ABSTRACT
Treatment for multiple myeloma (MM) has significantly advanced in the last decade with the introduction of proteasome inhibitors and immunomodulatory therapies. Unfortunately, MM continues to cause significant morbidity and most patients eventually succumb to the disease. As in other areas of cancer, immunotherapy in MM has also evolved and holds promise to deliver long-lasting remissions or even cure. The signaling lymphocyte activation molecules (SLAM) family of surface proteins represents a group of potential targets for immunotherapy in MM as some of the family members are expressed consistently on plasma cells and also on myeloma propagating pre-plasma cells. Here, we review the SLAM family members in detail, describe their tissue distribution, biologic pathways, as well as relevant pre-clinical studies and clinical trials in MM. Our review demonstrates the value of SLAM family receptors as potential targets for anti-myeloma immunotherapies and outlines how immunotherapeutic approaches can be developed.
Introduction
Clonal plasma cell dyscrasias represent a continuum of disorders from monoclonal gammopathy of unknown significance (MGUS) through smoldering myeloma (SMM) to multiple myeloma (MM) and plasma cell leukemia. In the case of MM, the last decade has witnessed unprecedented improvements in overall survival rates. However, patients still undergo multiple lines of treatment and most will eventually suffer a fatal relapse.Citation1 In particular, the treatment of MM patients with high-risk cytogenetics has been disappointing and so far we have not been able to identify a convincing way of dealing with this more therapy-resistant and aggressive subtype of myeloma. Immunotherapies targeting certain surface antigens carry the potential to overcome high relapse rates and the more aggressive clinical course of high-risk MM because these novel approaches potentially target the tumor cell irrespective of its biologic characteristics.
One major prerequisite for the design of novel immunotherapeutic approaches is the identification of promising target antigens. We have defined several criteria, which we deem essential for defining the most promising myeloma targets. We believe that an ideal myeloma-associated antigen for antibody-based approaches
must be expressed on the surface of myeloma cells
should be expressed by as few normal tissues as possible
should be expressed homogeneously on tumor cells by a sufficiently large proportion of myeloma patients.
should have a central function in the biology and/or pathophysiology of myeloma to prevent its downregulation under the selection pressure of an effective immunotherapy
Signaling lymphocyte activation molecule (SLAM) family receptors are expressed on the surface of different immune cells, they function as activating and inhibitory signal transducers, and they can potentially be used as targets for novel immunotherapies for MM and other lymphoid malignancies. In this review, we will determine whether the individual members of the SLAM family of receptors fulfill the criteria listed above, we will summarize the relevant pre-clinical data and the most recent clinical trials using SLAM family members as targets, and we will demonstrate how SLAM-targeting approaches can be designed. Overall, our summary will help to answer the question if and how members of the SLAM family of receptors can become part of the growing armamentarium in the fight against MM.
The signaling lymphocyte activation molecule (SLAM) family of receptors
Most SLAM family receptors including CD84, CD150, CD229, CD244, CD319 and CD352 are type 1 transmembrane receptors, while only SLAM family member CD48 has a glycosyl phosphatidyl inositol (GPI) membrane anchor.Citation2 All SLAM family receptors show a broad expression on different immune cells (), and they share a similar structure with an extracellular domain consisting of an Ig variable (V)-like domain, an Ig constant 2 (C2)-like domain, a transmembrane domain, and a cytoplasmic tail.Citation3 SLAM family member CD229 represents somewhat of an exception because it has 4 Ig-like domains with two tandem repeats of the basic V-like and C2-like organization. The cytoplasmic domain of the SLAM family proteins consists of one or more copies of the immunoreceptor tyrosine-based switch motif (ITSM), which can bind to adaptor molecules SAP (SLAM associated protein) and EAT-2 (Ewing sarcoma-associated transcript-2) and Src homology 2 (SH2)-containing phosphatase.Citation4,5 Surface receptors CD48, SLAMF8 and SLAMF9 do not have the cytoplasmic ITSM and are, therefore, not regarded as “true” SLAM family members. Engagement of the N-terminal Ig domains of SLAMF receptors with their cognate ligands results in the recruitment of these intracellular molecules, leading to signaling transduction events that ultimately modulate different types of immune responses.Citation5
Table 1. Tissue distribution of SLAM proteins and their ligands.
Most SLAM family receptors are self-ligands and are involved in homotypic associations through the extracellular domain. One exception is CD244, which binds to CD48 expressed on the surface of lymphocytes and other immune cells.Citation6,7 SAP adaptors are essential components of the SLAM pathway and in their absence, SLAM receptors function as inhibitory signals preventing cellular activation. SAP adaptors prevent coupling of the SLAM receptor to the inhibitory pathway mediated by SH2 domain-containing containing protein tyrosine phosphatase (SHP)-1, SHP-2 and SH2 domain-containing inositol phosphatase (SHIP)-1. They also recruit Src-related tyrosine kinase Fyn that leads to downstream phosphorylation and activation of the cells. EAT-2 has a similar mechanism of preventing SLAM binding to inhibitor pathway mediators and at the same time recruiting phospholipase C, which leads to granule polarization enhancing natural killer (NK) cell activity.Citation8 Other mechanisms for activation of individual SLAM family proteins have also been elucidated.Citation9
The SLAM family and multiple myeloma
The SLAM family of receptors has been implicated in the pathogenesis of different diseases including chronic infections, autoimmune diseases and cancer.Citation10,11 The SLAM family has a total of nine members; however, studies in MM have been limited to CD150 (SLAMF1), CD48 (SLAMF2), CD229 (SLAMF3), CD244 (SLAMF4), CD352 (SLAMF6) and CS1 (SLAMF7). We will review these SLAM family members in the context of MM including an overview of their tissue distribution, postulated mechanisms of action, and clinical studies that have been reported or are ongoing.
CD48 (SLAMF2) and CD244 (SLAMF4, 2B4)
CD48 is a GPI-linked glycoprotein expressed on the surface of immune cells and acts as the ligand for CD244. It does not have a cytoplasmic tail with ITSMs like other SLAM family proteins but modulates NK cell functions together with CD244. CD48 is highly expressed on T cells, B cells and plasma cells, and at a lower level on monocytes, neutrophils and CD34+ cells but it is absent from platelets and red blood cells (). Hosen et al. have shown that CD48 is expressed on more than 90% of plasma cells in 22 out of 24 MM patients with MM they studied. In vitro analyses using murine anti-human CD48 antibody showed complement-mediated cytotoxicity against myeloma cells, T cells and B cells, while CD34+ stem cells were spared. A significant anti-myeloma effect was also seen in a xenograft MM model.Citation12
CD244 on NK cells can act both as a stimulatory and inhibitory signal; however, the exact pathways underlying this are not well understood. Different groups have demonstrated contradictory findings whether CD244 engagement with CD48 on target cells decreases or enhances NK cell cytotoxicity.Citation13-15 Recent studies on the role of CD244 on T cells show that it might act as an inhibitory signal. In chronic infections, like Hepatitis B and tuberculosis, T cells show an increased expression of CD244 and decreased CD8+ T-cell cytotoxic activity. In contrast, blocking of CD244 or CD48 resulted in an enhanced CD8+ T-cell cytotoxicity.Citation11,16 Fauriat et al. showed that CD244 is downregulated on NK cells of patients with MM. They analyzed six patients with MM and found that CD244 expression by flow cytometry was significantly lower compared with healthy donors and postulated that this could be one mechanism of immune escape by MM.Citation17 Costello et al. reported a decrease in the bone marrow NK cell expression of CD244 and NKG2D and NKp30 compared with the peripheral blood in patients with MGUS. Both of these studies suggest that there is an immune escape mechanism with downregulation of NK cell activating receptors in the bone marrow environment in MM.Citation18
Overall, we think that CD244 and CD48 are interesting targets for MM, even though their exact role in the pathogenesis of MM is not understood. Unfortunately, CD48 is expressed on myeloid lineage and CD34+ stem cells, albeit at a lower level compared with MM, and this could lead to undesired myelo- and immuno-suppression if CD48 is directly targeted using novel cytotoxic immunotherapies.
CD229 (SLAMF3, Ly9)
CD229 is expressed on the surface of normal T and B lymphocytes () as well as on NK cells.Citation19 CD229 is unique among members of the SLAM family as it has four extracellular Ig domains and the longest cytoplasmic tail containing two ITSMs. Signaling is mediated by SAP binding to the two ITSMs on the cytoplasmic tail and CD229 homophilic binding leads to interaction of SAP and EAT-2 similar to other SLAM family receptors. Knockdown of CD229 in mice results in normal development of T cells, B cells, NK cells and natural killer T (NKT) cells but mice show a mild defect in T-cell activation and induction of a Th2-type response.Citation20 In addition, CD229 is the only SLAM member that is endocytosed and recycled to the cell surface mediated by adaptor protein (AP)-2 and clathrin coated pits.Citation21 This internalization is mediated by interaction of tyrosine residues in the cytoplasmic tail of CD229 with Grb2. It has also been shown that CD229 activation can downregulate TCR signaling and that Grb2 expression by TCR signaling leads to internalization of CD229, thereby allowing TCR activation.Citation9 Finally, there is evidence that CD229 modulates the development of an innate-like T and B cells response.Citation22,23
Our group has studied the expression of CD229 in 77 patients with plasma cell dyscrasias, which included 49 patients with MM, 7 with SMM, 17 with MGUS and 4 with plasma cell leukemia.Citation24 The tumor cells of all patients including newly diagnosed and relapsed patients showed strong expression of CD229. Furthermore, we observed that even though CD229 is known to be expressed on other hematopoietic cells like NK, T and B cells, its expression was weaker on these normal lymphocytes compared with myeloma cells. In addition, CD229 was highly expressed in patients with plasma cells with aberrant expression of CD56. Finally, we also showed that CD229 is expressed on the CD19−CD138− population of myeloma cells, which can be regarded as the myeloma-propagating pre-plasma cells that contributes to relapse and refractory disease.Citation24,25
High CD229 expression on myeloma was also reported by Yamada et al. in a study of 144 newly diagnosed and 25 relapsed/refractory MM patients.Citation26 In myeloma cell lines they also showed that CD229high cells had a higher proliferation rate compared with CD229low cells. In addition, they demonstrated that anti-myeloma chemotherapy melphalan was less capable of inducing apoptosis in CD229high cells compared with CD229low cells. Finally, they confirmed that CD229 was expressed on the CD138-immature myeloma cell population similarly to our own study. Carulli et al. studied the expression of CD229 in 40 patients with MM, 8 at the time of diagnosis, 8 at stringent complete response and the other 24 at partial response (PR) or VGPR. Malignant plasma cells from all these patients showed CD229 expression irrespective of treatment or response.Citation27
Our group was the first to show that targeting CD229 using antibodies can induce significant NK cell-mediated ADCC as well as CDC in in vitro models of MM. Furthermore, knockdown of CD229 in myeloma cell lines led to increased apoptosis and decreased colony formation, implying that CD229 is essential for the growth of MM cells.Citation28 As of now there are no clinical studies with CD229-targeting antibodies or T-cell therapies, but the preclinical results available augur such novel immunotherapeutic approaches in the near future.
CD352 (SLAMF6, NTB-A)
CD352 is expressed on normal NK cells, T cells and B lymphocytes and signals through the ITSM-SAP pathway. CD352 signaling promotes Th1 responses by T cells and leads to proliferation and IFNγ production.Citation29 and CD352 receptors also enhance NK cell cytotoxicity.Citation30 Lewis et al. studied the expression of CD352 in myeloma and showed that 13 of 15 patients evidenced surface expression by flow cytometry.Citation31 Lewis et al. recently reported their preclinical study of a new CD352-targeting antibody drug conjugate SGN-CD352A, a humanized anti-CD352 antibody conjugated with two molecules of the DNA-damaging agent pyrrolobenzodiazepine dimer. They found that SGN-CD352A displayed potent cytotoxicity against human myeloma cell lines with minimal toxicity to normal human T cells and B cells. Similar efficacy was demonstrated in mouse xenograft models of MM with 90–100% complete responses using the antibody conjugate.Citation31 We consider CD352, an interesting target with an expression limited to the lymphoid compartment and high expression in MM, although it is less well studied in MM compared with SLAMF7 and SLAMF3.
CD319 (SLAMF7, CS1, CRACC)
CS1 exists as two isoforms CS1-long (CS1-L) and CS1-short (CS1-S) both of which are expressed () on the surface of different normal immune cells.Citation32 CS1 is unique among the SLAM family in that it does not bind to SAP but expression is mediated through the EAT-2 pathway.Citation33 CS1-S lacks the EAT-2 binding tyrosine residues and it is not clear how CS1 regulates cellular function. Knockdown of CS1 in NK cells leads to a decrease of their cytotoxic function toward both CS1-positive and CS1-negative target cells.Citation33 Most of the studies show that surface expression of CS1 among normal tissues is restricted to immune cells like NK, B and T cells. However, tissue-specific mRNA expression also shows expression of CS1 in the kidney, pituitary, heart, skeletal muscles and parts of the brain.Citation34 CS1 expression on B cells varies, and it is higher during the later stages of development including mature plasma cells. EAT-2 is absent from T cells, B cells and plasma cells and so the mechanism of CS1 signaling in these cells is not well understood. Tai et al. reported expression of CS1 on most MM cell lines and also observed its expression on plasma cells of patients with MM including patients with relapsed/refractory disease and adverse cytogenetic abnormalities.
Importantly, early preclinical results had indicated that serum levels of soluble CS1 correlate with the stage and activity of MM and that an anti-CS1 antibody (HuLuc90) inhibits adhesion of plasma cells to bone marrow stromal cells and contributes to anti-MM activity. Furthermore, antibody-dependent cell-mediated cytotoxicity (ADCC) could be induced both in vitro and in vivo using anti-CS1 antibody, and cytotoxicity was augmented when the effectors were pre-treated with lenalidomide.Citation35 In parallel, Hsi et al. reported similar findings with uniform CS1 expression on myeloma cells and the anti-CS1 antibody HuLuc63 inducing NK-cell-mediated ADCC in vitro and in vivo.Citation36 In addition to the direct ADCC by binding to myeloma cells, anti-CS1 antibody can bind to CS1 on NK cells and activate these effector cells, thereby enhancing ADCC to myeloma but not to autologous NK cells.Citation37. This combined pre-clinical work set the stage for clinical studies with anti-CS1 antibodies.
Elotuzumab (previously called HuLuc63) is an IgG1 humanized antibody against CS1, which has been evaluated in clinical trials. Despite the promising pre-clinical data, single agent elotuzumab did not result in any significant clinical benefit. In the first phase 1 multicenter dose escalation study, Zonder et al. treated 35 patients with relapsed/refractory MM: no objective response was seen and 9 patients had stable disease but MM progressed in the others.Citation38
Based on pre-clinical studies showing superior anti-MM activity when elotuzumab was combined with novel agents such as bortezomib, several combinatorial clinical trials were initiated.Citation39 Accordingly, Jakubowiak et al. studied the combination of elotuzumab with bortezomib in a dose-escalation phase 1 study in patients with relapsed/refractory MM. Of the 27 evaluable patients treated, a partial response or better was seen in 48% with a median progression-free survival (PFS) of 9.5 mo.Citation40 The same group later studied the effect of combining elotuzumab with bortezomib and dexamethasone in an open label randomized phase 2 study of 152 patients with relapsed/refractory MM. The addition of elotuzumab to bortezomib and dexamethasone improved median PFS (9.7 vs 6.9 mo, hazard ratio (HR) 0.72, p = 0.09)) and numbers of patients showing at least a very good partial response (VGPR) (36% vs 27%). In line with previously reported importance of NK cell ADCC activity, patients with homozygosity of the high affinity FcγRIIIa allele had a better median PFS (22.3 vs 9.8 mo) compared with those with the low-affinity allele.Citation41
Lonial et al. studied the combination of lenalidomide, elotuzumab and dexamethasone in relapsed/refractory MM. They showed a high-objective response rate of 82% even in patients who had previously received lenalidomide.Citation4 This led to further phase III studies using this combination. In the Phase III randomized ELOQUENT-2 study, Lonial et al. reported the efficacy and safety of the combination of elotuzumab, lenalidomide, dexamethasone combination compared with lenalidomide and dexamethasone alone in relapsed/refractory MM. Patients who had previously received lenalidomide were included in the study, but only if they had achieved a partial response or better during the therapy and had not progressed on or within 9 mo after lenalidomide treatment. These patients were essentially still sensitive to lenalidomide and they made up only 6% of the patients studied. Patients received elotuzumab at 10 mg/kg intravenously on days 1,8,15 and 22 of a 28-d cycle for the first two cycles and then days 1 and 15 from Cycle 3 on. Elotuzumab treatment was combined with lenalidomide at 25 mg daily on days 1–21 and dexamethasone 40 mg orally weekly when off elotuzumab and 8 mg intravenously and 28 mg orally during the week on elotuzumab. The study population included 32% of patients with high-risk cytogenetics and 54% of the patients had an autologous stem cell transplant before enrollment into the study. The elotuzumab group showed a superior median PFS of 19.4 mo (95% CI, 16.6–22.2 mo) compared with 14.9 mo (12.1–17.2 mo) for the control group with a hazard ratio of 0.70 (0.57–0.85). The overall response rate was 79% in the elotuzumab arm compared with 66% in the control arm but interestingly complete response rates were lower for the elotuzumab (4%) compared with the control arm (7%). This is thought to be due to the interference of the therapeutic antibody with the results of the serum protein electrophoresis. As expected, the elotuzumab group had an increased rate of infusion-related reactions but mostly with the first dose and this only led to discontinuation of the treatment in 2 out of 321 patients. Similarly, lymphocytopenia was more common in the elotuzumab arm but there was no associated increase in infections or autoimmune diseases with the treatment.Citation42 The ELOQUENT-2 study led to the FDA approval of elotuzumab in combination with lenalidomide and dexamethasone in the treatment of relapse and refractory MM who had one to three prior therapies in November 2015.
Elotuzumab has also been studied in combination with thalidomide and dexamethasone. Mateos et al. recently reported results of a phase 2 single arm study where patients were treated with elotuzumab, thalidomide and dexamethasone with the provision of adding cyclophosphamide in case of an insufficient response. They treated 40 heavily pretreated patients on this protocol and observed an overall response rate (ORR) of 38% with a median time to response of 1.9 mo. Interestingly a 32% ORR was observed in patients previously refractory to IMIDs. They observed a median PFS of 3.9 mo and a median survival of 16.3 mo, which for this patient population is very encouraging.Citation43
The role of elotuzumab in upfront treatment of newly diagnosed patients with MM is currently being studied. The ELOQUENT-1 study (NCT01891643) is an ongoing Phase III trial with lenalidomide, dexamethasone with and without elotuzumab in newly diagnosed MM patients who are not candidates for high-dose therapy and autologous stem cell transplantation due to either co morbidities or advanced age. Two studies have been opened with a CS1-specific antibody–drug conjugate (ABBV-838), one in combination with venetoclax and dexamethasone (NCT02951117) and the other as a dose-escalation study with ABBV-838 monotherapy in relapsed refractory MM (NCT02462525).
Antigen CS1 has also been used as a target for cellular anti-myeloma immunotherapies. For example, chimeric antigen receptors (CARs) targeting CS1 have been incorporated into T cells and NK cells (). This approach has resulted in remarkable efficacy in vitro and in a xenograft model of MM. Chu et al. retrovirally transduced T cells and NK cells with anti-CS1 CARs and were able to show significant survival advantage in a mice treated with these CS1-specific CAR T cells and NK cells compared with mock transduced T and NK cells. Even though CS1 is expressed on T cells and NK cells themselves, the CAR T cells did not have a significant cytotoxicity toward these normal lymphocytes, the mechanism of which is not completely understood.Citation44,45 On the contrary, Danhof et al. showed that there was toxicity to CS1-expressing T and NK cells upon co-culture with CS1-specific CAR T cells. They further went on to show that it was possible to generate potent cytotoxic CS1-specific CAR T cells from autologous T cells of MM patients.Citation46 Moreover, Bae et al. reported that CS-1 cannot only be targeted by antibody-based approaches but that a novel HLA-A2-restricted CS1 peptide induced CS1-specific CTLs against MM. These CTLs displayed significant cytotoxicity toward myeloma cell lines and also primary MM cells.Citation47
Figure 1. Different types of SLAM-specific immunotherapies. The figure shows different ways to target the SLAM family of receptors expressed on myeloma cells. The myeloma antigen can be targeted by monoclonal antibodies (mAb), antibody-drug conjugates (ADC), bispecific antibodies, specific T cells recognizing HLA/SLAM peptide complexes, and SLAM-specific chimeric antigen receptor (CAR) T cells.
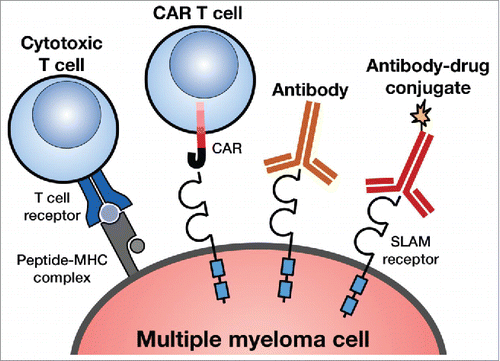
Overall, we consider CS1 a reasonable target for anti-myeloma immunotherapies as it is expressed in most patients with MM including relapsed/refractory. However, it is unknown whether the gene expression of CS1 in non-immune cells can lead to low-level surface expression not detected by available immunophenotyping techniques and toxicity especially with more potent cellular immunotherapies like CAR T cells and CTLs. The significant RNA expression of CS1 in different epithelial tissues also represents a potential toxicity issue. Furthermore, it is not known whether CS1 plays an essential role in the pathogenesis of MM and, therefore, it is possible that its downregulation under the selection pressure of an effective immunotherapy will lead to immune escape.
CD150 (SLAMF1), CD84 (SLAMF5), CD353 (SLAMF8, BLAME), CD2F10 (SLAMF9)
Studies on SLAM family receptors CD150, CD84, CD353 and CD2F10 in MM are limited and they do not seem to be interesting targets in MM. The available data for these four receptors are reviewed below.
CD150 is expressed on T cells, B cells, NK cells and dendritic cells.Citation48 In addition, CD150 seems to be expressed on normal and abnormal plasma cells (). Schoenhals et al. showed that CD150 was uniformly expressed on malignant plasma cells in all 10 MM patients studied.Citation49 However, Gordiienko et al. did not detect any CD150 expression in MM cell line RPMI-8226.Citation50 Thus, it remains an open question if and why CD150 is only expressed on a certain subset of myeloma cells. No therapeutic interventions have been pursued and no studies are available, considering CD150 a target for cancer immunotherapy.
CD84 is expressed on a wide range of hematopoietic cells including T cells, B cells, dendritic cells, monocytes, macrophages, neutrophils, eosinophils, mast cells, basophils, platelets and hematopoietic stem cells.Citation51,52 CD84 is differentially expressed on B cells and may play a role in the activation of memory B cells.Citation53 It has also been shown that CD84 plays an important role in the interaction between T cells and B cells by prolonging cell contact, enhancing T follicular helper cell function, as well as the formation of germinal centers.Citation54 Studies have been contradictory with regards to the expression on plasma cells. Tangye et al. showed a loss of expression of CD84 when plasmablasts were generated in vitro similar to their finding of absent CD84 expression on plasma cells and myeloma cell lines.Citation53 In contrast, De Salort et al. showed that CD84 was present in up to 70% of plasma cells residing in splenic or tonsillar tissue.[Citation55 There are no studies reporting the presence of CD84 on malignant plasma cells in MM. As CD84 is expressed on most hematopoietic cells including CD34+ stem cells, we do not think that it would be a preferable target for immune-based therapies.
CD353 is a type 1 transmembrane protein with an extracellular and transmembrane domain but unlike most other SLAM members has a short cytoplasmic tail that does not contain ITSMs that mediate SAP dependent activity.Citation56 CD353 is expressed on monocytes, dendritic cells and neutrophils and plays an important role as a negative regulator of reactive oxygen species (ROS) production and cell migration during inflammation.Citation57 Llinas et al. found that CD353 was only faintly expressed on B cells by flow cytometry and not expressed on T cells and NK cells. Expression on plasmablasts and plasma cells was also limited, with only 14% of cells expressing low level of CD353.Citation58 There are no studies that have specifically looked into the expression of CD353 on myeloma plasma cells and with its predominant expression on myeloid lineage we do not think that CD353 would be an interesting target for MM immunotherapy.
CD2F10 is the least studied of all the SLAM proteins and its function is not understood. Similar to CD353 its short cytoplasmic tail does not have ITSMs. Its tissue distribution as determined by RT-PCR is predominantly on immune cells like monocytes, dendritic cells, T and B cells but the role it plays in the biology of these cells is not understood.Citation59 There are no studies looking at the surface expression of CD2F10 on plasma cells in MM and so we do not consider CD2F10 a promising target for the immunotherapy in MM.
Conclusions and future directions
The SLAM family of receptors has nine members, which are mostly expressed on immune cells. To be efficient targets for immunotherapy the target antigens should have limited expression on normal immune cells and should be consistently expressed on the surface of malignant plasma cells. Of the nine SLAM proteins only certain members like CS1, CD48, CD352 and CD229 are highly expressed on plasma cells of patients with MM. The uniform and strong expression in all stages of MM, even the relapse refractory disease, makes these SLAM family proteins suitable targets for different immunotherapies such as monoclonal antibodies, bispecific antibodies, immunoconjugates, cytotoxic T cells, and CAR T cells (). The idea for SLAM-directed therapies to target the chemotherapy-resistant myeloma cell subpopulation in addition to the bulk of MM cells seems particularly attractive and needs to be explored in clinical trials. One could imagine, for example, that high-dose chemotherapy followed by autologous stem cell transplant could be used to perform tumor debulking followed by an anti-SLAM immunotherapy that will eradicate the remaining chemotherapy-resistant minimal residual disease.
The only caveat with SLAMF receptors as targets for immunotherapies is that the SLAM members are not exclusively expressed on MM but also on other hematopoietic cells albeit at a lower level. While it remains to be seen whether expression on healthy cell types would result in clinically relevant toxicities, several strategies to limit off-tissue reactivity have been developed. In the case of CAR T cells, for example, the incorporation of inducible suicide genes would allow the rapid deletion of engineered T cells as soon as toxicities are observed. In addition, different combinatorial strategies have been developed to improve the tissue specificity of receptor-transgenic T cells, which require either the binding of the CAR to multiple simultaneously expressed antigens or the absence of antigens binding to inhibitory CARs.
Based on their efficacy and potential long-term persistence we believe that engineered CAR T cells represent the preferable approach out of the different SLAM-targeting antibody-based approaches (). Unfortunately, downregulation of the target antigen with subsequent antigen escape has become a clinical issue. We believe that this problem can be overcome if we focus on targets, which play a central role in the biology of the tumor of interest. In the case SLAM family members, CD229 is probably the only candidate where available data point to an anti-apoptotic role in MM. In our view CD229 should, therefore, be among those SLAM family members preferably being investigated as targets for anti-myeloma immunotherapies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1):7-30; PMID:26742998; https://doi.org/10.3322/caac.21332
- Stefanova I, Horejsí V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 1991; 254(5034):1016-9; PMID:1719635; https://doi.org/10.1126/science.1719635
- Wu N, Veillette A. SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol 2016; 38:45-51; PMID:26682762; https://doi.org/10.1016/j.coi.2015.11.003
- Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL, Leleu X, Tsao LC, Westland C et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30(16):1953-9; PMID:22547589; https://doi.org/10.1200/JCO.2011.37.2649
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol 2011; 29:665-705; PMID:21219180; https://doi.org/10.1146/annurev-immunol-030409-101302
- Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J Immunol 1998; 161(11):5809-12; PMID:9834056
- Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med 1998; 188(11):2083-90; PMID:9841922; https://doi.org/10.1084/jem.188.11.2083
- Dong Z, Davidson D, Pérez-Quintero LA, Kurosaki T, Swat W, Veillette A. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity 2012; 36(6):974-85; PMID:22683124; https://doi.org/10.1016/j.immuni.2012.03.023
- Martin M, Del Valle JM, Saborit I, Engel P. Identification of Grb2 as a novel binding partner of the signaling lymphocytic activation molecule-associated protein binding receptor CD229. J Immunol 2005; 174(10):5977-86; PMID:15879090; https://doi.org/10.4049/jimmunol.174.10.5977
- Abadia-Molina AC, Ji H, Faubion WA, Julien A, Latchman Y, Yagita H, Sharpe A, Bhan AK, Terhorst C. CD48 controls T-cell and antigen-presenting cell functions in experimental colitis. Gastroenterology 2006; 130(2):424-34; PMID:16472597; https://doi.org/10.1053/j.gastro.2005.12.009
- Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, Zachoval R, Wächtler M, Spannagl M, Haas J et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010; 52(6):1934-47; PMID:21064032; https://doi.org/10.1002/hep.23936
- Hosen N, Ichihara H, Mugitani A, Aoyama Y, Fukuda Y, Kishida S, Matsuoka Y, Nakajima H, Kawakami M, Yamagami T et al. CD48 as a novel molecular target for antibody therapy in multiple myeloma. Br J Haematol 2012; 156(2):213-24; PMID:22098460; https://doi.org/10.1111/j.1365-2141.2011.08941.x
- Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol 1999; 29(5):1676-83; PMID:10359122; https://doi.org/10.1002/(SICI)1521-4141(199905)29:05%3c1676::AID-IMMU1676%3e3.0.CO;2-Y
- McNerney ME, Guzior D, Kumar V. 2B4 (CD244)-CD48 interactions provide a novel MHC class I-independent system for NK-cell self-tolerance in mice. Blood 2005; 106(4):1337-40; PMID:15870174; https://doi.org/10.1182/blood-2005-01-0357
- Lee KM, Forman JP, McNerney ME, Stepp S, Kuppireddi S, Guzior D, Latchman YE, Sayegh MH, Yagita H, Park CK, Oh SB et al. Requirement of homotypic NK-cell interactions through 2B4(CD244)/CD48 in the generation of NK effector functions. Blood 2006; 107(8):3181-8; PMID:15905190; https://doi.org/10.1182/blood-2005-01-0185
- Yang B, Wang X, Jiang J, Cheng X. Involvement of CD244 in regulating CD4+ T cell immunity in patients with active tuberculosis. PLoS One 2013; 8(4):e63261; PMID:23638187; https://doi.org/10.1371/journal.pone.0063261
- Fauriat C, Mallet F, Olive D, Costello RT. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia 2006; 20(4):732-3; PMID:16437151; https://doi.org/10.1038/sj.leu.2404096
- Costello RT, Boehrer A, Sanchez C, Mercier D, Baier C, Le Treut T, Sébahoun G. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology 2013; 139(3):338-41; PMID:23360454; https://doi.org/10.1111/imm.12082
- de la Fuente MA, Tovar V, Villamor N, Zapater N, Pizcueta P, Campo E, Bosch J, Engel P. Molecular characterization and expression of a novel human leukocyte cell-surface marker homologous to mouse Ly-9. Blood 2001; 97(11):3513-20; PMID:11369645; https://doi.org/10.1182/blood.V97.11.3513
- Graham DB, Bell MP, McCausland MM, Huntoon CJ, van Deursen J, Faubion WA, Crotty S, McKean DJ. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J Immunol 2006; 176(1):291-300; PMID:16365421; https://doi.org/10.4049/jimmunol.176.1.291
- Del Valle JM, Engel P, Martin M. The cell surface expression of SAP-binding receptor CD229 is regulated via its interaction with clathrin-associated adaptor complex 2 (AP-2). J Biol Chem 2003; 278(19):17430-7; PMID:12621057; https://doi.org/10.1074/jbc.M301569200
- Sintes J, Cuenca M, Romero X, Bastos R, Terhorst C, Angulo A, Engel P. Cutting edge: Ly9 (CD229), a SLAM family receptor, negatively regulates the development of thymic innate memory-like CD8+ T and invariant NKT cells. J Immunol 2013; 190(1):21-6; PMID:23225888; https://doi.org/10.4049/jimmunol.1202435
- Cuenca M, Romero X, Sintes J, Terhorst C, Engel P. Targeting of Ly9 (CD229) Disrupts Marginal Zone and B1 B Cell Homeostasis and Antibody Responses. J Immunol 2016; 196(2):726-37; PMID:26667173; https://doi.org/10.4049/jimmunol.1501266
- Yousef S, Kovacsovics-Bankowski M, Salama ME, Bhardwaj N, Steinbach M, Langemo A, Kovacsovics T, Marvin J, Binder M, Panse J et al. CD229 is expressed on the surface of plasma cells carrying an aberrant phenotype and chemotherapy-resistant precursor cells in multiple myeloma. Hum Vaccine Immunother 2015; 11(7):1606-11; PMID:26001047; https://doi.org/10.1080/21645515.2015.1046658
- Chaidos A, Barnes CP, Cowan G, May PC, Melo V, Hatjiharissi E, Papaioannou M, Harrington H, Doolittle H, Terpos E et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013; 121(2):318-28; PMID:23169779; https://doi.org/10.1182/blood-2012-06-436220
- Yamada A, HT, Ishibashi M, Isoda A et al. Expression and function of SLAM family molecule SLAMF3 (CD229) in myeloma. In: Proceedings of the 15th International Myeloma Workshop, 2015 ; Clinical Lymphoma, Myeloma and Leukemia; Volume 15:e242
- Carulli G, Buda G, Azzarà A, Ciancia EM, Sammuri P, Domenichini C, Guerri V, Petrini M. CD229 expression on bone marrow plasma cells from patients with multiple myeloma and monoclonal gammopathies of uncertain significance. Acta Haematol 2016; 135(1):11-4; PMID:26303094; https://doi.org/10.1159/000380939
- Atanackovic D, Panse J, Hildebrandt Y, Jadczak A, Kobold S, Cao Y, Templin J, Meyer S, Reinhard H, Bartels K et al. Surface molecule CD229 as a novel target for the diagnosis and treatment of multiple myeloma. Haematologica 2011; 96(10):1512-20; PMID:21606160; https://doi.org/10.3324/haematol.2010.036814
- Valdez PA, Wang H, Seshasayee D, van Lookeren Campagne M, Gurney A, Lee WP, Grewal IS. NTB-A, a new activating receptor in T cells that regulates autoimmune disease. J Biol Chem 2004; 279(18):18662-9; PMID:14988414; https://doi.org/10.1074/jbc.M312313200
- Stark S, Watzl C. 2B4 (CD244), NTB-A and CRACC (CS1) stimulate cytotoxicity but no proliferation in human NK cells. Int Immunol 2006; 18(2):241-7; PMID:16410313; https://doi.org/10.1093/intimm/dxh358
- Tim Lewis DJO, Gordon KA, Sandall SL, Miyamoto J, Westendorf L, Linares G, Leiske C, Kostner H, Stone I, Anderson M et al. Abstract 1195: SGN-CD352A: A novel humanized anti-CD352 antibody-drug conjugate for the treatment of multiple myeloma. In Proceedings of AACR 107th Annual Meeting, 2016.
- Kim JR, Mathew SO, Patel RK, Pertusi RM, Mathew PA. Altered expression of signalling lymphocyte activation molecule (SLAM) family receptors CS1 (CD319) and 2B4 (CD244) in patients with systemic lupus erythematosus. Clin Exp Immunol 2010; 160(3):348-58; PMID:20345977; https://doi.org/10.1111/j.1365-2249.2010.04116.x
- Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol 2009; 10(3):297-305; PMID:19151721; https://doi.org/10.1038/ni.1693
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 2004; 101(16):6062-7; PMID:15075390; https://doi.org/10.1073/pnas.0400782101
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112(4):1329-37; PMID:17906076; https://doi.org/10.1182/blood-2007-08-107292
- Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14(9):2775-84; PMID:18451245; https://doi.org/10.1158/1078-0432.CCR-07-4246
- Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling GC, Ciarlariello D, Bhaskar S, Briercheck EL et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 2013; 62(12):1841-9; PMID:24162108; https://doi.org/10.1007/s00262-013-1493-8
- Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J, Afar DE, Singhal AK. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012; 120(3):552-9; PMID:22184404; https://doi.org/10.1182/blood-2011-06-360552
- van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, Garg TK, Shi J, Moreno-Bost AM, Yun R et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Ther 2009; 8(9):2616-24; PMID:19723891; https://doi.org/10.1158/1535-7163.MCT-09-0483
- Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DE, Singhal AK, Anderson KC. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 2012; 30(16):1960-5; PMID:22291084; https://doi.org/10.1200/JCO.2011.37.7069
- Jakubowiak A, Offidani M, Pégourie B, De La Rubia J, Garderet L, Laribi K, Bosi A, Marasca R, Laubach J, Mohrbacher A et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016; 127(23):2833-40; PMID:27091875; https://doi.org/10.1182/blood-2016-01-694604
- Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373(7):621-31; PMID:26035255; https://doi.org/10.1056/NEJMoa1505654
- Mateos MV, Granell M, Oriol A, Martinez-Lopez J, Blade J, Hernandez MT, Martín J, Gironella M, Lynch M, Bleickardt E et al. Elotuzumab in combination with thalidomide and low-dose dexamethasone: a phase 2 single-arm safety study in patients with relapsed/refractory multiple myeloma. Br J Haematol 2016; 175(3):448-56; PMID:27434748; https://doi.org/10.1111/bjh.14263
- Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, Yi L, Kwon CH, Wang QE, Devine SM et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clin Cancer Res 2014; 20(15):3989-4000; PMID:24677374; https://doi.org/10.1158/1078-0432.CCR-13-2510
- Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014; 28(4):917-27; PMID:24067492; https://doi.org/10.1038/leu.2013.279
- Sophia Danhof TG, Koch S, Schreder M, Knop S, Einsele H, Hudecek M. CAR-engineered T cells specific for the elotuzumab target SLAMF7 eliminate primary myeloma cells and confer selective fratricide of SLAMF7+ normal lymphocyte subsets. Blood 2015; 126:115.
- Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC, Munshi NC. A novel immunogenic CS1-specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol 2012; 157(6):687-701; PMID:22533610; https://doi.org/10.1111/j.1365-2141.2012.09111.x
- Wang N, Morra M, Wu C, Gullo C, Howie D, Coyle T, Engel P, Terhorst . CD150 is a member of a family of genes that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics 2001; 53(5):382-94; PMID:11486275; https://doi.org/10.1007/s002510100337
- Schoenhals M, Frecha C, Bruyer A, Caraux A, Veyrune JL, Jourdan M, Moreaux J, Cosset FL, Verhoeyen E, Klein B. Efficient transduction of healthy and malignant plasma cells by lentiviral vectors pseudotyped with measles virus glycoproteins. Leukemia 2012; 26(7):1663-70; PMID:22318450; https://doi.org/10.1038/leu.2012.36
- Gordiienko IM, Shlapatska LM, Kovalevska LM, Sidorenko SP. Differential expression of CD150/SLAMF1 in normal and malignant B cells on the different stages of maturation. Exp Oncol 2016; 38(2):101-7; PMID:27356578
- Zaiss M, Hirtreiter C, Rehli M, Rehm A, Kunz-Schughart LA, Andreesen R, Hennemann B. CD84 expression on human hematopoietic progenitor cells. Exp Hematol 2003; 31(9):798-805; PMID:12962726; https://doi.org/10.1016/S0301-472X(03)00187-5
- Sintes J, Romero X, Marin P, Terhorst C, Engel P. Differential expression of CD150 (SLAM) family receptors by human hematopoietic stem and progenitor cells. Exp Hematol 2008; 36(9):1199-204; PMID:18495325; https://doi.org/10.1016/j.exphem.2008.03.015
- Tangye SG, van de Weerdt BC, Avery DT, Hodgkin PD. CD84 is up-regulated on a major population of human memory B cells and recruits the SH2 domain containing proteins SAP and EAT-2. Eur J Immunol 2002; 32(6):1640-9; PMID:12115647; https://doi.org/10.1002/1521-4141(200206)32:6%3c1640::AID-IMMU1640%3e3.0.CO;2-S
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 2010; 32(2):253-65; PMID:20153220; https://doi.org/10.1016/j.immuni.2010.01.010
- De Salort J, Sintes J, Llinàs L, Matesanz-Isabel J, Engel P. Expression of SLAM (CD150) cell-surface receptors on human B-cell subsets: from pro-B to plasma cells. Immunol Lett 2011; 134(2):129-36; PMID:20933013; https://doi.org/10.1016/j.imlet.2010.09.021
- Kingsbury GA, Feeney LA, Nong Y, Calandra SA, Murphy CJ, Corcoran JM, Wang Y, Prabhu Das MR, Busfield SJ et al. Cloning, expression, and function of BLAME, a novel member of the CD2 family. J Immunol 2001; 166(9):5675-80; PMID:11313408; https://doi.org/10.4049/jimmunol.166.9.5675
- Wang G, van Driel BJ, Liao G, O'Keeffe MS, Halibozek PJ, Flipse J, Yigit B, Azcutia V, Luscinskas FW, Wang N et al. Migration of myeloid cells during inflammation is differentially regulated by the cell surface receptors Slamf1 and Slamf8. PLoS One 2015; 10(3):e0121968; PMID:25799045; https://doi.org/10.1371/journal.pone.0121968
- Llinas L, Lázaro A, de Salort J, Matesanz-Isabel J, Sintes J, Engel P. Expression profiles of novel cell surface molecules on B-cell subsets and plasma cells as analyzed by flow cytometry. Immunol Lett 2011; 134(2):113-21; PMID:20951740; https://doi.org/10.1016/j.imlet.2010.10.009
- Zhang W, Wan T, Li N, Yuan Z, He L, Zhu X, Yu M, Cao X. Genetic approach to insight into the immunobiology of human dendritic cells and identification of CD84-H1, a novel CD84 homologue. Clin Cancer Res 2001; 7(3 Suppl):822s-829s; PMID:11300479
