ABSTRACT
Purpose: To determine feasibility and explore the clinical efficacy of concurrent radiotherapy and carboplatin as adjuvant treatment of triple negative breast cancer (TNBC).
Patients and Methods: Women with Stage I-II TNBC were treated after surgery in a phase I–II prospective trial [NCT01289353]. Weekly carboplatin (AUC = 2.0) was delivered for 6 weeks. Concurrent radiotherapy was delivered in the prone position during weeks 2–4, for a total dose of 40.5 Gy in 15 fractions to the breast, and 46.5 Gy in 17 fractions to the tumor bed. Adverse events (AE) were assessed weekly during treatment, once at 45–60 d, and every 6 mo thereafter, using the Common Terminology Criteria for AE (CTCAE) v3.0.
Results: A total of 39 patients accrued and 36 received treatment. Eight patients (22%, exact 95% CI: 10%, 39%) developed grade 2 or greater acute radiation dermatitis. Overall, grade 2 AE were seen in nine and grade 3 in two patients. Twenty-three patients (64%) received additional adjuvant chemotherapy. With a median follow-up of 48 mo, 34/36 (94%) are alive and disease free. One patient died of pulmonary failure with possible but unproven breast cancer recurrence, and one patient died of pelvic malignancy. One patient recurred locally and is alive and disease free after surgical management. Brisk lymphocytic infiltrate was present pre-treatment in 39% of 18 patients with evaluable tumor.
Conclusions: Adjuvant concurrent carboplatin and prone accelerated radiotherapy is a well-tolerated and promising treatment of early stage TNBC. The observed 3% compares favorably with the expected 30% recurrence rate within 1–4 y from treatment, warranting further studies.
Abbreviations
| AE | = | adverse events |
| BC | = | breast cancer |
| CTCAE | = | Common Terminology Criteria for AE |
| DAMP | = | damage-associated molecular pattern |
| ICD | = | immunogenic cell death |
| LPBC | = | lymphocyte-predominant BC |
| pCR | = | pathological complete response |
| PDL-1 | = | programmed death ligand-1 |
| pPR | = | pathological partial response |
| TILs | = | tumor-infiltrating lymphocytes |
| TLR | = | toll-like receptor |
| TNBC | = | triple negative breast cancer |
Introduction
The contribution of standard cancer therapies such as chemotherapy and radiotherapy to the induction of a type of cell death that is sensed by the immune system as immunogenic was originally described in a series of reports from Laurence Zitvogel and Guido Kroemer's laboratories.Citation1-5 Their work identified three critical molecular signals that were required to induce an immunogenic cell death (ICD): translocation of calreticulin to the cell membrane to deliver an “eat me” signal,Citation2 release of HMGB-1, a damage-associated molecular pattern (DAMP) that binds to toll-like receptor-4 (TLR4) to promote cross-presentation of tumor-derived antigens,Citation4 and Adenosine triphosphate (ATP) released by dying cells that binds to P2RX7 purinergic receptor leading to inflammasome activation and IL-1β production.Citation5
We developed an in vitro assay to expedite the testing of different combinations of chemotherapy and radiotherapy, to select those that best achieve ICD. The assay confirmed the dose-dependent effect of radiotherapy, for each of the three component of ICD. In addition, the combination of carboplatin and radiation was found to be a potent inducer of ICD.Citation6 This finding led to the hypothesis that in vivo carboplatin and concurrent radiotherapy may trigger an adaptive antitumor immune response. This background inspired the design of a phase I–II clinical trial (NCT01289353) to test feasibility and explore clinical efficacy of this combination in the adjuvant setting of triple negative breast cancer (TNBC).
The rationale for combining radiation with concurrent carboplatin was also based on the specific vulnerability of TNBC. Several studies postulated that BRCA gene inactivation might also have a role in sporadic TNBCs and have defined as “BRCA-ness” the multiplicity of DNA repair deficiencies associated with these tumors.Citation7-9 Repair deficiencies of TNBC can be exploited by treatment with platinum salts, cisplatin, or carboplatin.Citation10-12 Platinum agents cause covalent crosslinks within the DNA double helix and interfere with the progression of the replication fork. In a setting of defective BRCA1 and BRCA2, breast cancer cells either attempt to repair the damaged DNA by mechanisms like non-homologous end joining further acquiring genomic instability, or fail to repair the DNA damage caused by platinum agents, resulting in programmed cell death.Citation9 An analogous vulnerability to ionizing radiation among BRCA mutation carriers and in tumors with BRCA-ness has been established.Citation12,13
In other tumor types than breast cancer, concurrent chemo-radiation with a platinum compound and radiotherapy has demonstrated efficacy and superiority to a sequential approach.Citation14,15 In this study, we used the prone accelerated radiotherapy regimen, delivered over 3 weeks that we have extensively tested in the adjuvant setting of breast cancer,1617 and slightly modify it to isolate the tumor bed boost on two Sundays during the 3 weeks, to reduce the risk of skin toxicity when radiation was used with a powerful radio-sensitizer such as carboplatin.
Moreover, because of the promising clinical results at 48 mo of median follow-up, we quantified tumor-infiltrating lymphocytes (TILs) in patients who had available material from surgical specimens. TILs are a parameter that has been shown to have prognostic value in TNBC patients treated with adjuvant anthracycline-based chemotherapy.Citation18,19 Interestingly, in the neo-adjuvant GeparSixto trial, patients with TN and HER-2+ tumors with >60% stromal TILs had 3.71-fold increase in the odds of pathological complete response (pCR) if they had received carboplatin.Citation20 These results support the hypothesis that carboplatin has a significant interaction with the immune system.
The results of NCT01289353 are reported. The trial demonstrates the optimal feasibility of the combination and preliminary efficacy at a median follow-up of 4 y, confirming the translation to the clinic of preclinical predictions.
Results
Patient characteristics
Thirty-nine patients signed an informed consent to participate to the trial. Three patients elected to withdraw consent before initiation of any treatment, and 36 patients completed the trial as designed and were evaluable. Among these, 36 patients 61% were older than 50 (age median 55.5 y, range 27–82 y), 44.3% had tumors larger than 2 cm in diameter, and 16% were node positive ().
Table 1. Baseline patient characteristics (n = 36).
Feasibility
Only two patients (5.5%) had grade 3 acute AE (one wet desquamation and one pain), demonstrating the feasibility of the concurrent administration of carboplatin and radiation used.
Safety
The primary end point of the study was the occurrence of grade 2 or greater acute carbo-radiation toxicity. Eight patients (22%, exact 95% CI: 10%, 39%) developed grade 2 or greater acute radiation dermatitis (). Grade 2–3 pain occurred in two patients (5.6%) and grade 2 fatigue in one patient (2.8%). All acute toxicities resolved within 60 d from inception of combined chemoradiation. Although the trial end point was limited to acute toxicity, late effects of the combined carboplatin and radiation were acceptable, without grade 3–4 toxicity detected at last follow-up.
Table 2. Maximum grade acute carboplatin-radiation AE (n = 36).
Efficacy
At a median follow-up of 4 y, one patient has died from fulminant pulmonary failure and thrombotic angiopathy 5.6 mo from trial completion. As there was no biopsy or autopsy-proven tumor, a breast cancer recurrence could not be excluded. A second patient developed an in-breast recurrence, contiguous to the original tumor bed, 27 mo after treatment. She underwent salvage mastectomy and is alive without evidence 47 mo from diagnosis. One patient succumbed to disseminated pelvic malignancy more than 2 y after the completion of treatment. The remainder 33 patients are alive and have remained disease free ().
Analysis of the tumor lymphocytic infiltrate and PDL-1 expression
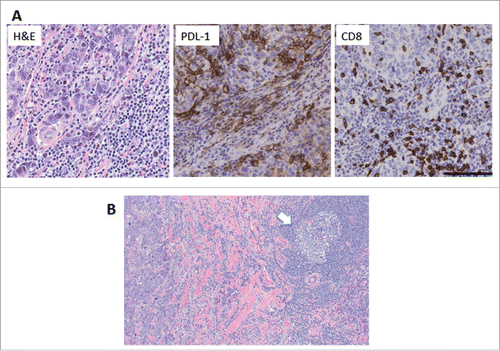
Eighteen patients had original tumor slides available for evaluation. No significant differences were observed with respect to baseline and clinical characteristics between patients with available tumor slides and patients without available slides (Table S1). Total 7/18 tumors showed >60% stromal TILs, fulfilling the definition of lymphocyte-predominant BC (LPBC)Citation20 ( and ). One patient had tertiary lymphoid structures (TLS) adjacent to the tumor, a feature associated with improved outcomeCitation21 (). Among the non-LPBC, 8/18 had >10% TILs, indicating that most patients had favorable prognostic characteristic.Citation18,19 Interestingly, the only patient with a proven recurrence had low TILs ().
Table 3. TILs infiltration and PDL-1 expression (n = 18).
For 11 of the 18 cases, sufficient tissue was available for the evaluation of programmed death ligand-1 (PDL-1) expression in the tumor. PDL-1 is a ligand for the immune-checkpoint receptor programmed death-1 (PD-1) expressed on T cells and represents a major mechanisms of immune escape and a therapeutic target in many tumors including breast cancer.Citation22,23 PDL-1 was expressed at significant levels in most of the tumors with high TILs ( and ), consistent with the phenomenon of induced resistance whereby cancer cells upregulate PDL-1 in response to IFNγ produced by the infiltrating antitumor T cells.Citation24
The neutrophil-to-lymphocyte ratio (NLR) was analyzed for this cohort. None of the 36 patients had a baseline ratio >4: The NLR mean value was 2.19 (range 0.93–3.76). A mixed effects longitudinal analysis of neutrophil and lymphocyte levels in peripheral blood over time was also conducted for all patients. Neutrophil levels decreased significantly during treatment (Pre–During: −0.90 with 95% CI: −1.4, −0.42) and from pre-treatment to post-treatment (−1.27 with 95% CI: −1.78, −0.76) (Table S2). Similarly, lymphocyte levels decreased significantly during treatment (Pre–During: −0.54 with 95% CI: −0.69, −0.42) and from pre-treatment to post-treatment (−0.50 with 95% CI: −0.66, −0.34) (Table S3). Furthermore, longitudinal analyses were conducted to assess changes in the NLR over time. The ratio increased during treatment (Pre–During: 0.15 with 95% CI: −0.11, 0.41) and decreases from pre-treatment to post-treatment (−0.19 with 95% CI: −0.47, 0.09). Both of these changes were not significant (Table S4).
Additional systemic chemotherapy
A total of 23 of 36 patients (64%) received some additional systemic chemotherapy, after concurrent carboplatin and radiation. Specifically, 13 patients (36%) underwent dose dense ACT (four cycles of doxorubicin and cyclophosphamide followed by paclitaxel every 2 weeks), and four patients underwent CMF (cyclophosphamide, methotrexate, and 5fluorouracil, every 3 weeks for eight cycles), three patients received docetaxel and cyclophosphamide every 3 weeks for four cycles, one patient received AC (doxorubicin and cyclophosphamide for four cycles), one patient received nab-paclitaxel for five cycles, and one patient received liposomal doxorubicin for four cycles.
Discussion
TNBC characterizes an aggressive group of breast cancers with a high risk of distant recurrence and death after initial surgical treatment. In a seminal paper that describes the natural history of the different subtypes of breast cancer, Dent et al. demonstrated the increased risk of death from disease among TNBC patients (42.2%) in the period between 1 and 4 y from diagnosis when compared to hormone receptors-positive and/or HER2-positive breast cancer, and a sharp decline of the recurrence rate of TNBC after 5 y of follow-up.Citation25 At a median follow-up of 4 y, only two patients (5.3%) in the current study have died, one without confirmed recurrent disease and another with a metastatic pelvic malignancy. Only one patient developed an isolated in breast recurrence, contiguous to the original tumor bed, and she is alive and free of disease after surgical management of the recurrent tumor. The meaning of a local recurrence in TNBC is different from that of other breast cancer subtypes. In the original series from Dent et al., a local recurrence heralded distal recurrence in 25% of TNBC carriers versus 44% of the patients with the other subtypes of breast cancer (p = 0.02).Citation25
Inclusion of radiotherapy to the breast in the management of TNBC who had undergone breast conservation was associated with statistically significant increase in survival in a retrospective analysis of 249 patients from Washington University.Citation26 The adjuvant regimen of concurrent carboplatin and radiotherapy tested in this study was chosen to translate to the clinic the preclinical findings of increased ICD of the combination of carboplatin and ionizing radiation.Citation6 The concurrent administration of carboplatin and accelerated radiotherapy was well tolerated with no increase in acute radiation dermatitis above the baseline expected from radiation alone, and only 2/36 (5.6%) patients with any grade 3 AE, demonstrating feasibility and safety of this treatment.
Previous experience in the neo-adjuvant setting of locally advanced breast cancer both with continuous infusion 5FluoruracilCitation27 and with twice weekly paclitaxelCitation28 supports the benefit of the use of concurrent chemo-radiation for TNBC. The latter combination was tested at three institutions, USC, NYU, and Vanderbilt in 105 patients with locally advanced breast cancer treated with paclitaxel (30 mg/m2 intravenously twice a week) for 10–12 weeksCitation28 with daily breast and nodal radiotherapy during weeks 2–7. Pathological response (pCR and pPR) after neoadjuvant chemo-radiation was achieved in 36/105 patients (34%, 95% CI: 25–44%). TNBC patients had a 54.2% pathological response rate. Patients with pathologic response had a lower risk of recurrence or death compared with non-responders (hazard ratio = 0.35, 95% CI: 0.15–0.80, log-rank p-value = 0.01).Citation29 In a companion study conducted in the same population to identify molecular markers of pathologic response to neoadjuvant paclitaxel/radiation treatment by protein and gene expression profiling performed on pretreatment biopsies, a significant enrichment in immune-related gene was found in patients with pCR.Citation30
Thus, based on this clinical experience and growing experimental evidence that the therapeutic success of cytotoxic treatments relies on their ability to induce antitumor immune responses,Citation31 we originally hypothesized that the success of concurrent chemo-radiation may derive from the induction of ICD by the combination.Citation32,33 The adjuvant setting of TNBC offered the opportunity for testing this approach in a clinical situation of minimal tumor burden, ideal for the success of an immune response, and in a disease with generally shorter median time to recurrence.
The role of TILs as predictors of outcome in TNBC has been demonstrated in several adjuvant and neo-adjuvant studies.Citation18-20,34 In this study, we were able to retrieve the original tumor specimen and evaluate TILs in only half of the patients. This analysis showed that in 39% of the patients tested, the tumor was associated with >60% TIL infiltrate. This type of TIL-rich breast cancer has been designated as LPBC.Citation34 In a study in which 28.3% of 314 TNBC and 19.9% of 266 HER2+ tumors were LPBC, addition of carboplatin to neoadjuvant chemotherapy increased the odds of pCR 3.71-fold in LPBC, but only 1.01-fold in non-LPBC, suggesting a strong interaction of carboplatin with the immune system.Citation20 Because of the generalized good outcome of our cohort of patients at the current median follow-up of 4 y and the fact that in only half we could analyze the pretreatment tumor tissue, it is impossible to demonstrate whether with the degree of TILs infiltration correlates with outcome. Interestingly, the only patient in this series who recurred locally had low TIL infiltrate in the original segmental mastectomy specimen.
A recent re-classification of TNBC by Lehmann et al. has demonstrated the heterogeneity of this subset of breast cancer and introduced opportunities to identify distinct and specific therapeutic targets. Gene expression of 587 TNBC cases from 21 breast cancer data sets was conducted. Based on distinct gene ontology, six subtypes were identified and, pre-clinically, this genetic classification informed selection of therapeutic modalities.Citation35 No genetic analysis of the original tumor was conducted in the current study, but it is possible that the cohort of patients that accrued to this trial was enriched for the Basal-like group. This group encompasses the BL1 and BL2 subtypes that are enriched in the activation cell cycle and cell division and the DNA damage response pathways. Future studies should characterize TNBC to diversify its therapeutic approach and should further test the role of concurrent platinum compounds with radiation for the basal-like group.
In conclusion, this Phase I–II study of concurrent carboplatin and radiotherapy in the adjuvant setting of TNBC resulted in optimal tolerance and lack of significant alopecia with an excellent outcome at a median follow-up of 4 y, despite the fact that 36% of the patients in this series did not receive any additional adjuvant chemotherapy. It is intriguing to hypothesize that in a subset of TNBC a brief regimen of chemo-radiation may be recruiting an adaptive immune response producing a long-lasting immunological equilibrium and impacting disease-free survival. Testing this combination in a pre-surgical setting could enable a better understanding of the effects on the tumor and elucidate the role of different TNBC subtypes in the response to carboplatin and radiation.
Patients and methods
Patient characteristics
Patients with newly diagnosed Stage I–II (pT1-T2, pN0, and N1) TNBC were eligible to be enrolled into this clinical study, if they had refused or were not prescribed standard adjuvant chemotherapy (dose-dense ACT or TC), after breast cancer surgery (segmental mastectomy or mastectomy). Triple-negative status was defined at the time of study entry as estrogen and progesterone receptor expression in less than 1% of the cancer cells, according to the American Society of Clinical Oncology (ASCO)/College of American pathologists (CAP) guidelinesCitation36 and HER2-negative at HercepTest [Dako] score 0 or 1+ or the gene amplification ratio <2·2 by in situ hybridization. The study was approved by NYU Institutional Review Board and was conducted in compliance with the Declaration of Helsinki. Clinical trial registration number is NCT01289353. Written informed consent was obtained from all patients.
Study design
This was a phase I–II single institution study. Weekly carboplatin (AUC = 2.0) was delivered for 6 weeks, with RT to the whole breast concurrently delivered during weeks 2–4, via a 3D-CRT or IMRT technique, in the prone position, as previously reported.Citation16,17 A total dose to the breast of 40.5 Gy was delivered in 15 fractions (Monday–Friday). The tumor bed received a boost of two additional fractions of 3 Gy, delivered on two consecutive Sundays (before the 2nd and 3rd weeks of RT), for a total dose of 46.5 Gy to the tumor bed in 17 fractions over 19 d (). Additional adjuvant chemotherapy was administered at the discretion of the treating medical oncologist.
Figure 1. Treatment schema. The trial consisted of 6 weeks of weekly carboplatin (AUC = 2) delivered with concurrent breast radiotherapy (top panel), during week 2–4, as detailed in the bottom panel. Additional adjuvant chemotherapy was administered at the discretion of the treating medical oncologist, for the patients who accepted additional treatment.
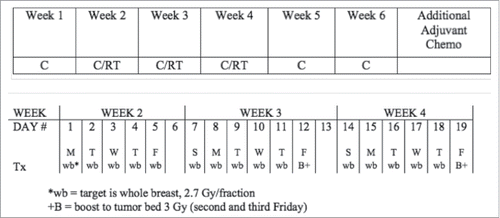
Figure 2. Kaplan–Meier plots of progression-free survival and disease-free survival (n = 36) of patients with TNBC treated with adjuvant concurrent carboplatin and accelerated radiotherapy.
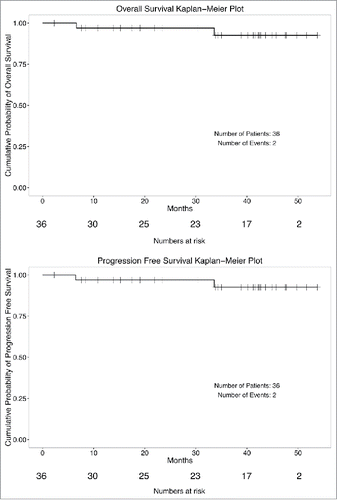
Figure 3. TILs, PDL-1 expression, CD8+ distribution and TLS in the tumor of patient ID#32. (A) Example of tumor with 80% stromal TILs, as assessed on H&E sections. The tumor showed strong expression of PDL-1 on the cancer cells and infiltrating immune cells, and CD8+ T cells infiltrating tumor cell nests. Magnification 200X, bar = 100 μm. (B) TLS (arrow) was present at the periphery of the tumor. Magnification 100X.
Feasibility, safety, and efficacy
This trial was designed to test the feasibility of the combined regimen, defined as <10% Grade 3 acute AE assessed within 60 d from the initiation of concurrent carboplatin and radiation. Exploratory endpoints were local and systemic recurrence. Safety of the regimen was defined as grade 2 or greater acute radiation dermatitis. AE were assessed weekly during treatment, and once at 45–60 d, using the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Patients were followed up every 6 mo after completion of treatment of local and systemic recurrence.
Evaluation of tumor-infiltrating lymphocytes (TILs), and Programmed cell death 1 ligand 1 (PDL-1) expression
TIL evaluation was performed on a single full-face hematoxylin and eosin (H&E)-stained section, which was available from 18 of the 36 patients who completed treatment. The percentage of stromal TILs was estimated by a pathologist with experience in this methodology (SD) following the consensus guidelines published by the TIL working group.Citation37 Results are reported as described previouslyCitation19 with small modifications, in increments of 10 for values of 10% or above; <1% is considered 0, and values >1 but <10% were rounded up to 5%. Patients with tumors with >60% stromal TILs were defined as LPBC.Citation20
Tumor tissue was analyzed for PD-L1 expression by immunohistochemistry (IHC), performed on formalin-fixed, paraffin-embedded, 4-µm tissue sections using unconjugated, rabbit anti-human PDL-1 (CD274) clone SP142 (PDL1, Spring Biosciences catalog number M4420).Citation38 Staining was performed on a Ventana Medical Systems Discovery XT instrument with online deparaffinization and using Ventana's reagents and detection kits. PDL1 was antigen retrieved in Ventana Cell Conditioner 1 (Tris-Borate-EDTA) for 20 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 4 min. PDL1 was diluted 1:50 in phosphate buffered saline and incubated for 60 min at 37 °C. Primary antibody was detected with hapten linked, anti-rabbit multimer incubated for 20 min followed by anti-hapten horseradish peroxidase conjugate for 20 min. The complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate. A tissue microarray containing placental tissue was used as positive control. PDL-1 expression was quantified as a percentage of tumor cells with positive membrane staining and intensity classified on a scale of 1–3. In some samples, CD8+ T cell infiltration was assessed by staining with rabbit anti-human CD8+ clone SP57 (Ventana Medical Systems catalog number 790–4460).
Statistical analysis
The primary objective of this trial was to estimate the proportion of patients with grade 2 or greater acute radiation dermatitis. A difference of +/− 18% could be detected (from a baseline rate of 25% of patients with grade 2 or greater acute radiation dermatitis) with a two-sided α = 0.05 and power of 80% using an exact binomial test. If 15 or more events among these 37 patients were observed, the null hypothesis that the rate is 25% was to be rejected (Calculations from PASS 2008, NCSS).
Patient demographic and disease characteristics are summarized using frequency distributions for categorical variables and summary statistics and graphical displays for quantitative variables, including TILs and PDL-1 expression. To examine the changes in circulating leukocytes over time, we used a mixed effects longitudinal regression models for neutrophils (N) and lymphocytes (L) separately, and the N/L ratio in which we considered both the within patient and between patient variability.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Authors SCF, YN, JDG, and SD contributed to conception and design; SCF, SD, JDG, and YN to the development of methodology; SCF, YN, MFK, JT, SD, SC, and JS to acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.); SCF, EBG, SD, YN, XL, and JDG to analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis); SCF, SD, YN, XL, and JDG to writing, review, and/or revision of the manuscript; and SCF, SC, MFK, and XL to administrative, technical, or material support (i.e., reporting or organizing data, constructing databases).
KONI_A_1274479-s02.docx
Download MS Word (100.1 KB)Acknowledgment
The authors are grateful to Luis Chriboga for his expert help with IHC.
Funding
The NYU Experimental Pathology Immunohistochemistry Core Laboratory is supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH/NCI P30CA016087, and the National Institutes of Health S10 Grants; NIH/ORIP S10OD01058 and S10OD018338.
References
- Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol 2010; 22:113-24; PMID:20403709; http://dx.doi.org/10.1016/j.smim.2010.03.001
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:17187072; http://dx.doi.org/10.1038/nm1523
- Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14:1848-50; PMID:17657249; http://dx.doi.org/10.1038/sj.cdd.4402201
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID:17704786; http://dx.doi.org/10.1038/nm1622
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170-8; PMID:19767732; http://dx.doi.org/10.1038/nm.2028
- Golden EB, Frances D, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. OncoImmunology 2014; 3:e28518; PMID:25071979; http://dx.doi.org/10.4161/onci.28518
- Turner N, Tutt A, Ashworth A. A Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 2004; 4:814-9; PMID:15510162; http://dx.doi.org/10.1038/nrc1457
- Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 2007; 26:2126-32; PMID:17016441; http://dx.doi.org/10.1038/sj.onc.1210014
- Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer 2016; 16:110-20; PMID:26775620; http://dx.doi.org/10.1038/nrc.2015.21
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst 2004; 96:1659-68; PMID:15547178; http://dx.doi.org/10.1093/jnci/djh312
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434:917-21; PMID:15829967; http://dx.doi.org/10.1038/nature03445
- Moynahan ME, Chiu JW, Koller BH, Jasin M. BRCA1 controls homology-directed DNA repair. Mol Cell 1999; 4:511-8; PMID:10549283; http://dx.doi.org/10.1016/S1097-2765(00)80202-6
- Formenti SC, Preston-Martin S, Haffty BG. BRCA1/2 germline mutations: a marker for radioresistance or radiosensitivity? J Clin Oncol 2000; 18:1159-60; PMID:10694571; http://dx.doi.org/10.1200/jco.2000.18.5.1159
- Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:2181-90; PMID:20351327; http://dx.doi.org/10.1200/JCO.2009.26.2543
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, Williams CJ. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001; 358:781-6; PMID:11564482; http://dx.doi.org/10.1016/S0140-6736(01)05965-7
- Formenti SC, DeWyngaert JK, Jozsef G, Goldberg JD. Prone vs supine positioning for breast cancer radiotherapy. JAMA 2012; 308:861-3; PMID:22948692; http://dx.doi.org/10.1001/2012.jama.10759
- Osa EO, DeWyngaert K, Roses D, Speyer J, Guth A, Axelrod D, Fenton Kerimian M, Goldberg JD, Formenti SC. Prone breast intensity modulated radiation therapy: 5-year results. Int J Radiat Oncol Biol Phys 2014; 89:899-906; PMID:24867535; http://dx.doi.org/10.1016/j.ijrobp.2014.03.036
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31:860-7; PMID:23341518; http://dx.doi.org/10.1200/JCO.2011.41.0902
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ et al. Prognostic value of tumor-infiltrating lymphocytes (TILs) in Triple Negative Breast Cancers (TNBC) from two Phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J CLin Oncol 2014; 32:2959-66; PMID:25071121; http://dx.doi.org/10.1200/JCO.2013.55.0491
- Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015; 33:983-91; PMID:25534375; http://dx.doi.org/10.1200/JCO.2014.58.1967
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873-92; PMID:23778140; http://dx.doi.org/10.1172/JCI67428
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450-61; PMID:25858804; http://dx.doi.org/10.1016/j.ccell.2015.03.001
- Emens, LA, Braiteh, FS, Cassier, P, DeLord, J, Eder, JP, Shen, X, Xiao, Y, Wang, Y, Hedge, PS, Chen, D et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res 2014; 75: Abs PD1-6; http://dx.doi.org/10.1158/1538-7445.SABCS14-PD1-6
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13:4429-34; PMID:17671126; http://dx.doi.org/10.1158/1078-0432.CCR-06-3045
- Steward LT, Gao F, Taylor MA, Margenthaler JA. Impact of radiation therapy on survival in patients with triple-negative breast cancer. Oncol Lett 2014; 7:548-52; PMID:24396485; http://dx.doi.org/10.3892/ol.2013.1700
- Formenti SC, Dunnington G, Uzieli B, Lenz H, Keren-Rosenberg S, Silberman H, Spicer D, Denk M, Leichman G, Groshen S et al. Original p53 status predicts for pathological response in locally advanced breast cancer patients treated pre-operatively with continuous infusion 5-fluorouracil and radiation therapy. Int J Radiation Oncology Biol Phys 1997; 39:1059-68; PMID:9392545; http://dx.doi.org/10.1016/S0360-3016(97)00506-3
- Formenti SC, Volm M, Skinner KA, Spicer D, Cohen D, Perez E, Bettini AC, Groshen S, Gee C, Florentine B et al. Preoperative twice-weekly paclitaxel with concurrent radiation therapy followed by surgery and postoperative doxorubicin-based chemotherapy in locally advanced breast cancer: a phase I/II tria. J Clin Oncol 2003; 21:864-70; PMID:12610186; http://dx.doi.org/10.1200/JCO.2003.06.132
- Adams S, Chakravarthy AB, Donach M, Spicer D, Lymberis S, Singh B, Bauer JA, Hochman T, Goldberg JD, Muggia F et al. Preoperative concurrent paclitaxel-radiation in locally advanced breast cancer: pathologic response correlates with five-year overall survival. Breast Cancer Res Treat 2010; 124:723-32; PMID:20878462; http://dx.doi.org/10.1007/s10549-010-1181-8
- Bauer JA, Chakravarthy AB, Rosenbluth JM, Mi D, Seeley EH, De Matos Granja-Ingram N, Olivares MG, Kelley MC, Mayer IA, Meszoely IM et al. Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation. Clin Cancer Res 2010; 16:681-90; PMID:20068102; http://dx.doi.org/10.1158/1078-0432.CCR-09-1091
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008; 118:1991-2001; PMID:18523649; http://dx.doi.org/10.1172/JCI35180
- Formenti SC, Demaria S. Effects of chemoradiation on tumor-host interactions: the immunologic side. J Clin Oncol 2008; 26:1562-3; PMID:18349411; http://dx.doi.org/10.1200/JCO.2007.15.5499
- Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res 2008; 10:215; PMID:19014406; http://dx.doi.org/10.1186/bcr2160
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. [Erratum appears in J Clin Oncol. 2010 Feb 1;28(4):708]. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/10.1200/JCO.2009.23.7370
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121:2750-67; PMID:21633166; http://dx.doi.org/10.1172/JCI45014
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28:2784-95; PMID:20404251; http://dx.doi.org/10.1200/JCO.2009.25.6529
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/10.1093/annonc/mdu450
- Bianchini G, Pusztai L, Pienkowski T, Im YH, Bianchi GV, Tseng LM, Liu MC, Lluch A, Galeota E, Magazzù D et al. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol 2015; 26:2429-36; PMID:26387142; http://doi.org/10.1093/annonc/mdv395
