ABSTRACT
Lessons learned over decades on the use of gene and cell therapies have found clinical applicability in the field of cancer immunotherapy. On December 16th, 2016 a symposium was held in Pamplona (Spain) to analyze and discuss the critical points for the clinical success of adoptive cell transfer strategies in cancer immunotherapy. Cellular immunotherapy is being currently exploited for the development of new cancer vaccines using ex vivo manipulated dendritic cells or to enhance the number of effector cells, transferring reinvigorated NK cells or T cells. In this meeting report, we summarize the main topics covered and provide an overview of the field of cellular immunotherapy.
Introduction
Novel findings in cancer immunology are being translated to the clinic at an unprecedented pace. Therefore, it is necessary to create new educational instruments to train and build networks between researchers and clinicians. On December 16th, 2016 a symposium was held in Pamplona (Spain) as a preamble to the closing ceremony of the second edition of the University degree in Immuno-Oncology organized by the University of Navarra. This course trained 60 oncologists and hematologists in the advances in cancer immunology/immunotherapy and was taught by leading experts in the field.
Renzo Canneta (Former Vice President Global R&D Oncology Policy, Bristol-Myers Squibb Company) gave a lecture in the closing ceremony providing his personal clinical-development views on the key milestones that have resulted in the cancer immunotherapy revolution with the development of monoclonal antibodies (mAbs) against CTLA-4 and PD-1.Citation1,2 In his view, the next step will be the combination of different immunotherapy strategies, a paradigm that has been materialized in the first approval of a combined treatment with ipilimumab and nivolumab for metastatic melanoma.Citation3 This revolution has been possible due to the contributions of different stakeholders and collaboration rather than competition is needed to materialize complex immunotherapy strategies such as cellular immunotherapies as routine clinical practice.
Understanding and exploiting cytotoxicity
Most cancer immunotherapies rely on the induction of cytotoxic cells that recognize and kill tumor cells. The first talk was devoted to further mechanistically understand this critical process. Salvatore Valitutti (University of Toulouse, Toulouse, France) focused his lecture on the lytic synapse formed by cytotoxic T lymphocytes (CTLs) and target cells illustrating the power of live microscopy techniques to unravel the mechanisms of heterotopic interactions in immunity. He showed that lytic granule secretion is independent of CTL polarization against target cellsCitation4 and that tumor cells can exhibit resistance to CTL killing at the immune synapse. His group has published evidence that melanoma cells need longer interaction times with CTLs and multiple interactions with CTLs to be lysed.Citation5,6 This resistance mechanism involves two interesting phenomena: less intake of granzyme B into the cytoplasm of tumor cells and the ability of melanoma cells to polarize late endosomal lysosomal (LLE) vesicles toward interacting CTLs. Moreover, this group's experimentation showed that the proteases in these vesicles were able to degrade the perforin directionally secreted by CTLs. This is the first evidence that proteases such as cathepsins released from LLE vesicles are able to degrade proteins at the lytic synapse. These proteins could be cytolytic proteins but also proteins involved in cell-to-cell adhesion.Citation7 This immune-escape mechanism originally identified in melanoma cells might also be present other tumor types. Indeed, target cells expressing high CD63 as a surrogate marker for a high content of LLE vesicles exhibit more pronounced resistance to CTL killing. Therefore, overcoming this resistance mechanism can potentially be a new target to enhance the efficacy of immunotherapies based on CTL activity.
Induction of immunogenic cell death (ICD) is a way to induce T-lymphocyte-mediated cytotoxicity in cancer immunotherapy. Lionel Apetoh (INSERM, Dijon, France) presented new data providing a rational for the combination of ICD-promoting chemotherapy agents with mAb blocking the PD-1/PD-L1 pathway. ICD is characterized by the presence of calreticulin (CRT) on the plasma membrane and the release of ATP and HMGB1 by the dying tumor cells. Thus, those drugs that induce ICD contribute to the activation of antitumor immune responses.Citation8 However, Apetoh showed that tumor treatment in a colon cancer mouse model with a combination of 5-FU and oxaliplatin, that promotes ICD, induced PD-L1 expression on the surface of cancer cells. IFNγ produced by CD8+ T lymphocytes infiltrating the tumors was the driver molecule in the upregulation of PD-L1. Moreover, such ICD-inducing chemotherapy favors the presence of PD1+Tim3+ CD8+ T lymphocytes in the tumor microenvironment. This population of tumor-infiltrating lymphocytes (TILs) is able to produce IFNγ and on the cell surface expresses CD107a, a well-known marker of degranulation, at early time points following ICD-inducing chemotherapy. Importantly, this population becomes dysfunctional after 12 d. The combination of ICD-inducing chemotherapies and the blockade of PD-L1 or PD-1 by mAbs restored the production of IFNγ by TILs and enhanced the overall antitumor effect.
Advances toward a new generation of cancer vaccines
Jolanda de Vries (Radboud University Medical Center, Nijmegen, The Netherlands) introduced one of the most important applications of cellular therapies against cancer: antitumor vaccines based on dendritic cells (DCs). DC-based vaccines aim at priming tumor-specific T cells but clinical research in recent years has highlighted important limitations that must be overcome by the next generation of such vaccines. For instance, the limited migration to lymph nodes of DCs injected subcutaneously can be bypassed by direct injection into the lymph nodes.Citation9 To make the most of coming generations of DC vaccines, we must consider the nature of the antigens carried by the DC (shared antigens, neoantigens, embryonic antigens, viral antigens, etc) and the functional subsets of DCs. DCs can be generated by culturing bone marrow progenitors present in blood, they may be derived from purified monocytes or they can be directly isolated from the blood of patients. Jolanda de Vries focused on this last approach. By taking advantage of immunomagnetic selection, her group has performed clinical trials with two naturally occurring DC populations: plasmacytoid DCs and conventional myeloid DCs. A clinical trial with plasmacytoid DCs showed some clinical benefit for advanced melanoma patients. Plasmacytoid DCs were matured with commercially available tick-borne encephalitis virus vaccine (FSME) and as early as 4 h following intranodal injection, IFN type I mRNA was detected in peripheral blood of these pDC-vaccinated patients. Furthermore, CD8+ T cell responses against the antigen loaded on pDCs (gp100) were observed,Citation10 both in peripheral blood as well as in T cell cultures from DTH skin challenged sites.
In a subsequent clinical trial, myeloid DCs were purified based on their BDCA-1 marker. DCs were cultured with GM-CSF and loaded with gp100 peptides. In a minority of the melanoma patients, specific CD8+ T cell responses were observed, correlating with increased progression-free survival (PFS), and objective clinical responses.Citation11 Interestingly, it was observed that the presence of CD1c+CD14+ cells in the vaccine impaired effectiveness. This double positive population corresponded to a monocyte/macrophage-like cell that expressed high levels of PD-L1 and impaired T-cell proliferation in vitro in an antigen-specific manner. Interestingly, higher percentages of these cells were present in advanced melanoma patients than in patients in earlier stages of the disease.Citation12 Overall, Jolanda de Vries' data showed that vaccination with circulating DCs is feasible. Future directions have to include cooperative vaccination with distinct DC subsets to simultaneously promote CD4+ and CD8+ T cell responses and will include BDCA-3 DC that are reported to be specialized in cross-priming.Citation13
Making the most of adoptive cell therapies for cancer
Adoptive T-cell transfer was pioneered in its clinical development using TILs for the treatment of metastatic melanoma by the group of Steve Rosenberg. While TIL-based therapy continues to undergo improvements, the field has been revolutionized by the advent of lymphocytes transfected with transgenic T cell receptors (TCRs) and with chimeric antigen receptors (CARs). Several talks in this meeting were devoted to understanding the recent advances in the field of adoptive transfer of T lymphocytes. Several lines of evidence suggest that TIL-based therapy can be more successful if the infused lymphocytes recognize tumor-specific mutant antigens (neoantigens). Alena Gros (Surgery Branch, National Cancer Institute (NCI), National Institutes of Health, Bethesda, Maryland, USA) presented a non-invasive strategy to identify and isolate neoantigen-reactive T cells. This group had demonstrated that CD8+ and PD-1+ identify patient-specific tumor-reactive T cells, including those recognizing neoantigens in cancer melanoma patients.Citation14 In an attempt to develop T cell-based therapies specifically targeting neoantigens, they described a novel personalized screening approach to identify neoantigen-specific lymphocytes in peripheral blood. Tumor-reactive T cells were prospectively identified in circulating CD8+PD1+ lymphocyte subsets in three out of the four melanoma patients evaluated. The methodology involves identification of neoantigens by performing tumor whole exome sequencing, and co-culture of circulating in vitro—expanded CD8+PD1+ cells with autologous DCs transfected with tandem minigene (TMG) constructs containing mutated gene sequences. Subsequently, the TMG-reactive T cells were enriched by selecting activated CD137+ cells and co-culturing with DCs pulsed with each of the mutated 25-mers peptides encoded by the corresponding TMG. Tumor reactivity was demonstrated by autologous tumor recognition of the selected peripheral T cells and by gene-engineered lymphocytes expressing their cloned neoantigen-specific TCRs.Citation15
Troels Holz Borch, from the laboratory of Inge Marie Svane (Herlev University Hospital, Herlev, Denmark) provided an overview of the European experience in cancer treatment with TILs. Adoptive cell transfer of autologous TILs in patients with metastatic melanoma has shown promising responses, resulting in prolonged survivals. However, such clinical success is being overshadowed by the complexity of TIL production and the severe treatment-associated toxicities. Preconditioning by standard lymphodepleting chemotherapy before TIL infusion and IL-2 administration after cell transfer have been shown to induce higher persistence and proliferation of the infused cells.Citation16,17 Both interventions are currently necessary for the achievement of durable clinical responses but are the principal causes of the treatment-related toxicities.
Borch showed data from a phase I/II trial of adoptive cell transfer based on TIL infusion before lymphodepleting chemotherapy and followed by a reduced regimen of IL-2 administration in patients with advanced melanoma. Treatment resulted in long-lasting clinical responses with a persistence of tumor-reactive lymphocytes (<2%) over one year, mild treatment-related toxicities and an objective response rate of 42%. However, only 24 out of the initial 33 patients were eligible for treatment with ex vivo cultured TILs.Citation18 In addition, it was suggested that treatment with an anti-CTLA4 antibody or other checkpoint inhibitors before adoptive transfer of TILs might be beneficial to increase tumor infiltration and efficacy. This important issue needs to be addressed in the future.Citation19
Ongoing research from the Danish group intends to improve TIL efficacy. Low-dose IFNγ pretreatment of the tumor cells is proposed to selectively enhance TIL responses to tumor-associated antigens by means of increasing the density of peptide-MHC complexes.Citation20 A second strategy to improve TIL efficacy is based on the transient transfection of TILs with CXCR2 mRNA to improve TILs migration toward tumor-secreted chemokines, since infiltration into tumors seems to be a limiting factor,.Citation21
Patient-specific minor histocompatibility antigens, possibly expressed by the tumor and its associated stroma, remain an interesting target. Nevertheless, treatment of solid tumors with non-myeloablative allogeneic hematopoietic stem cell transplantation (Allo-HSCT) in combination with adoptive T-cell therapy has not achieved in solid tumors the same success as in hematologic neoplasias. Alloreactivity of T cells from donors has been the cause of the toxicity and graft versus host disease (GVHD) effects found upon treatment with such therapy.Citation22 Anna Mondino (San Raffaele Scientific Institute, Italy) focused on avoiding this fatal outcome while potentiating the graft-vs.-tumor (GVT) effect, which is the principal cause of Allo-HSCT success.Citation22 In her experiments, male mice bearing a spontaneous prostate adenocarcinoma model (TRAMP) were immunized with the tumor-specific vaccine (DCs pulsed with the tumor-specific peptide SV40-Tag), following Allo-HSCT and T lymphocyte infusion derived from syngeneic female donors.Citation23,24 The HY-mismatch permits the recognition by donor T-cells of minor histocompatibility antigens (H), which are encoded in the Y chromosome of the recipient mice. Such H-specific T cell responses were instrumental in promoting the recognition of the tumor associated SV-40 T large antigen (Tag) in transgenic TRAMP mice and to prompt tumor-specific lymphocytes to reject tumors and prevent relapse.Citation23 Importantly, this allogenic T cell transfer strategy in combination with a tumor vaccine potentiated the generation of IFNγ-producing lymphocytes specific for both, H- and SV40-T antigens. This was observed not only in lymphoid tissue but also in the tumor microenvironment.Citation23,24 As a logical next step, autologous T cells were TCR-gene engineered to accomplish similar functions. Mondino's team transduced TRAMP-derived lymphocytes with a retrovirus encoding a TCRs specific for a SV-40 large T antigen and a HY antigen-derived peptide (Uty). TRAMP mice were thus preconditioned and transplanted with T-depleted autologous HSCT, and treated with adoptive T-cell transfer based on autologous Tag- and Uty-specific T lymphocytes followed by a tumor vaccination.Citation25 In this setting, only the combination of TCR-transduced T lymphocytes led to antitumor synergistic effects. In these phenomena, TNFα was identified to be a key mediator. Inspired by these findings, the authors showed that the infusion of the TNFα derivate (NGR-TNFα) mimicked the antitumor role of the H-specific T lymphocyte and greatly improved efficacy of T cells redirected to tumor antigens.Citation26 Thus, dual tumor/tumor-stroma targeting strategies might reveal themselves to be useful to enhance the efficacy of ACT against solid tumors.
Adoptive transfer of NK cells
Antonio Perez-Martinez (Hospital Universitario La Paz, Madrid, Spain) focused his presentation on the ongoing efforts to improve NK cell-based cancer treatments. According to the missing-self hypothesis, virally infected or malignant cells decrease MHC class I expression to avoid antigen presentation. NK cells are suppressed by autologous MHC class I molecules and release more efficiently the cytotoxic granules when failing to detect MHC class I. NK cytotoxic mechanisms are triggered by the expression of stress-associated molecules, which are activatory ligands for NK receptors. These changes in target cell phenotype tilt the balance between inhibition and activation of NK cells toward activation and target killing. Missing self-recognition is thereby exploited in allogenic bone-marrow transplantation. The KIR mismatch between adoptive-transferred haploidentical NK cells and donors' transformed cells induces a graft-vs.-leukemia effect capable of eradicating the disease in some instances. For these techniques, NK cells need to be expanded ex vivo and activated with cytokines and feeder cells.Citation27 Adoptive transfer of NK cells can be enhanced by combination with substances augmenting NK cell activation and cytotoxicity, such as TLR ligands, IL-15, or copolymers like Arabinoxylan.Citation28,29
Hematologic malignancies are highly sensitive to NK cell-based therapies. Antonio Perez-Martinez presented data demonstrating that most leukemia cell lines and primary pediatric leukemia cells express NK receptor ligands at different levels. In particular, acute myeloid leukemia and acute lymphocytic leukemia blasts express variable levels of HLA-I and ULBP4 (NKG2D ligand). Acute lymphocytic leukemia blasts are those expressing the highest levels thus explaining their susceptibility to NK cytotoxicity.Citation30,31 In addition to its role in cytotoxicity, NKG2D and its ligands seem to play a relevant role also in osteosarcoma, promoting tumorigenicity, and sarcosphere formation.Citation32
Due to the increasing importance of NKG2D in the tumor, Antonio Perez-Martinez presented in vitro and in vivo data on an innovative chimeric NKG2D receptor, encompassing a cytoplasmic CD137 activatory domain and CD3ζ chain. The introduction of the chimeric NKG2D receptor into memory T cells represents a new strategy to overcome osteosarcoma relapse.
Conclusions and future perspectives
mAbs targeting checkpoint inhibitors, such as CTLA-4 and PD-1/PD-L1 have demonstrated that immunotherapy is an effective alternative for cancer treatment. Novel antibody formats and antibody combinations hold promise for the future of cancer treatment. In parallel, cellular immunotherapies are being developed and are seeking their niche for the treatment of different indications (). Several clinical trials are ongoing or planned to identify the best DC subset and the best manufacturing procedures to boost tumor-specific immune responses. Adoptive transfer of T-cells is achieving in some instances excellent clinical success in spite of the technological complexities and serious toxicities. Transfer of TILs is being improved with different strategies, while genetic manipulation of the lymphocytes to express transgenic TCRs or CARs is soon expected to receive the first regulatory approvals. The lessons learned with T lymphocytes are being transferred to the adoptive transfer of NK cells and new waves of DC-based cancer vaccines are in the making. In conclusion, cellular immunotherapies are real candidates as techniques to expand our therapeutic arsenal in the near future.
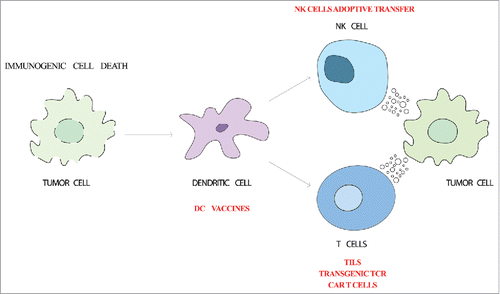
Figure 1. Cellular immunotherapy strategies. Cancer immune response starts with the release of tumor-associated antigens and danger signals by dying tumor cells (immunogenic cell death). Dendritic cells capture tumor-associated antigens and mature due to the detection of danger signals by pattern-recognition receptors. Upon migration to the lymph node, dendritic cells are able to trigger the effector antitumor response. Cytotoxic T lymphocytes and NK cells recognize and kill the tumor cells. Adoptive transfer of dendritic cells, T cells or NK cells are being used to boost this process.
Disclosure of potential conflicts of interest
I.M. has participated in advisory boards serving Roche-Genentech, Bristol-Myers Squibb, Incyte, Medimmune, Novartis, Alligator Biosciences, Bioncotech, and LeadArtis. The rest of the authors have no conflict of interest to declare.
Acknowledgments
We are in debt to Belen Palencia, Esther Guirado, and Cibeles Pinto for excellent meeting logistics.
Funding
This work was supported by Foundation for Applied Medical Research (FIMA), BBVA foundation, AIRC, Asociación Española contra el Cancer (AECC), Red Temática de Investigación Cooperativa en Cáncer (RD12/0036/0040 and RD12/0036/0062), Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional (FEDER, PI14/01686, PI13/00207, PI16/00668), FAECC and H2020 PROCROP project, under grant agreement 635122. P.B. was supported by a Miguel Servet and Miguel Servet II (CPII15/00004) contract from Instituto de Salud Carlos III. The international meeting was supported by grants from Miltenyi Biotec and Bristol-Myers Squibb.
References
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID: 20525992; https://doi.org/10.1056/NEJMoa1003466
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30; PMID: 25399552; https://doi.org/10.1056/NEJMoa1412082
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID: 23724867; https://doi.org/10.1056/NEJMoa1302369
- Bertrand F, Muller S, Roh KH, Laurent C, Dupre L, Valitutti S. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci USA 2013; 110:6073-8; PMID: 23536289; https://doi.org/10.1073/pnas.1218640110
- Vasconcelos Z, Muller S, Guipouy D, Yu W, Christophe C, Gadat S, Valitutti S, Dupré L. Individual human cytotoxic T Lymphocytes exhibit intraclonal heterogeneity during sustained killing. Cell Rep 2015; 11:1474-85; PMID: 26027932; https://doi.org/10.1016/j.celrep.2015.05.002
- Halle S, Keyser KA, Stahl FR, Busche A, Marquardt A, Zheng X, Galla M, Heissmeyer V, Heller K, Boelter J et al. In vivo killing capacity of cytotoxic T Cells is limited and involves dynamic interactions and T Cell cooperativity. Immunity 2016; 44:233-45; PMID: 26872694; https://doi.org/10.1016/j.immuni.2016.01.010
- Petit AE, Demotte N, Scheid B, Wildmann C, Bigirimana R, Gordon-Alonso M, Carrasco J, Valitutti S, Godelaine D, van der Bruggen P. A major secretory defect of tumour-infiltrating T lymphocytes due to galectin impairing LFA-1-mediated synapse completion. Nat Commun 2016; 7:12242; PMID: 27447355; https://doi.org/10.1038/ncomms12242
- Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014; 3:e955691; PMID: 25941621; https://doi.org/10.4161/21624011.2014.955691
- Aarntzen EH, Srinivas M, Bonetto F, Cruz LJ, Verdijk P, Schreibelt G, van de Rakt M, Lesterhuis WJ, van Riel M, Punt CJ et al. Targeting of 111In-labeled dendritic cell human vaccines improved by reducing number of cells. Clin Cancer Res 2013; 19:1525-33; PMID: 23382117; https://doi.org/10.1158/1078-0432.CCR-12-1879
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013; 73:1063-75; PMID: 23345163; https://doi.org/10.1158/0008-5472.CAN-12-2583
- Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res 2016; 22:2155-66; PMID: 26712687; https://doi.org/10.1158/1078-0432.CCR-15-2205
- Bakdash G, Buschow SI, Gorris MA, Halilovic A, Hato SV, Skold AE, Schreibelt G, Sittig SP, Torensma R, Duiveman-de Boer T et al. Expansion of a BDCA1+CD14+ Myeloid cell population in melanoma patients may attenuate the efficacy of dendritic cell vaccines. Cancer Res 2016; 76:4332-46; PMID: 27325645; https://doi.org/10.1158/0008-5472.CAN-15-1695
- Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207:1247-60; PMID: 20479116; https://doi.org/10.1084/jem.20092140
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-59; PMID: 24667641; https://doi.org/10.1172/JCI73639
- Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 2016; 22:433-8; PMID: 26901407; https://doi.org/10.1038/nm.4051
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988; 319:1676-80; PMID: 3264384; https://doi.org/10.1056/NEJM198812223192527
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233-9; PMID: 18809613; https://doi.org/10.1200/JCO.2008.16.5449
- Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Hölmich LR, Hendel HW, Met Ö, Andersen MH et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 2016; 22:3734-45; PMID: 27006492; https://doi.org/10.1158/1078-0432.CCR-15-1879
- Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, Kubi A, Shoshani N, Zikich D, Ohayon Y et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: Intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013; 19:4792-800; PMID: 23690483; https://doi.org/10.1158/1078-0432.CCR-13-0380
- Donia M, Hansen M, Sendrup SL, Iversen TZ, Ellebaek E, Andersen MH, Straten Pt, Svane IM. Methods to improve adoptive T-cell therapy for melanoma: IFN-gamma enhances anticancer responses of cell products for infusion. J Invest Dermatol 2013; 133:545-52; PMID: 23014345; https://doi.org/10.1038/jid.2012.336
- Idorn M, Thor Straten P, Svane IM, Met O. Transfection of tumor-infiltrating T Cells with mRNA encoding CXCR2. Methods Mol Biol 2016; 1428:261-76; PMID: 27236805; https://doi.org/10.1007/978-1-4939-3625-0_17
- Bonini C, Mondino A. Adoptive T-cell therapy for cancer: The era of engineered T cells. Eur J Immunol 2015; 45:2457-69; PMID: 26202766; https://doi.org/10.1002/eji.201545552
- Hess Michelini R, Freschi M, Manzo T, Jachetti E, Degl'Innocenti E, Grioni M, Basso V, Bonini C, Simpson E, Mondino A et al. Concomitant tumor and minor histocompatibility antigen-specific immunity initiate rejection and maintain remission from established spontaneous solid tumors. Cancer Res 2010; 70:3505-14; PMID: 20388780; https://doi.org/10.1158/0008-5472.CAN-09-4253
- Hess Michelini R, Manzo T, Sturmheit T, Basso V, Rocchi M, Freschi M, Listopad J, Blankenstein T, Bellone M, Mondino A. Vaccine-instructed intratumoral IFN-gamma enables regression of autochthonous mouse prostate cancer in allogeneic T-cell transplantation. Cancer Res 2013; 73:4641-52; PMID: 23749644; https://doi.org/10.1158/0008-5472.CAN-12-3464
- Manzo T, Sturmheit T, Basso V, Petrozziello E, Hess Michelini R, Riba M, Freschi M, Elia AR, Grioni M, Curnis F et al. T Cells redirected to a minor histocompatibility antigen instruct intratumoral TNFalpha expression and empower adoptive cell therapy for solid tumors. Cancer Res 2017; 77(3):658-671; PMID: 27872095; https://doi.org/10.1158/0008-5472.CAN-16-0725.
- Manzo T, Sturmheit T, Basso V, Petrozziello E, Hess Michelini R, Riba M, Freschi M, Elia AR, Grioni M, Curnis F et al. T Cells redirected to a minor histocompatibility antigen instruct intratumoral TNFalpha expression and empower adoptive cell therapy for solid tumors. Cancer Res 2017; 77:658-71; PMID: 27872095; https://doi.org/10.1158/0008-5472.CAN-16-0725
- Perez-Martinez A, de Prada Vicente I, Fernandez L, Gonzalez-Vicent M, Valentin J, Martin R, Maxwell H, Sevilla J, Vicario JL, Díaz MÁ. Natural killer cells can exert a graft-vs-tumor effect in haploidentical stem cell transplantation for pediatric solid tumors. Exp Hematol 2012; 40:882-91 e1; PMID:22771496; https://doi.org/10.1016/j.exphem.2012.07.004
- Perez-Martinez A, Iyengar R, Gan K, Chotsampancharoen T, Rooney B, Holladay M, Ramírez M, Leung W. Blood dendritic cells suppress NK cell function and increase the risk of leukemia relapse after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17:598-607; PMID: 20977942; https://doi.org/10.1016/j.bbmt.2010.10.019
- Perez-Martinez A, Valentin J, Fernandez L, Hernandez-Jimenez E, Lopez-Collazo E, Zerbes P, Schwörer E, Nuñéz F, Martín IG, Sallis H et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2015; 17:601-12; PMID: 25541298; https://doi.org/10.1016/j.jcyt.2014.11.001
- Nanbakhsh A, Pochon C, Mallavialle A, Amsellem S, Bourhis JH, Chouaib S. c-Myc regulates expression of NKG2D ligands ULBP1/2/3 in AML and modulates their susceptibility to NK-mediated lysis. Blood 2014; 123:3585-95; PMID: 24677544; https://doi.org/10.1182/blood-2013-11-536219
- Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar-Filipowicz A. Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res 2007; 31:1393-402; PMID: 17391757; https://doi.org/10.1016/j.leukres.2007.02.020
- Fernandez L, Valentin J, Zalacain M, Leung W, Patino-Garcia A, Perez-Martinez A. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett 2015; 368:54-63; PMID: 26276724; https://doi.org/10.1016/j.canlet.2015.07.042
