still lacking.
NK-cell function is the result of the synergistic action of activating and inhibitory receptor signaling. Inhibitory receptor diversity is mostly dependent on the genetic background, whereas activating receptors appear to be more influenced by environmental factors.Citation7 Increased attention has been paid to the inhibitory receptor function and involvement in the process of NK-cell education to self-tolerance and anti-viral and antitumor immune response,Citation7,8 whereas less is known about the regulation of activating NK-cell receptors. The importance of NK-cell receptors in mediating antitumor immune response is further supported by the identification of several mechanisms of immune escape, mainly consisting in the downregulation of activating receptors and their ligands, or alternatively in the upregulation of inhibitory receptor–ligand pairs.Citation9-12 Additional mechanisms of immune evasion have also been described, such as escape from NKG2D-mediated immunosurveillance through shedding of NKG2D ligand MICA.Citation13
Few data are available about the role played by NK receptor expression and signaling in the determination of HCC outcome. Previous reports have shown the involvement of activating NKG2D-mediated signaling in the immune response toward HCC,Citation11,12 and loss of expression of NKG2D ligand ULBP1 upon tumor progression has been related to early recurrence after surgical resection.Citation11 In a recent study, we identified a relationship between better HCC prognosis and KIR2DS5, HLA-C1 and compound KIR2DL2-C1/ KIR3DS1-Bw4T80 genotypes,Citation14 supported by increased cytotoxic capacity of NK-cells from subjects with HLA-C1 alone or combined with KIR2DL2/KIR2DL3. NK-cell receptor signaling is also involved in the functional regulation by the tumor microenvironment. NK-cell inhibition through direct interaction of myeloid derived suppressor cells with the activating receptor NKp30 has been observed in patients with HCC6, and tumor monocytes have been shown to drive sequential activation and exhaustion/apoptosis of NK-cells through CD48/2B4 interaction without involvement of NKp30 nor of NKG2D4.
Persistent infection by hepatitis C virus (HCV) represents one of the main risk factors for HCC and has been shown to impair NK-cell response,Citation15 mainly exerting a suppressive function that might play a role in tumor promotion or progression. The present study was undertaken to investigate the phenotype and function of peripheral blood NK in HCV-linked HCC and their possible prognostic implications. To this aim, we have evaluated a cohort of patients with HCV-related HCC undergoing curative treatments by either surgical resection or RTA.
Results
HCC outcome according to clinical parameters
Seventy consecutive Caucasian patients with diagnosis of HCV-related HCC, 12 patients with HCV-related cirrhosis without HCC and 18 healthy volunteers were included in this study (). Mean age was significantly higher in patients with HCC (p < 0.0001).
Table 1. Demographic and clinical characteristics of patients and controls
HCC patients were allocated to different types of treatment (SR or RTA) according to established clinical criteria. The median OS was 64 mo and the median time to the first HCC recurrence (TTR) was 16.5 mo. Older age at diagnosis, RTA treatment and Child-Pugh class B were related to shorter survival, whereas Child-Pugh class was the only clinical parameter predictive of TTR () at univariate statistical analysis.
Table 2. TTR and OS of HCC patients according to clinical characteristics, NK-cell frequency and phenotype cluster.
NK-cell phenotype and function
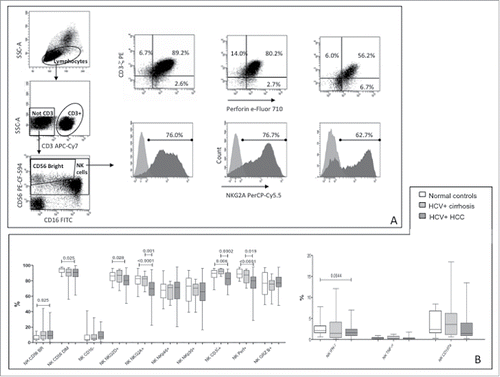
NK-cells from healthy subjects showed lower CD56BR NK-cells content and higher percentage of CD56DIM compared to HCC patients (). No difference was observed compared to cirrhotic patients suggesting that higher levels of CD56BR NK-cells could be related either to HCV infection or to HCC. Frequencies of NK-cells carrying NKG2D and NKG2A receptors were lower in patients with HCC compared to healthy subjects. Significantly lower frequency of cells expressing CD3ζ and perforin were observed in HCC patients, compared to healthy and cirrhotic subjects. NK-cells from patients with HCC exhibited lower capacity to produce IFNγ than those from normal controls, whereas levels of TNF-α and CD107a production were similar to control groups.()
Figure 1. Phenotype and function of NK-cells in patients and controls. (A) Dot plots showing gating strategy and phenotype for CD3ζ, perforin and NKG2A in a representative healthy subject, a patient with HCV-related liver cirrhosis and a patient with HCC (left to right). (B) Differential distribution of NK-cell phenotypes and functional parameters in HCC patients and controls. CD107a expression was evaluated on unstimulated NK-cells. Significance levels are shown on top of each panel.
Unsupervised clustering based on the frequency of cells expressing the different phenotypic NK-cell markers segregated HCC patients into two main cohorts, clusters 1 and 2 (), each one including two main branches, indicated as 1a (22 patients) and 1b (29 patients), 2a (2 patients) and 2b (17 patients). Due to the low number of patients in branch 2a, cluster 2 was considered as a single entity in further statistical analysis. OS and TTR of patients included in the two main clusters 1 and 2 did not differ significantly (data not shown), whereas when branches 1a and 1b were compared to cluster 2, significantly different OS was detected, with close similarity between groups 1b and 2 (, left panels). By contrast, patients included in cluster 1a showed significantly shorter OS (mean: 29.5 mo compared to 78 mo in cluster 1b and 76 mo in cluster 2) and TTR (mean: 11 mo compared to 17 mo in cluster 1b and 28 mo in cluster 2). Significantly different outcomes were therefore observed between patients in cluster 1a and all remaining patients (clusters 1b+2; , right panels).
Figure 2. Hierarchical clustering analysis of 24 NK-cell phenotypic markers in patients with HCV-linked HCC. The heat map represents the frequency of each phenotype (higher expression in red and lower expression in green). Clustering analysis was done after median centering on both the tested markers and patients using Ward's linkage and Euclidean distance.
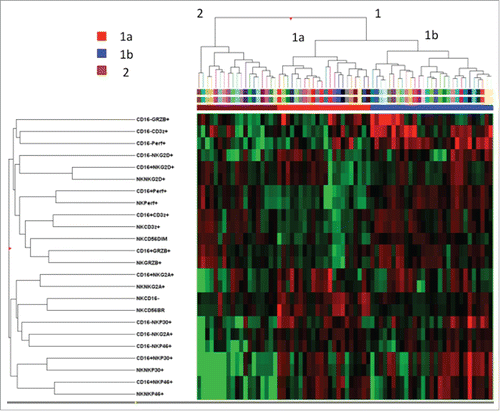
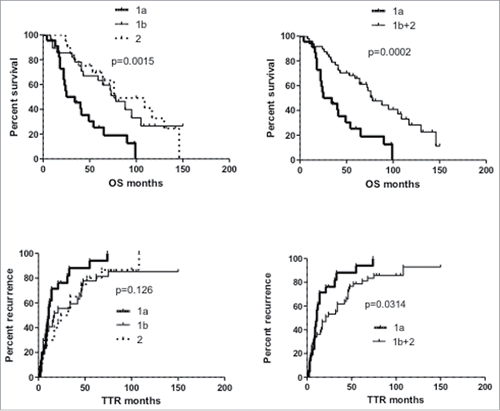
Figure 3. Kaplan–Meier curves of overall survival (OS) and time to recurrence (TTR) of HCC patients according to NK-cell phenotypic clustering. Left panels: comparison of patient clusters 1a, 1b and 2 (); right panels: comparison between patient cluster 1a and clusters 1b+2. Significance values (log-rank test) are shown on top of each panel.
To dissect which combination of phenotypic markers had a significant impact on HCC outcome, the frequency of cells expressing each marker was compared according to cluster grouping (1a vs. all remaining HCC patients; ). Peripheral blood NK-cells from patients with worse outcome (cluster 1a) were characterized by lower frequency of total CD3− CD56+ NK-cells and of CD56DIM NK-cells, with significantly higher prevalence of CD56BR and CD16− cells. Among patients with worse prognosis, expression of CD3ζ and perforin was lower in all NK-cell subsets (total, CD16+ and CD16−), whereas GRZB was less expressed in total and in CD16+, but not in CD16− NK-cells. NKG2A+ and NKp46+ cells were more frequent in all NK-cell subsets from patients with worse outcome, whereas NKp30+ cells were increased in the CD16− subset only.
Table 3. Comparison of NK-cell phenotypic subsets according to hierarchical clustering of HCC patients. Mann–Whitney test. SD: standard deviation; TL: total lymphocytes; BR: bright; NS: not significant
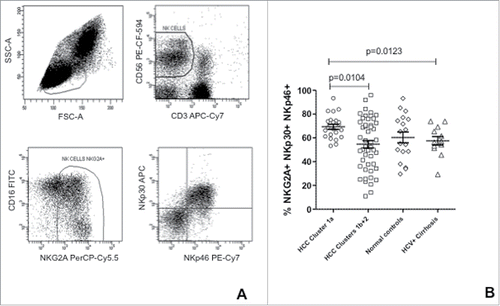
For a better understanding of the receptor expression profile, the rate of activating and inhibitory receptor co-expression was determined in patients and controls. The frequency of NK-cells co-expressing inhibitory NKG2A and activating NKp46-p30 was significantly higher in HCC patients with worse outcome and in healthy subjects compared to the remaining two groups (HCCs with better outcome and patients with HCV-positive liver cirrhosis).()
Figure 4. Co-expression of NKG2A, NKp30 and NKp46 in NK-cells from HCC patients and controls. (A) Strategy for the flow cytometric analysis of receptor co-expression in NK-cells; (B) Frequency of NK-cells co-expressing NKG2A, NKp30 and NKp46 in HCC patients' clusters and in controls. Significance levels are reported.
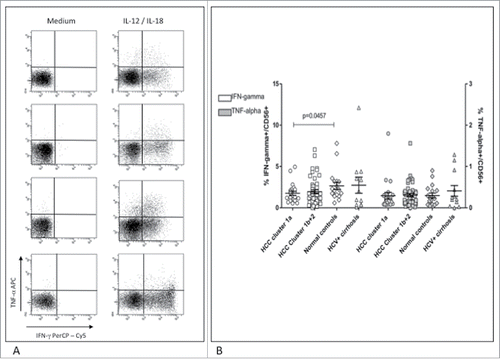
To evaluate the functional correlates of NK-cell phenotypes, we determined CD107a expression in the two subgroups of HCC patients with different prognosis (). After overnight incubation with target cells, NK-cells from patients with worse outcome showed significantly lower CD107a expression compared to normal subjects and to patients with liver cirrhosis. IL-15 was able to restore CD107a expression in NK-cells from this group of HCC patients, but to a lesser extent compared to patients with better clinical outcome (p = 0.055). Cytokine expression analysis showed a significantly lower frequency of IFNγ positive cells in HCC patients with worse prognosis compared to normal controls, whereas TNF-α expression was comparable among all groups (). Cytokine production was further analyzed in a subset of 45 HCC patients (12 in group 1a and 33 in group 1b+2) with and without stimulation with IL-15. Results showed lower levels of TNF-α (not significant) and IFNγ (p = 0.015) in group 1a compared to group 1b+2. Upon IL-15 stimulation differences were not significant any more although maintaining higher cytokine production in group 1b+2 compared to 1a.
Figure 5. Cytotoxic function of NK-cells from HCC patients and controls. (A) Dot plots show upregulation of CD107a in NK-cells after incubation with K562 target cells with and without IL-15 activation in representative patients and controls. Top to bottom: HCC cluster 1a, HCC cluster 1b+2, healthy control, HCV-related cirrhosis. (B) Results are shown with or without overnight activation with IL-15. Percent cytotoxicity was normalized to the frequency of circulating CD3−CD56+ cells. Significance levels are reported.
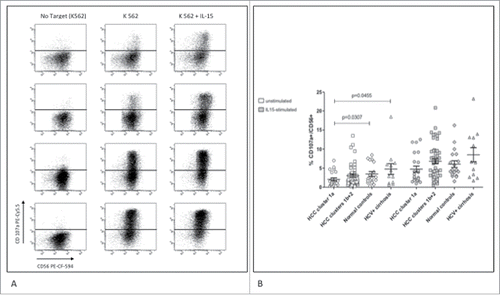
Figure 6. Cytokine production in NK-cells from patients and controls. (A) Dot plots show staining of NK-cells for IFNγ and TNF-α upon IL-12/IL-18 stimulation in representative patients and controls. Top to bottom: HCC cluster 1a, HCC cluster 1b+2, healthy control, HCV-related cirrhosis. (B) Frequency of NK-cells producing IFNγ (left axis, white symbols) and TNF-α (right axis, gray symbols) was normalized to the frequency of circulating CD3−CD56+ cells. Significance levels are reported.
To further clarify the role of NKG2A/NCR co-expression, we correlated this phenotype to NK-cell function. A trend to negative correlation was observed in HCCs with worse outcome, reaching significance only for CD107a expression after IL-15 stimulation. Conversely, in group 1b-2 HCCs, cirrhotic patients and normal controls the NKG2A-p30-p46 co-expression did not negatively affect the NK-cell function (data not shown).
Expression of IL-15 receptor α on NK-cells
To further understand the differences in NK-cell function between patients with different clinical outcome, we performed phenotypic staining for CD122 and CD132 on CD56BR and DIM NK-cells on the same subset of 45 samples. Comparison between groups 1a and 1b+2 showed similar levels of IL-15 receptor (CD122+CD132+) expression in total peripheral NK-cells; however, significantly higher levels (p = 0.02) were observed in CD56BR NK-cells from patients with better outcome. These results might in part explain the lower levels of functionality in NK-cells from group 1a patients.
Multivariate survival analysis
Multivariate survival analysis was carried out by Cox regression on parameters significantly related to OS by univariate statistical analysis: age at diagnosis, Child-Pugh class, type of treatment, clustering of NK phenotypic characteristics. All parameters except the type of treatment were independently associated with OS (). Indeed patients undergoing SR were significantly younger than patients undergoing RTA treatment (p < 0.01, not shown).
Multivariate analysis failed to identify any independent parameter associated with TTR ().
Discussion
The prognostic implication of NK-cell phenotype and function was analyzed in a cohort of HCC patients undergoing curative treatment. At univariate analysis, OS of patients was influenced by age at diagnosis, Child-Pugh score and type of treatment, as already reported,Citation14 but was independent of clinical parameters such as nodule number, size and BCLC, that have been identified as major determinants of HCC outcome,Citation16 and of the frequency of total circulating T and NK-cells. Among clinical parameters, only Child-Pugh score was associated with TTR. Decreased expression of inhibitory receptor NKG2A and of molecules linked to effector function (CD3ζ, perforin) was present in peripheral blood NK-cells from HCC patients. A prevalent CD56BR phenotype, as previously reported,Citation3 and decreased expression of activating receptor NKG2D was also observed in HCC patients, even though the lack of a significant difference compared to cirrhotic patients does not rule out the role of HCV infection as responsible for this phenotype.
Peripheral blood NK-cells from HCC patients also showed decreased of inhibitory receptor NKG2A and of molecules linked to effector function (CD3ζ, perforin). A decreased expression of NKG2DCitation11 and of perforinCitation3 has already been described in HCC. Conversely, low expression of NKG2A is in contrast to previous data generated from patients with advanced disease,Citation3 showing similar levels of expression compared to healthy controls. The decreased expression of NKG2A observed in the present study might be a consequence of downregulation upon interaction with MHC ligand in the context of an exhausted NK-cell phenotype, consistent with the decreased expression of CD3ζ, perforin and NKG2D. The mean age of HCC patients included in the present study was significantly older compared to controls (both normal volunteers and cirrhotic patients with chronic HCV infection). However, this feature is likely unrelated to the differences detected in NK-cell phenotype, since the NK-cell population in the elderly appears to be characterized by a reduction of CD56BR NK-cells, of NKp30 and NKp46 expressionCitation17 with unchanged expression of NKG2A and perforin compared to younger individuals.Citation18 From a functional standpoint, peripheral NK-cells from patients with HCC showed lower IFNγ production, but similar TNF-α and CD107a expression levels compared to controls.
Hierarchical clustering of NK-cell phenotypes identified a subgroup of HCC patients (cluster 1a) with shorter OS and TTR. HCC patients with worse outcome were characterized by lower total number of NK-cells and specifically of the CD56DIM subset, with parallel increase of CD56BR NK-cells, and by lower expression of effector molecules (CD3ζ, perforin and GRZB). CD3ζ is responsible for the signal transduction after ligand binding to NKp30 and NKp46, which lack functional intracellular signaling domain. Diminished CD3ζ expression or function has been observed in T and NK-cells from patients with infections, auto-immune diseases and cancer.Citation19,20 The loss of CD3ζ function is, therefore, a likely cause of reduced NKp46-p30 signaling capacity and a potential predictor of poor prognosis and survival,Citation20 consistent with our observation of significantly decreased CD3ζ expression in HCC patients with worse outcome.
The higher frequency of NKG2A+ NK-cells might be another clue to explain the worse prognosis of HCC patients in cluster 1a, since increased expression of NKG2A and of its ligand HLA-E is a common mechanism of NK-cell dysfunction in solid tumors.Citation10,21 HLA-E expression has been observed in hepatocytes and appears to be increased in human HCC by Granulin–epithelin precursor, a hepatic oncofetal protein overexpressed in HCC.Citation22 By contrast, the enrichment of NKp46- and NKp30-positive NK-cells in HCCs with worse prognosis is in apparent contradiction with the activating function of these receptors, even though the above mentioned low expression of CD3ζ may reflect poor or absent receptor signaling. The higher percentage of NKG2A, NKp30 and NKp46 co-expressing cells that we observed in the subgroup of HCCs with shorter survival may also be implicated, due to the ability of NKG2A co-engagement to block NKp46-mediated cytotoxicity and NKp30-induced IFNγ production that was previously reported in uterine decidual NK-cells during early pregnancy.Citation23 In agreement with this observation, a negative correlation was observed between this phenotypic subset and NK-cell function only in HCCs with worse prognosis. Consistent with the observation of NKp46 editing by its unknown ligand in a mouse model,Citation24 the high levels of NKp46 might also reflect very low or absent expression of specific ligands at the tumor cell surface. Indeed NK-cell cytotoxic function, apparently conserved in patients with HCC, was significantly reduced in the subgroup of patients with worse prognosis but could be restored after treatment of NK-cells with IL-15, although to a lesser extent compared to HCCs with a better outcome. In a similar fashion, IL-15 could partially restore cytokine production. These observations not only provides a background to the different clinical prognosis of HCC patient clusters, but gives indication that the NK-cell function may potentially be restored in vivo.
IL-15 receptor (CD122+CD132+) expression was reduced on CD56BR NK-cells of patients with worse outcome, suggesting an in vivo defect in activation and differentiation toward an effector phenotype playing a role in cancer control.
To identify independent predictors of HCC outcome, clinical and phenotypic parameters significantly related to OS were evaluated by Cox regression. Age at diagnosis, Child-Pugh class and phenotypic clustering were identified as independently related to OS, while the type of treatment was likely excluded since it was significantly associated with patients' age, which is one of the most important clinical discriminants for treatment decision. By contrast, the median age of patients included in the different phenotypic clusters was similar (data not shown). Even though both NK-cell phenotype and Child score were associated with outcome at univariate analysis, no independent variable significantly related to TTR was identified. It is difficult to provide a clear explanation for this finding; however, we may speculate that NK-cell phenotype may have influenced not only HCC progression but also deterioration of liver function because of the underlying viral disease, which might in turn affect NK-cell function. Alternatively, other yet unknown variables affecting both NK-cell phenotype and liver function might represent independent parameter(s) influencing TTR.
Altogether the results of the present study support the prognostic relevance of NK-cell phenotype and function in HCC. Combined analysis of NK phenotypes by hierarchical clustering allowed the identification of a patient subset with worse TTR and OS, characterized by a predominant CD56BR NK-cell phenotype and lower expression of effector molecules. The functional correlate of this NK-cell phenotype consisted of reduced cytotoxic capacity and cytokine secretion that could be restored by IL-15 treatment, further providing a rational background for therapeutic interventions aimed at recovering NK-cell antitumor responses.
Materials and methods
We evaluated 70 consecutive Caucasian HCC patients with HCV-related liver disease that underwent treatment by either surgical resection or radiofrequency thermal ablation (RTA) at the University Hospital of Parma (Parma, Italy). HCC diagnosis had been made by ultrasonography and computed tomography or magnetic resonance imaging (MRI) in selected cases. The type of treatment was decided on the basis of liver function (Child-Pugh score), comorbidities, age and location of HCC nodules. Patients with HCC were evaluated by a multidisciplinary group to decide treatment allocation based on liver function, portal hypertension, number, site and size of hepatocellular carcinoma lesions. Peripheral mononuclear cells were obtained 24 h before treatment.
Hepatitis B surface antigen (HBsAg) and anti-human immunodeficiency virus were negative in all cases. All patients had the same postoperative follow-up based on bidimensional and contrast-enhanced ultrasonography every 4 mo and dynamic CT or contrast-enhanced MRI in any case of appearance of new liver nodules more than 1 cm in diameter or arterial enhancement at the site of previous ablation. Twelve patients with HCV-related liver cirrhosis and 18 healthy volunteers were also included in the study as control groups. The demographic and clinicopathologic features of patients and healthy subjects are shown in .
The study was approved by the local ethical committee (Comitato Etico Indipendente of the Azienda Ospedaliero-Universitaria of Parma, Parma, Italy). All participants gave written informed consent to participate in the study.
Phenotypic analysis of NK-cells
Peripheral blood mononuclear cells (PBMCs) were resuspended in RPMI-1640 containing 8% human serum (4 × 105 /tube) and stained with monoclonal antibodies specific for CD3-APC/Cy7, CD16-FITC, CD56-PE-CF594, NKG2A (CD94)-PerCP/Cy5.5, Granzyme-B-AlexaFluor647 (BD Biosciences-PharMingen), NKG2D-PE, CD3ζ-PE, Perforin-eFluor710 (eBioscience), NKp30-APC (Miltenyi Biotec), NKp46-PE-Cy7, CD122-PE and CD132-APC (Biolegend). For detection of intracellular antigens, cells were fixed and permeabilized with Fix and Perm reagents (Caltag) in accordance with manufacturer's instructions.
Analysis was performed on a FACSCanto II flow cytometer (BD Biosciences) by using the FACSDiva Software. Frequency of CD56DIM and CD56BR NK-cells was defined for each patient evaluating different fluorescence intensity of CD3−CD56+cells. NK-cell phenotypes were analyzed on total NK, CD16+ and CD16− subsets.
Functional analysis of NK-cells
The cytotoxic capacity was assessed by incubating PBMCs with K562 target cells and measuring the modification of CD107a expression on the effector cells. After overnight incubation with or without 1 ng/mL IL-15 (Sigma), PBMCs were co-cultured with K562 target cells in the presence of CD107a-PE/Cy5.5 antibody for 3 h at 37°C, then labeled with anti-CD3-APC/Cy7, CD16-FITC and CD56-PE-CF594 antibodies (BD Biosciences-PharMingen) and analyzed by flow cytometry.
The capacity of producing cytokines was tested on NK-cells after overnight incubation with or without IL-12 (10 ng/mL) and IL-18 (10 ng/mL) in the presence of 10 μg/mL Brefeldin A (BFA) added during the last 3 h of culture. In a subgroup of 45 HCC patients, PBMCs were pre-incubated overnight with or without IL-15 (1 ng/mL) and then placed in contact with the K562 target cells for the last 3 h in the presence of Brefeldin A in order to measure cytokine production also by this experimental approach.
After overnight incubation cells were stained with anti-CD3-APC/Cy7, CD16-FITC and CD56-PE-CF594 antibodies, fixed and permeabilized as described above, stained with IFNγ-PerCP/Cy5 and TNF-α-APC (Biolegend) antibodies and analyzed by flow cytometry. All functional parameters (IFNγ and TNF-α production, CD107a expression) were normalized to the frequency of circulating CD3− CD56+ NK-cells.
Statistical analysis
The differences between groups of continuous variables were analyzed by t-test, Mann–Whitney or Wicoxon test, as appropriate. Survival curves were estimated by the Kaplan–Meier method and compared by log-rank test. Cox proportional hazards regression model was conducted for multivariate survival analysis. Only variables significantly associated with survival in the univariate analysis were included in the multivariate model. Prism (Graph Pad) and StatPlus (AnalystSoft Inc.) statistical softwares were used for analysis. p < 0.05 (two tailed) was considered significant.
Unsupervised hierarchical clustering was performed by the Genespring GX 12.6 statistical software (Agilent Technologies), after a median baseline transformation of all the samples and using Ward's linkage and Euclidean distance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from Fondazione CARIPARMA (Parma, Italy) and by the grant RBAP10TPXK from Fondo per gli Investimenti della Ricerca di Base (Ministry of Education, University and Research, Italy).
References
- Larsen SK, Gao Y, Basse PH. NK-cells in the tumor microenvironment. Crit Rev Oncog 2014; 19:91-105; PMID:24941376; http://dx.doi.org/10.1615/CritRevOncog.2014011142
- Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun 2011; 3:355-64; PMID:21502747; http://dx.doi.org/10.1159/000325465
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H et al. Functional impairment in circulating and intrahepatic NK-cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129:428-37; PMID:18824414; http://dx.doi.org/10.1016/j.clim.2008.08.012
- Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57:1107-16; PMID:23225218; http://dx.doi.org/10.1002/hep.26192
- Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer 1998; 83:58-63; PMID:9655293; http://dx.doi.org/10.1002/(SICI)1097-0142(19980701)83:1%3c58::AID-CNCR8%3e3.0.CO;2-A
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799-807; PMID:19551844; http://dx.doi.org/10.1002/hep.23054
- Marras F, Bozzano F, Ascierto ML, De Maria A. Baseline and dynamic expression of activating NK-cell receptors in the control of chronic viral infections: the paradigm of HIV-1 and HCV. Front Immunol 2014; 5:305; PMID:25071766; http://dx.doi.org/10.3389/fimmu.2014.00305
- Cariani E, Missale G. KIR/HLA immunogenetic background influences the evolution of hepatocellular carcinoma. Oncoimmunol 2013; 2:e26622; PMID:24501686; http://dx.doi.org/10.4161/onci.26622
- Peng YP, Zhu Y, Zhang JJ, Xu ZK, Qian ZY, Dai CC, Jiang KR, Wu JL, Gao WT, Li Q et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med 2013; 11:262; PMID:24138752; http://dx.doi.org/10.1186/1479-5876-11-262
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK-cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/10.1172/JCI45816
- Kamimura H, Yamagiwa S, Tsuchiya A, Takamura M, Matsuda Y, Ohkoshi S, Inoue M, Wakai T, Shirai Y, Nomoto M et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J Hepatol 2012; 56:381-8; PMID:21756848; http://dx.doi.org/10.1016/j.jhep.2011.06.017
- Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43:1013-20; PMID:16168521; http://dx.doi.org/10.1016/j.jhep.2005.05.026
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impari expression of NKG2D and T-cell activation. Nature 2002; 419:734-8; PMID:12384702; http://dx.doi.org/10.1038/nature01112
- Cariani E, Pilli M, Zerbini A, Rota C, Olivani A, Zanelli P, Zanetti A, Trenti T, Ferrari C, Missale G. HLA and killer immunoglobulin-like receptor genes as outcome predictors of hepatitis C virus-related hepatocellular carcinoma. Clin Cancer Res 2013; 19:5465-73; PMID:23938290; http://dx.doi.org/10.1158/1078-0432.CCR-13-0986
- Saha B, Szabo G. Innate immune cell networking in hepatitis C virus infection. J Leukoc Biol 2014; 96:757-66; PMID:25001860; http://dx.doi.org/10.1189/jlb.4MR0314-141R
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19:329-38; PMID:10518312; http://dx.doi.org/10.1055/s-2007-1007122
- Solana R, Campos C, Pera A, Tarazona R. Shaping of NK-cell subsets by aging. Curr Opin Immunol 2014; 29:56-61; PMID:24792889; http://dx.doi.org/10.1016/j.coi.2014.04.002
- Campos C, Pera A, Sanchez-Correa B, Alonso C, Lopez-Fernandez I, Morgado S, Tarazona R, Solana R. Effect of age and CMV on NK-cell subpopulations. Exp Gerontol 2014; 54:130-7; PMID:24440462; http://dx.doi.org/10.1016/j.exger.2014.01.008
- Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 2004; 4:675-87; PMID:15343367; http://dx.doi.org/10.1038/nri1434
- Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother 2004; 53:865-78; PMID:15118842; http://dx.doi.org/10.1007/s00262-004-0521-0
- Bossard C, Bézieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F, Mosnier JF. HLA-E/β2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int J Cancer 2012; 131:855-63; PMID:21953582; http://dx.doi.org/10.1002/ijc.26453
- Cheung PF, Yip CW, Wong NC, Fong DY, Ng LW, Wan AM, Wong CK, Cheung TT, Ng IO, Poon RT et al. Granulin-epithelin precursor renders hepatocellular carcinoma cells resistant to natural killer cytotoxicity. Cancer Immunol Res 2014; 2:1209-19; PMID:25315249; http://dx.doi.org/10.1158/2326-6066.CIR-14-0096
- El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O, Clouet-Delannoy M, Lombardelli L, Jabrane-Ferrat N, Rukavina D et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK-cells in early pregnancy. J Immunol 2008; 181:3009-17; PMID:18713971; http://dx.doi.org/10.4049/jimmunol.181.5.3009
- Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G, Mandelboim O. Tumor immunoediting by NKp46. J Immunol 2010; 184:5637-44; PMID:20404273; http://dx.doi.org/10.4049/jimmunol.0901644
