ABSTRACT
CD28H is a newly discovered co-receptor of the human B7 family. CD28H interacts with its ligand B7-H5 and regulates T cell response. Here we showed that CD28H was not expressed on granulocytes, monocytes, myeloid dendritic cells (MDCs), and B cells, but constitutively expressed with moderate levels on memory T cells and with high levels on naïve T cells, innate lymphoid cells (ILCs), natural killer (NK) cells, and plasmacytoid dendritic cells (PDCs) in human peripheral blood. Similar CD28H+ cell profile existed in secondary lymphoid organs and pathological tissues including multiple types of cancers. Further analysis demonstrated that CD28H+ naïve and CD28H+ memory T cells were characterized with increased naïve feature and less effector functional phenotype, respectively. High levels of constitutive CD28H expression on naïve T cells and innate immune cells suggest a potential role of CD28H in innate and adaptive immunity.
Introduction
The B7-CD28 co-signaling pathway is crucial in acquisition of effector functions by the immune system. Recent studies have demonstrated that inhibitory B7 family members and their receptor signaling pathways mediate dysfunctional T cell immunity in the tumor microenvironmentsCitation1-3 and blockade of PD-L1 (B7-H1) and PD-1 pathway induces important objective clinical responses in many types of human cancer.Citation4,Citation5 It is well known that CD28 ligation in the context of TCR engagement is regarded as the most important co-signaling pathway to activate T cells and to prevent T cell anergy. In addition, CD28 may promote NK cell cytolytic activity and cytokine production.Citation6,Citation7 A recent report shows that ILCs can express CD28 at different levels in inflammatory conditions,Citation8 however, the potential immunological role of CD28 in ILCs remains unknown.
In an effort to identify novel B7-CD28 co-signaling member and particularly explore potential new B7 target for cancer immunotherapy, CD28H (TMIGD2) is a newly discovered co-receptor of the human B7 family that interacts with its ligand B7-H5 (B7-H7, HHLA2) and regulates T cell responses.Citation9 CD28H is constitutively expressed on naive T cells. Repetitive antigenic exposure induces a complete loss of CD28H on T cells.Citation9 It has been reported that CD28H signaling pathways promotes T cell proliferation and effector responses.Citation9 However, additional studies demonstrate an inhibitory role of B7-H5 signaling in T cell activation Citation10 and show a negative association between tumor tissue B7-H5 expression and patient outcomes.Citation11,Citation12 In this study we systemically analyzed CD28H expression profile, the phenotype and the distribution of CD28H+ cells in different immune cell subsets in peripheral blood, secondary lymphoid organs, and multiple types of human pathogenic tissues including cancer tissues. Our study suggests a potential role of CD28H in innate and adaptive immunity.
Results
CD28H expression on immune cell subsets in human peripheral blood
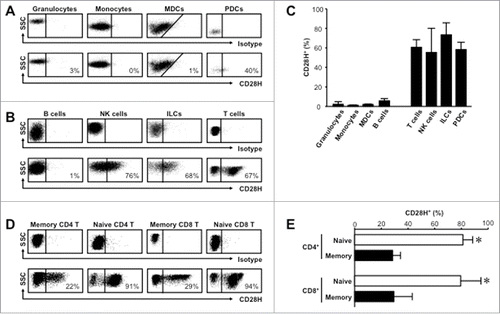
We systemically examined the CD28H expression profile across immune cell subsets in healthy human peripheral blood following mononuclear cell enrichment. We found that CD28H was negligible on granulocytes, monocytes, myeloid dendritic cells (MDCs), and B cells (). As expected, NK and T cells expressed CD28H,Citation9 and naïve T cells expressed higher levels of CD28H than memory T cells (). Interestingly, we detected high levels of CD28H expression on plasmacytoid dendritic cells (PDCs) and innate lymphoid cells (ILCs), which had not been previously reported (). We compared the phenotypic and potential functional characteristics of CD28H expression on different immune cell subsets in peripheral blood, secondary lymphoid organs, and pathological tissues including human cancer tissues.
Figure 1. CD28H is expressed on immune cell subsets in healthy human peripheral blood. (A) Representative flow cytometric plots showing staining against CD28H or isotype control on FSC/SSCSINGLETSCD45+CD3−CD19− granulocytes (CD33+CD14−CD15+), monocytes (CD33+CD14−CD15+), myeloid dendritic cells (MDCs) (CD33+CD14−CD15−CD11 c+HLA-DR+) and plasmacytoid cells (PDCs) (CD33−HLA-DR+CD123+). (B) Representative flow cytometric plots showing staining against CD28H or isotype control on FSC/SSCLOWCD45+CD3− B cells (CD19+), NK cells (CD7+CD19−CD16+NKp46+), innate lymphoid cells (ILCs) (lin−CD7+CD19−CD16−CD127+), and FSC/SSCLOWCD45+CD19− T cells (CD3+). (C) Bar graphs summarize CD28H expression from (A) and (B). (D) Representative flow cytometric plots showing staining against CD28H or isotype control on FSC/SSCLOWCD45+CD33−CD19−CD3+ naïve (CD45RA+CD45RO−CCR7+CD62 L+) and memory (CD45RA−CD45RO+ CCR7−CD62 L−) CD4+ and CD8+ T cells. (E) Bar graphs summarize CD28H expression from (D). *, P < 0.05. 6–10 donors.
CD28H+ naïve T cells possess enriched naïve characteristics
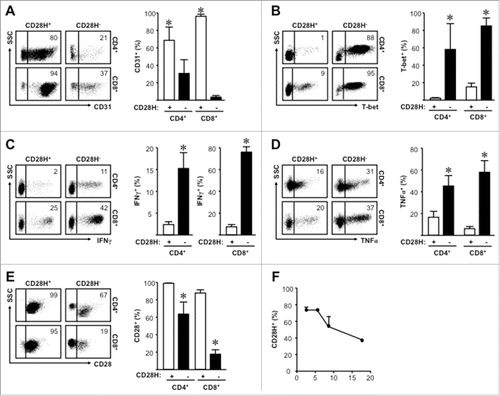
6–15% naïve T cells were CD28H− (, ). We analyzed and compared the potential phenotypic differences between CD28H+ and CD28H− naïve T cells. We found that the majority of CD28H+, but not CD28H− naïve T cells expressed CD31 (), a recent thymic emigrant marker.Citation16 In line with this, CD28H− naïve cells expressed higher levels of TH1-regulating transcription factor T-bet (), as well as effector cytokines IFNγ () and TNFα (). Similar levels of IL-2 and CXCL8 were detected in CD4+CD28H+ and CD4+CD28H− naïve and memory T (Sup. ). Interestingly, CD28 surface expression was lower on CD28H− than CD28H+ naive cells (). Furthermore, enriched naïve CD4+ T cells cultured in vitro progressively lost CD28H surface expression (). Taken together, compared with CD28H− naïve T cells, CD28H+ naïve T cells show enriched naïve characteristics.
Figure 2. CD28H+ naïve T cells show increased naïve characteristics. (A) Expression of CD31 on naïve T cells. Representative plots and the mean percentages + SEM showing CD31 expression on CD28H+ and CD28H− naïve T cells. (B) Expression of T-bet on naïve T cells. Representative plots and the mean percentages + SEM showing T-bet expression on CD28H+ and CD28H− naïve T cells. (C) Expression of IFNγ on naïve T cells. Representative plots and the mean percentages + SEM showing IFNγ expression on CD28H+ and CD28H− naïve T cells. (D) Expression of TNFα on naïve T cells. Representative plots and the mean percentages + SEM showing TNFα expression on CD28H+ and CD28H− naïve T cells. (E) Expression of CD28 on naïve T cells. Representative plots and the mean percentages + SEM showing CD28 expression on CD28H+ and CD28H− naïve T cells. 6–8 donors, *, P < 0.05. (F) Kinetic CD28H expression in in vitro cultured enriched naïve T cells. Enriched peripheral blood CD4+ naïve T cells were stimulated with anti-CD3 and anti-CD28 every 3 d for up to 20 d as described. CD28H surface expression was measured by FACS. 3 donors with repeats.
CD28H+ memory T cells show less effector function and differentiation
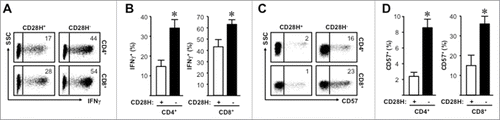
20% memory T cells expressed CD28H(, ). A similar approach was used to compare the phenotype of CD28H+ and CD28H− memory T cells. We found that IFNγ levels were higher in CD28H− T cells than CD28H+ T cells (, ). CD28H− T cells might be replicative senescent following cellular division.Citation9 In line with this, we detected higher levels of CD57 expression on CD28H− T cells than CD28H+ T cells (, ). CD57 may serve as a marker of T cell terminal differentiation.Citation17 Interestingly, we found higher levels of CD28 expression on RORγ-t+CD4+ and Foxp3+CD4+ T cells as compared with T-bet+CD4+ and GATA-3+CD4+ T cells (Sup. ). Thus, CD28H+ memory T cells show less effector function and differentiation.
Figure 3. CD28H+ memory T cells show less effector function marks. (A, B) Expression of IFNγ on memory T cells. Representative plots and the mean percentages + SEM showing IFNγ expression on CD28H+ and CD28H− memory T cells. (C, D) Expression of CD57 on memory T cells. Representative plots and the mean percentages + SEM showing CD57 expression on CD28H+ and CD28H− memory T cells. 6–8 donors, *, P < 0.05.
CD28H+ T cells exist in lymphoid organs and pathological tissues
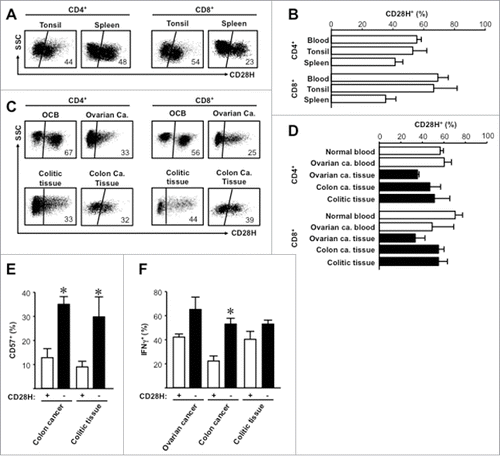
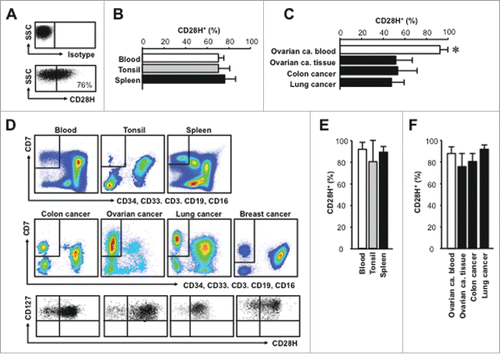
CD28H is expressed in the majority of naïve T cells. Naïve T cells are largely located in secondary lymphoid organs. CD28H ligation by B7-H5 may have a stimulatory Citation9 or inhibitory Citation10 effect on T cells. We analyzed CD28H+ T cells in the human secondary lymphoid organs including tonsil and spleen. CD28H expression was not different between blood, tonsil, and spleen (). Next we examined CD28H expression on T cells from ovarian cancer patient blood, ovarian cancer tissues, colon cancer tissues, and colon colitic tissues. We found CD28H+ T cells in different pathological tissues (). The levels of CD28H+ T cells were slightly or moderately reduced in different pathological tissues as compared with healthy blood and ovarian cancer patient blood (). We further evaluated the effector state of CD28H-expressing tissue-infiltrating T cells. We observed that the expression levels of CD57 () and IFNγ () were significantly () and moderately () lower in CD28H+ T cells than CD28H− CD8+ T cells in different tissues. Thus, CD28H+ T cells can be recruited into tumor and inflammatory tissue sites and tissue CD28H+ T cells show less activation and differentiation.
Figure 4. CD28H expression on tissue T cell subsets. (A, B) Representative flow cytometric plots for isotype and anti-CD28H staining (A) and the percentages + SEM of CD28H expression (D) on secondary lymphoid tissue T cells. 4–6 donors. (C, D) Representative flow cytometric plots for isotype and anti-CD28H staining (C) and the percentages + SEM of CD28H expression (D) on pathological tissue T cells. 4–6 donors. (E, F) The mean percentages + SEM of CD57 (E) and IFNγ (F) expressing CD8+ T cells in CD28H+ and CD28H− subsets from pathological tissue T cells. 4–6 donors, *, P < 0.05. OCB, blood from ovarian cancer patient; Ca, Cancer.
CD28H+ ILCs and NK cells exist in lymphoid organs and pathological tissues
Similar to peripheral blood, we analyzed CD28H expression on NK cells in secondary lymphoid organs including tonsil and spleen and different types of human cancer tissues. We detected high levels of CD28H expression on NK cells in tonsil and spleen (, ) and several types of cancer tissues (). It appeared that the percentage of CD28H+ NK cells was lower in tissues than in peripheral blood (). We also analyzed CD28H expression on ILCs in secondary lymphoid organs including tonsil and spleen and different types of human cancer tissues (). ILCs expressed high levels of CD28H in secondary lymphoid organs () and different types of human cancer tissues (). Notably, the percentages of CD28H+ ILCs were comparable in different pathological tissues (). Further studies should measure the functional consequences of CD28H ligation on pDCs, ILCs and NK cells.
Figure 5. CD28H expression on NK cells and ILCs. (A-C) NK cells were gated on CD45+CD3−CD7+CD16+CD19−CD33−CD34−CD127−. (A) Representative flow cytometric plots for isotype and anti-CD28H staining in NK cells. (B) The mean percentage + SEM of CD28H positive cells in NK cells in peripheral blood of healthy donors (n = 8), tonsil (n = 5) and spleen (n = 4). (C) The mean percentage + SEM of CD28H positive cells in NK cells in ovarian cancer patient blood (n = 5) and cancer tissues from patients with ovarian cancer (n = 5), colon cancer (n = 5), and lung cancer (n = 4). *, P < 0.05. (D-F) ILCs were gated on CD45+ CD3− CD7+ CD16− CD19− CD33− CD34− CD127+. (D) Representative flow cytometric plots of ILCs in secondary lymphoid organs and cancer tissues for isotype and anti-CD28H staining. (E) The mean percentage + SEM of CD28H positive cells in ILCs in peripheral blood of healthy donors (n = 8), tonsil (n = 5) and spleen (n = 4). (F) The mean percentage + SEM of CD28H positive cells in ILCs in ovarian cancer patient blood (n = 5) and cancer tissues from patients with ovarian cancer (n = 5), colon cancer (n = 5), lung cancer (n = 4), and breast cancer (n = 3).
Discussion
In this work we have systemically examined CD28H expression in different immune cell subsets. In line with the previous report,Citation9 we have shown that CD28H is constitutively expressed on the majority of naive T cells and a small fraction of memory T cells in human peripheral blood, secondary lymphoid organs, and different pathological tissues including breast cancer, colon cancer, lung cancer, ovarian cancer, and colitic colon tissues. Furthermore, we have not detected CD28H expression on B cells. Based on the phenotype and effector cytokine profile, we have found that CD28H+ memory T cells show less effector function and minimal differentiation features. It has been described that engagement of CD28H Citation9 and its ligand B7-H5 Citation10 result in T cell activation and inhibition, respectively. This discrepancy is poorly understood in literature. Non-existence of murine CD28H and B7-H5 and lack of reliable, consistent, and reproducible reagents including specific neutralizing antibodies against human CD28H and B7-H5 significantly dampen our efforts toward a comprehensive understanding of this B7 family pathway. Nonetheless, CD28H expression profile and tissue distribution suggest a potential role of CD28H in adaptive T cell immunity.
In addition to T cells and B cells, we have examined CD28H expression on antigen presenting cell subsets and innate immune cell subsets. Granulocytes, monocytes, and MDCs do not express CD28H. However, 50% PDCs express CD28H. As peripheral blood Citation18 and tumor associated Citation19 PDCs are major type-I IFN producers, PDCs are considered innate immune cells. CD28H expression is highly expressed on NK cells and ILCs. CD28 can support NK cell function.Citation6,Citation7 ILCs may express different levels of CD28 but the effect of its ligation has not yet been elucidated.Citation8 Given the homology of CD28 and CD28H, CD28H may carry out a potential stimulatory and/or survival signal to ILCs and NK cells. Although high levels of CD28H expression on PDCs, ILCs, and NK cells in blood, secondary lymphoid organs, and pathological tissues suggest a potential role of CD28H in innate immunity, functional studies are in urgent need to define biologic and pathological relevance of CD28H and B7-H5 signaling pathway. Similar to the CD28 and B7 signaling pathway, we speculate that the CD28H and B7-H5 signaling pathway may add a novel layer of immune regulation in innate and/or adaptive immunity. Nonetheless, functional studies are essential to determine the basic immunological activity of this interaction and to assess whether targeting this pathway is an effective therapeutic approach for cancer immunotherapy.
Materials and methods
Human subjects and human samples
Peripheral blood was obtained from healthy volunteers and patients with ovarian cancer. Mononuclear cells were enriched by Ficoll-Hypaque (GE Healthcare and Life Sciences) or Lymphoprep (STEMCELL Technologies) density gradient centrifugation. Naïve CD4+ T cells were enriched from healthy donors using CD4+ RossetteSep enrichment cocktail (STEMCELL Technologies) followed by anti-CD45RA microbeads (Miltenyi Biotec) for in vitro T cell cultures. Pathological tissues from breast cancer, colon carcinoma, lung cancer, ovarian cancer, and ulcerative colitis were used. Human tissues were from patients presenting for diagnostic biopsy, prophylactic colectomy, or tumor debulking. Mononuclear cells from healthy or patient donors were used fresh unless described otherwise. All donors provided written, informed consent. In addition to these pathological tissues, human spleen and tonsil tissues were obtained from the tissue procurement core at the University of Michigan Hospital. Human pathological tissue cells were obtained as described.Citation1,Citation13,Citation14 The Institutional Review Boards of the University of Michigan School of Medicine approved the study.
Reagents
Antibodies for flow cytometry analysis were CD3 (cat. 557943, 562280, 340662, clone UCHT1), CD4 (cat. 470049, clone RPA-T4), CD8 (cat. 557760, clone RPA-T8), CD11 c (cat. 559877, clone B-Ly6), CD14 (cat. 555397, clone M5E2), CD15 (cat. 642917, clone MMA), CD28 (cat. 555729, clone CD28.2), CD31 (cat. 561654, clone WM59), CD33 (cat. 341640, clone P67.6), CD45RA (cat. 562298, 560675, clone HI100), CD45RO (cat. 340438, 555492, 560608, clone UCHL1), CD127 (cat. 562436, clone HIL-7R-M21), CXCL8 (cat. 554720, clone G265–8), IFN-γ (cat. 557844, 557995, 562392, clone 4 S.B3), TNF-α (cat. 557996, 554514, clone MAb11), NKp46 (cat. 562101, clone 9E2), T-bet (cat. 561264, clone 4B10), GATA-3 (cat. 563510, clone L50–823), Rorγ-t (cat. 563081, clone Q21–559) (BD Biosciences), CD28H (Amplimmune), CD7 (cat. 47–0079, clone 124–1D1), CD16 (cat. MHCD1630, clone 3G8), CD19 (cat. 25–0199–42, clone HIB19), CD45 (cat. MHCD4530, clone HI30), CD57 (cat. 48–0577, clone NK-1), Foxp3 (cat. 48–4776, clone PCH101), HLA-DR (cat. 47–9956–42, clone LN3), IL-2 (cat. 46–7029, MQ1–17H12) (ThermoFischer), Armenian Hamster isotype control (cat. 400916, clone HTK888), and anti-Armenian Hamster (cat. 405503, clone Poly4055) (Biolegend). Antibodies for T cell activation were anti-CD3 (cat. 555329/14–0038–82, clone UCHT1) and anti-CD28 (cat. 555728/16–0289–81, clone CD28.2) (BD Biosciences and ThermoFischer).
Flow cytometry
Single-cell suspensions from tissue were stained for extracellular surface antigens with specific antibodies, followed by fixation and permeabilization using Perm/Fix solution (ThermoFischer), then stained against intracellular antigens as described.Citation14,Citation15 Viable single cells were analyzed.
T cell culture
Naïve CD4+ T cell subsets were stimulated with 2.5 µg/mL anti-CD3 and 1.25 µg/mL anti-CD28 mAb and 3 ng/mL rhIL-2 for up to 20 d. Medium was refreshed every 3 d with 2.5 ug/mL anti-CD3, 1.25 ug/mL anti-CD28 and rhIL-2 3 ng/mL. T cells were then were measured against CD28H at indicated time-points.
T cell stimulation
Freshly isolated mononuclear cells were stimulated in vitro with PMA/Ionomycin, GolgiStop and GolgiPlug (BD Biosciences) for 4 hrs then stained against extracellular and intracellular antigens as described above.
Disclosures
The authors have no financial conflicts of interest.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
supp.zip
Download Zip (20.9 KB)Acknowledgment
This work was supported in part by research grants from the NIH/NCI R01 grants (W.Z) (CA217510, CA123088, CA099985, CA193136 and CA152470) and the NIH through the University of Michigan Cancer Center Support Grant (CA46592). We thank Linda Liu, D. Postiff, M. Vinco, R. Craig, and J. Barikdar for their assistance.
References
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562-7. doi:10.1038/nm863. PMID:12704383
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263-74. doi:10.1038/nrc1586. PMID:15776005
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467-77. doi:10.1038/nri2326. PMID:18500231
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64. doi:10.1038/nrc3239. PMID:22437870
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi:10.1126/scitranslmed.aad7118
- Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J, et al. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol. 1999;163:62-70.
- Nandi D, Gross JA, Allison JP. CD28-mediated costimulation is necessary for optimal proliferation of murine NK cells. J Immunol. 1994;152:3361-9.
- Roan F, Stoklasek TA, Whalen E, Molitor JA, Bluestone JA, Buckner JH, Ziegler SF. CD4+ Group 1 Innate Lymphoid Cells (ILC) Form a Functionally Distinct ILC Subset That Is Increased in Systemic Sclerosis. J Immunol. 2016;196:2051-62. doi:10.4049/jimmunol.1501491
- Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M, Ruff W, et al. B7-H5 costimulates human T cells via CD28 H. Nat Commun. 2013;4:2043. doi:10.1038/ncomms3043. PMID:23784006
- Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, Ohaegbulam KC, Ghosh K, Zhao A, Scharff MD, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Nati Acad Sci U S A. 2013;110:9879-84. doi:10.1073/pnas.1303524110. PMID:23716685
- Cheng H, Janakiram M, Borczuk A, Lin J, Qiu W, Liu H, Chinai JM, Halmos B, Perez-Soler R, Zang X. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin Cancer Res. 2017;23:825-32. doi:10.1158/1078-0432.CCR-15-3071. PMID:27553831
- Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, Castano, Ohaegbulam KC, Zhao R, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res. 2015;21:2359-66. doi:10.1158/1078-0432.CCR-14-1495. PMID:25549724
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-9. doi:10.1038/nm1093. PMID:15322536
- Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L, Lu F, Wei S, Maj T, Peng D, et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell. 2016;165:1092-105. doi:10.1016/j.cell.2016.04.009. PMID:27133165
- Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249-53. doi:10.1038/nature15520. PMID:26503055
- Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, Thiel A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789-94. doi:10.1084/jem.20011756. PMID:11901204
- Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin immunol. 2013;25:214-21. doi:10.1016/j.coi.2012.12.003. PMID:23298609
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835-7. doi:10.1126/science.284.5421.1835. PMID:10364556
- Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339-46. doi:10.1038/nm1201-1339. PMID:11726975
