ABSTRACT
Monocytosis is considered a poor prognostic factor for many cancers, including B cell lymphomas. The mechanisms by which different monocyte subsets support the growth of lymphoma is poorly understood. Using a pre-clinical mouse model of B cell non-Hodgkin's lymphoma (B-NHL), we investigated the impact of tumor progression on circulating monocyte levels, subset distribution and their activity, with a focus on immune suppression. B-NHL development corresponded with significant expansion initially of classical (Ly6Chi) and non-classical (Ly6Clo) monocytes, with accumulation and eventual predominance of Ly6Clo cells. The lymphoma environment promoted the conversion, preferential survival and immune suppressive activity of Ly6Clo monocytes. Ly6Clo monocytes expressed higher levels of immunosuppressive genes including PD-L1/2, Arg1, IDO1 and CD163, compared to Ly6Chi monocytes. Both monocyte subsets suppressed CD8 T cell proliferation and IFN-γ production in vitro, but via different mechanisms. Ly6Chi monocyte suppression was contact dependent, while Ly6Clo monocytes suppressed via soluble mediators, including IDO and arginase. Ly6Clo monocytes could be selectively depleted in tumor-bearing hosts by liposomal doxorubicin treatment, further enhanced by co-administration of anti-4-1BB monoclonal antibody. This treatment led to a reduction in tumor growth, but failed to improve overall survival. Analogous immunosuppressive monocytes were observed in peripheral blood of diffuse large B cell lymphoma patients and actively suppressed human CD8 T cell proliferation. This study highlights a potential immune evasion strategy deployed by B cell lymphoma involving accumulation of circulating non-classical monocytes with immunosuppressive activity.
Introduction
B cell lymphomas are the most common hematological malignancy. Whilst most patients are initially responsive to front-line therapy of Rituximab or R-CHOP (Rituximab + cyclophosphamide + doxorubicin + vincristine + prednisolone), relapse is common and is associated with poor outcomes. Elevated levels of circulating monocytes (monocytosis) has long been considered a poor prognostic factor for many cancers, including B cell lymphoma.Citation1–3 Monocytes may play a role in promoting tumor progression via promotion of angiogenesis,Citation4,5 metastasisCitation6–8 and inhibition of T cell responses.Citation9,10 However, the mechanisms of monocyte mobilization, immunosuppressive activity of different sub-populations and differentiation into myeloid-derived suppressor cells (MDSC) in B cell lymphoma remain poorly defined.
Tumor cells can manipulate the immune response to create a favorable environment for tumor growth. The inherent plasticity of the monocytic compartment enables these cells to respond rapidly to infectious threats or tissue damage, but also makes them sensitive to tumor regulation. In mice, monocytes are defined as CD11b+ CD115+ and further characterized as either ‘classical’ Ly6Chi monocytes or ‘non-classical’ Ly6Clo monocytes (sometimes called patrolling monocytes).Citation11 The human equivalents are divided into classical CD14+ monocytes and non-classical CD14dim CD16+ monocytes.Citation12 Several studies have investigated associations between monocyte subset distribution and tumor progression. For example, breast cancer progression is associated with expansion of a non-classical CD16+ monocyte population.Citation13 Similarly, pancreatic cancer patients with high circulating classical CD14+ monocytes have a poorer prognosis.Citation14 CD16+ monocytes and correspondingly secreted CD163 is a poor prognostic marker in multiple myelomaCitation15 and Hodgkin's lymphoma.Citation16 Supporting these observations, CCL2 blockade (which prevents global monocyte egress from the bone marrow) successfully improved responses to immunotherapy in experimental models of solid tumors, arguing that monocytes are detrimental to anti-tumor immune responses against solid tumors.Citation17 Furthermore, in a murine model of chronic lymphocytic leukemia (CLL) non-classical Ly6Clo monocytes were shown to promote tumor growth.Citation18 In contrast to these studies showing detrimental effects of monocytes in cancer, there is evidence to suggest that monocytes may also protect against cancer development. For example, a recent study showed an immune-supportive role for Ly6Clo monocytes, contributing to immune surveillance of melanoma.Citation19 In CLL patients, high baseline levels of classical CD14+ monocytes in those was associated with increased time to commencement of initial treatment.Citation20 These inconsistencies in the literature warrant further investigation into the role of both classical and non-classical monocytes in the immune microenvironment of B cell lymphoma.
Monocytes can extravasate into the tissue where they respond to local environmental cues and differentiate into macrophages or MDSC. MDSC describe a heterogeneous population of immature myeloid cells that are present in tumor stroma and can reduce T cell function and promote tumor vascularization. Generally MDSC are divided into two subsets, a granulocytic Ly6G+ Gr-MDSC and a monocytoid Ly6G- Mo-MDSC. It is well accepted that MDSC numbers increase with tumor burden and associate with poor prognosis for melanoma,Citation21 pancreatic, esophageal and gastric cancers.Citation22 This is also true for hematological malignancies such as B cell lymphomaCitation23 and CLL.Citation24 However, the distinction between MDSC and monocytes in lymphoma is poorly understood, and may well be one of the same. Mo-MDSCs can be further divided into two different subsets of cells based on Ly6C expression. The connection between monocytes and MDSC remains to be completely understood in B cell lymphoma.
In this study we characterize monocytes during B cell lymphoma disease progression in the pre-clinical Eµ-myc mouse model of B cell lymphoma,Citation25 to better understand the impact of malignant B cells on the monocytic compartment and formation of an immunosuppressive, tumor-permissive microenvironment. We show marked expansion and accumulation of non-classical CD11b+/Ly6G-/CD115+/Ly6Clo monocytes with immune suppressive and pro-tumoral activity. Together, these data provides new insight into lymphoma-evoked changes to the monocyte compartment, leading to an increase in immunosuppressive populations that support tumor growth, and unveils potential pathways that could be targeted to improve immune control of B cell lymphomas.
Results
Circulating monocytic and granulocytic cell populations increase with B lymphoma development
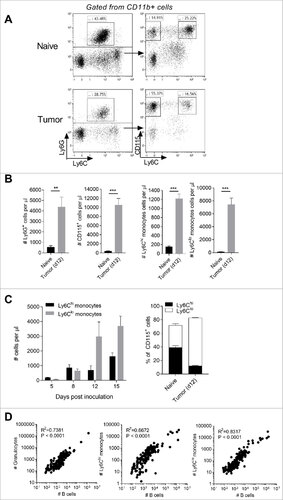
We divided CD11b+ myeloid cells in the peripheral blood of healthy and Eµ-myc tumor-transplanted mice into three broad populations based on expression of Ly6G, CD115 and Ly6C. Granulocytes were identified as CD11b+ Ly6G+. Monocytes were broadly identified as CD11b+ Ly6G- CD115+ and then further characterized into two sub-populations based on Ly6C expression (Ly6Chi and Ly6Clo) (). Compared to healthy mice, tumor-bearing mice contained substantially higher numbers of circulating granulocytes (Ly6G+) and monocyte (CD115+) populations (). The numbers of monocytes steadily increased with lymphoma progression. Initially there was an equivalent increase of both Ly6Chi and Ly6Clo monocytes, however by day 12 the numbers of Ly6Clo cells had increased significantly becoming the dominant monocyte population (). To address whether these changes in the peripheral myeloid compartment occurred during spontaneous lymphoma development we monitored equivalent myeloid populations in Eµ-myc Tg mice. Tumor growth was assessed by increases in CD19+ B cells in the blood. Monitoring these mice for 5 months we observed a significant correlation between circulating CD19+ cells and granulocytes (R2 = 0.7381, P<0.0001), Ly6Chi monocytes (R2 = 0.6672, P<0.0001) and Ly6Clo monocytes (R2 = 0.8317, P<0.0001) ().
Figure 1. Expansion of monocyte and granulocyte populations with B cell lymphoma progression. (A) Gating strategy for identifying granulocytes, Ly6Chi and Ly6Clo monocytes. (B) Numbers of granulocytes, monocytes, Ly6Chi monocytes and Ly6Clo monocytes from blood of day 12 Eµ-myc tumor-bearing mice versus healthy (naïve) mice. (C) Numbers of Ly6Chi and Ly6Clo monocytes in the blood of Eµ-myc tumor-bearing mice over time. Right panel – Ly6Chi and Ly6Clo monocytes expressed as a percentage of all CD115+ monocytes. (D) Correlations between granulocytes / Ly6Chi monocytes / Ly6Clo monocytes and B cells in Eµ-myc transgenic mice. (## p < 0.01 ### p < 0.001)
Depletion of monocytic, but not granulocytic populations suppresses tumor growth
We depleted monocytic or granulocytic cells in Eµ-myc tumor-bearing hosts to determine whether reducing the levels of these cells in circulation impacted on tumor growth. Recent reports have shown that although increases in granulocytic MDSC are observed in DLBCL patients, these cells do not correlate with elevated risk factors or reduced survival.Citation26 Similarly, upon depletion of granulocytes with anti-Ly6G antibody (1A8) (), we observed no alterations in Eµ-myc tumor growth () or mouse survival ().
Figure 2. Myeloid cell depletions and monocyte conversion in lymphoma-bearing mice.(A-C) Eµ-myc tumor-bearing mice were given anti-Ly6G mAb or control mAb (c-Ig) treatments i.p. on days 5, 8, 11 and 14 to deplete granulocytes and monitored over time for granulocyte levels and tumor burden in the blood, and survival. (D-F) Eµ-myc tumor-bearing mice were administered clodronate-containing liposomes (Clod Lipo) or control PBS liposomes (PBS Lipo) i.v. on days 2 and 9 and the numbers of circulating macrophages (Macs), monocyte subsets and granulocytes (Grans) were assessed on day 12 (D). Tumor levels in blood (E), and survival (F) were monitored. Ly6Chi and Ly6Clo monocytes were isolated from blood of Eµ-myc tumor-bearing CD45.1+ mice at day 12 and adoptively transferred into either healthy (G) or Eµ-myc tumor-bearing (H) CD45.2+ hosts. Data shows recovery transferred monocytes at 48 hours in blood as a percentage of total CD11b+ myeloid cells (granulocytes excluded) – left panels, and the proportions of Ly6Chi and Ly6Clo monocytes within the transferred population – right panels. (# p < 0.05; ## p <0.01; ### p < 0.001; n = 5 per group)
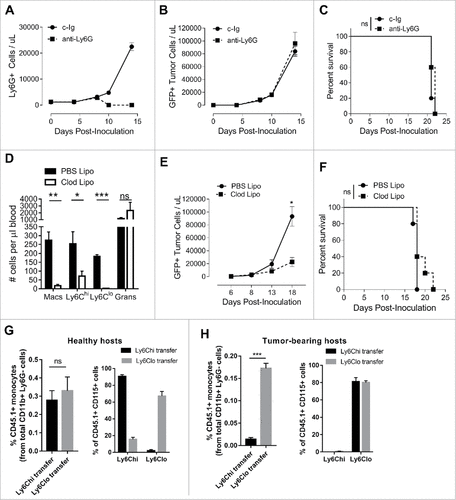
Clodronate liposomes have been used extensively to deplete phagocytic myeloid cell populations in mice, including macrophages and monocytes. Consistent with these findings we observed non-specific, but high level depletion of Ly6Chi and Ly6Clo monocytes and F4/80+ macrophages with Clodronate liposomes in mice challenged with Eµ-myc lymphoma. Granulocyte numbers were not affected (). This treatment led to early suppression of tumor development (), however did not result in improved survival (). Based on these observations, we focused our efforts on understanding the impact of monocytic-MDSCs in lymphoma.
B lymphoma environment promotes Ly6Chi to Ly6Clo monocyte conversion
In the steady-state Ly6Chi monocytes convert into Ly6Clo monocytes.Citation27 To determine whether increased conversion of Ly6Chi monocytes to Ly6Clo monocytes could account for the significant expansion of circulating Ly6Clo monocytes in tumor-bearing mice (, ), we adoptively transferred populations of purified Ly6Chi and Ly6Clo monocyte subsets into healthy or Eµ-myc lymphoma-bearing mice and observed for changes in Ly6C expression. In healthy hosts, CD45.1+ monocytes from both Ly6Chi and Ly6Clo transfers could be recovered equally in the blood at day 2, and their phenotype in respect to Ly6C expression remained largely unchanged (). In contrast, only Ly6Clo monocytes could be consistently recovered from blood of Eµ-myc tumor-bearing hosts, irrespective of whether the original cells transferred were Ly6Chi or Ly6Clo (). This demonstrates that the lymphoma microenvironment promotes preferential survival or expansion of Ly6Clo monocytes and/or rapid conversion of Ly6Chi monocytes to become Ly6Clo. Culturing Ly6Chi monocytes with tumor-conditioned media in vitro did not result in conversion or downregulation of Ly6C (Supplementary Figure 1), suggesting that a cell contact signal or undefined stromal-derived soluble signal in vivo was supporting conversion and/or survival.
Ly6Clo monocytes differentially express immunosuppressive genes
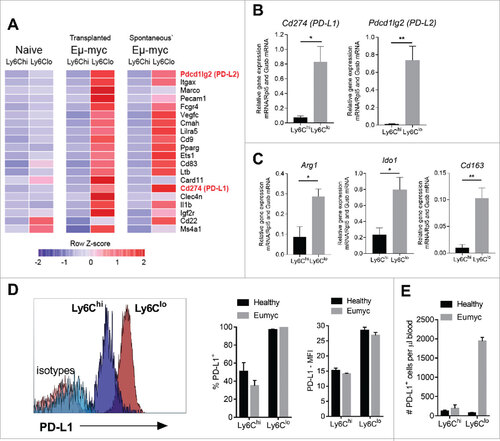
Programmed death ligand (PD-L) expression on myeloid cells can inhibit PD-1+ T cell function.Citation28 Both Cd274 (PD-L1) and Pdcd1lg2 (PD-L2) were included within the 20 most highly differentially expressed genes in Ly6Clo vs Ly6Chi monocytes, analyzed by NanoString RNA nCounter system. Gene changes in monocytes derived from either of the transplantable or spontaneous Eu-myc tumors were similar (). Differential Cd274 and Pdcd1Ig2 was confirmed by qRT-PCR analysis of day 12 transplantable tumor-derived monocytes (). Ly6Clo monocytes also differentially expressed higher levels of other genes associated with myeloid cell immunosuppression, including Arg1 (Arginase), Ido1 (Indoleamine 2,3-dioxygenage) (IDO) and Cd163 (CD163) when directly compared to Ly6Chi monocytes in qRT-PCR analysis (). Expression levels of Arg1, Ido1 and Cd163 in tumor-derived Ly6Chi and Ly6Clo monocytes were not significantly altered when compared to equivalent monocyte populations from healthy mice (Supplementary Figure 2). Focusing on PD-L1, we confirmed that the majority of Ly6Clo monocytes express surface PD-L1 at significantly higher levels than Ly6Chi monocytes. Monocyte PD-L1 protein expression was comparable when isolated from healthy or tumor-bearing mice, indicating that Eµ-myc tumor environment did not affect surface PD-L1 expression (). Due to specific expansion, the majority of circulating PD-L1+ monocytes in tumor-bearing mice are of Ly6Clo phenotype (). We have previously shown that Eµ-myc lymphoma induces PD-1 upregulation on CD8 T cells, thereby creating potential PD-L1/2 : PD-1 inhibitory interactions.Citation29
Figure 3. Monocyte expression of immunosuppressive genes and PD-L1 surface protein levels. (A) Top 20 genes showing largest fold-differences between Ly6Clo – Ly6Chi cells from Eµ-myc 4242 (transplanted) tumor-bearing mice in descending order, and equivalent comparisons in Eµ-myc transgenic (spontaneous) tumor-bearing mice and healthy (naïve) mice. DESeq count normalization was applied to NanoString nCounter count data and the normalized expression data is plotted as a heat map along a relative z-scale (average values of each row is normalized to zero). The colour scheme of the heat map ranges from the minimum and maximum values within each row/gene and represented as a colour gradient from blue to red. (B-C) qRT-PCR assessment of immunosuppressive genes (relative expression) in Ly6Chi and Ly6Clo monocytes from Eµ-myc tumor-bearing mice (n = 4 biological replicates for each group). (D) Representative histograms of PD-L1 expression on monocyte subsets, compared to isotype control antibody staining (as indicated). Graphs show percentages (left panel) and mean fluorescence intensity (MFI) (right panel) of surface PD-L1 expression. (E) Numbers of PD-L1+ Ly6Chi and Ly6Clo monocytes (day 12). (# p < 0.05; ## p < 0.01).
Both Ly6Clo and Ly6Chi monocytes suppress CD8 T cell activity, but via distinct mechanisms
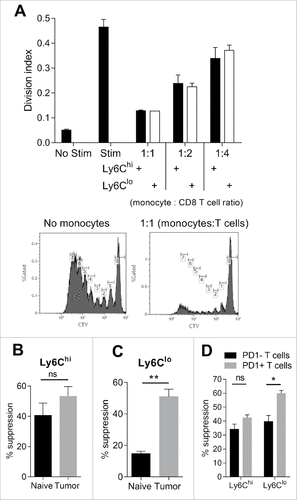
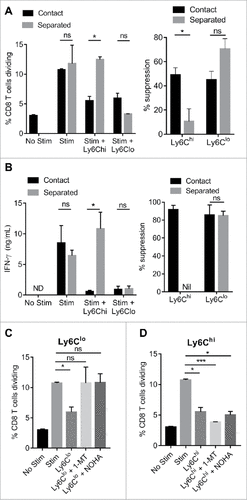
Using in vitro co-cultures we demonstrated that both Ly6Chi and Ly6Clo monocytes were capable of suppressing CD8 T cell proliferation in response to CD3/CD28 stimulation (). Ly6Chi monocytes derived from healthy mice were equally as suppressive as the equivalent tumor-derived population (). In contrast, suppressive activity of Ly6Clo monocytes was elevated in tumor-derived cells (). Ly6Chi monocytes suppressed PD-1 positive and PD-1 negative CD8 T cells to an equivalent extent, suggesting that suppression via the PD-1 axis was not the dominant determinant of T cell sensitivity to monocyte suppression. Ly6Clo monocytes did however display significantly higher suppressive activity against PD-1 positive T cells ().
Figure 4. Immunosuppressive activity of monocyte subsets against CD8 T cells. (A) CD8 T cell proliferation shown as division index, with or without 3 days stimulation with CD3/CD28 beads and co-culture with tumor-derived Ly6Chi or Ly6Clo monocytes at the indicated monocyte to CD8 T cell ratios. Histograms show representative proliferation (CTV dye dilution) of CD8 T cells cultured in presence of absence of monocytes. (B/C) Percent suppression of CD8 T cell proliferation by Ly6Chi and Ly6Clo monocytes derived from healthy (naïve) or Eµ-myc tumor-bearing mice at 1 monocyte : 1 T cell ratio. (D) Percent suppression of PD-1 positive and PD-1 negative CD8 T cell proliferation in the presence of tumor-derived Ly6Chi and Ly6Clo monocytes at 1:1 ratio. (# p < 0.05; ## p < 0.01; n = 5 per group).
By physically separating monocytes from CD8 T cells using transwells, we observed that the suppressive activity of Ly6Chi monocytes was abolished in the absence of cell-to-cell contact; however the suppressive activity of Ly6Clo monocytes was not perturbed. This was shown for suppression of both CD8 T cell proliferation () and IFNγ production (). This suggested that the mechanism of suppression of PD-1 negative CD8 T cells by Ly6Clo monocytes is distinct from that of Ly6Chi monocytes, and was dependent on a soluble factor. Given the observation that tumor-derived Ly6Clo monocytes had high expression of immunosuppressive Ido1 and Arg1 genes (), we blocked these enzymes with specific inhibitors (1-MT and nor-NOHA, respectively) to determine the effect on suppressive activity. Inhibition of IDO or arginase eliminated Ly6Clo monocyte suppression of CD8 T cell proliferation (). Conversely, the suppressive activity of Ly6Chi monocytes was not altered by these agents ().
Figure 5. Mechanisms of monocyte immunosuppressive activity. Purified CD8 T cells were stimulated for 3 days with CD3/CD28 beads in the presence or absence of tumor-derived Ly6Chi and Ly6Clo monocytes co-cultured at 1:1 ratio in physical contact, or separated by transwells. CD8 T cell proliferation (A) and IFN-γ production (B) is shown and the percent suppression induced is indicated on the right. (C/D) Percentage of CD8 T cells dividing when co-cultured with the indicated tumor-derived monocyte subsets at 1:1 ratio, in the presence of absence of IDO inhibitor (1-MT) or Arginase inhibitor (nor-NOHA). (#p < 0.05; ### p < 0.001; n = 4 per group)
Liposomal chemotherapy selectively depletes circulating Ly6Clo monocytes, and synergizes with anti-4-1BB antibody treatment to suppress early tumor growth
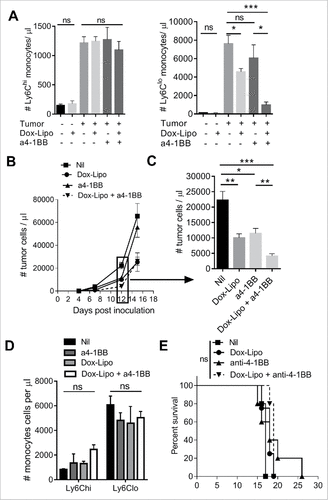
Liposomal doxorubicin (Doxil®) has immunomodulatory effects on myeloid cells,Citation30 however the effect on monocyte subset depletion is largely unknown. We investigated the impact of Doxil treatment on circulating monocyte numbers and Eµ-myc tumor growth with or without co-administration of anti-4-1BB mAb, shown by us previously to promote anti-tumor T cell immunity against Eµ-myc lymphoma.Citation29,31 Doxil treatment significantly reduced Ly6Clo monocyte numbers without affecting Ly6Chi monocytes in tumor-bearing mice (), at the time (day 12) where we typically observe rapid expansion of monocytes in blood. Treatment with anti-4-1BB mAb alone did not overtly affect monocyte numbers, however the combination of Doxil plus anti-4-1BB mAb further reduced circulating levels of Ly6Clo monocytes (). The decrease in Ly6Clo monocytes was associated with reduction in peripheral tumor burden ( and ). Anti-4-1BB mAb treatment led to a specific increase in circulating CD8 T cells, causing a significant shift in CD8 T cell to CD4 T cell ratio, which was not altered by addition of Doxil (Supplementary Figure 3A). Tumor growth increased rapidly by day 15 (), and this was associated with recovery of Ly6Clo monocyte numbers (), and reduction in CD8 T cells (Supplementary Figure 3B). As a result, no survival advantage was observed with this treatment ().
Figure 6. Depletion of Ly6Clo monocytes with liposomal doxorubicin and anti-4-1BB antibody. (A) Numbers of circulating Ly6Chi and Ly6Clo monocytes in healthy or day 12 Eµ-myc tumor-bearing mice after treatment with Doxil (Dox-Lipo) i.v. on days 2 and 9, and/or anti-4-1BB mAb i.p. on days 5 and 12. (B) Tumor burden in the blood over time following the indicated treatments, and (C) numbers of tumor cells in blood at day 12. (D) Numbers of circulating Ly6Chi and Ly6Clo monocytes in tumor-bearing mice on day 15. (E) Survival of Eµ-myc tumor-bearing mice receiving the indicated treatments. (# p < 0.05; ## p < 0.01; ### p < 0.001; n = 5 per group).
Elevated CD14+HLA-DRlo monocytes in DLBCL patients share similar T cell suppressive activity
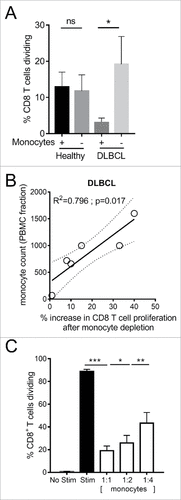
Phenotypically Ly6Clo monocytes in Eµ-myc tumor-bearing mice resemble CD14+HLA-DRlo monocytes which are elevated in the circulation of DLBCL patients.Citation9,32 Both express higher levels of CD163, arginase and PD-L1. Here we assessed whether they also share similar immunosuppressive activity against CD8 T cells. In the presence of monocytes, CD8 T cells from DLBCL patients proliferated less in response to CD3/CD28/4-1BB stimulation than healthy donor CD8 T cells. When CD14+ monocytes were depleted from PBMCs, the proliferation of DLBCL patient CD8 T cells was significantly enhanced (). Increases in CD8 T cell proliferation after monocyte depletion significantly correlated with original patient monocyte counts within the PBMC fraction (). The addition of patient-derived CD14+HLA-DRlo monocytes to healthy CD8 T cells resulted in cell dose-dependent suppression of CD8 T cell proliferation (). These data provide evidence for comparable immunosuppressive activity of an elevated circulating monocyte population found in both mouse and human B lymphoma.
Figure 7. Immunosuppressive activity of CD14+HLA-DRlo monocytes from DLBCL patients. (A) CD8 T cell proliferation after 3 days stimulation of healthy donor (n = 8) or DLBCL patient (n = 6) PBMC with anti-CD3/CD28/4-1BB beads, with or without depletion of CD14+ monocytes. (B) Correlation of DLBCL patient CD14+ monocyte counts within the PBMC fraction versus the percentage increase in CD8 T cell proliferation after CD14+ monocyte depletion. (C) Proliferation of purified CD8 T cells from healthy donor PBMC (n = 18) following stimulation for 3 days with anti-CD3/CD28/4-1BB beads and co-culture with CD14+HLA-DRlo monocytes isolated from DLBCL patients PBMC, at the indicated ratios. (#p < 0.05; ## p < 0.01; ### p < 0.001)
Discussion
B cell lymphomas are aggressive cancers with high levels of disease relapse and mortality following treatment. Tumor-evoked immunosuppression is a major impediment to successful treatment outcomes in many haematological malignancies, including B cell lymphoma. Advances in technology have allowed us to better understand and appreciate the interactions between tumors and the immune system in complex microenvironments. Utilising a pre-clinical mouse model of B cell lymphoma, this study has extended our understanding of the characteristics and immunosuppressive activities of tumor-evoked monocyte populations, and provides the basis for appropriate immunotherapeutic intervention.
Evidence in B cell malignancies suggests that systemic immunosuppression is maintained largely by myeloid lineage cells including Mo-MDSCs, Gr-MDSCs and M2-like tumor-associated macrophages (M2-TAMs).Citation33–35 Recent studies have identified a CD14+HLA-DRlo population of monocytes in B-NHL that may represent a distinct population of moMDSC. Patients with increased levels of circulating CD14+HLA-DRlo monocytes had more aggressive disease, displayed suppressed adaptive immunity and were more likely to relapse post-therapy.Citation9,32 Further to this, we have previously identified up-regulated expression of the scavenger receptor, CD163 as a distinguishing marker of this highly immunosuppressive subset of monocytes in poor-prognosis DLBCL, that are distinct to CD163 negative monocytes.Citation36 CD163+ monocytes were elevated in the circulation of pre-therapy DLBCL patients and were also markedly elevated in patients that showed persistence of disease after 4 cycles of R-CHOP treatment. CD163+ monocytes from DLBCL patients also displayed significantly greater activity levels of the immunosuppressive enzyme, arginase, and expressed higher levels of the immune-inhibitory ligand, PD-L1.Citation36 Whether these CD163+ monocytes are precursor cells to the CD68+/CD163+ M2-TAMs that have been associated with poor clinical outcomes in patients with DLBCLCitation37 or Hodgkin's lymphomaCitation16,38,39 remains unclear. Our data here reveals that circulating CD14+HLA-DRlo monocytes from DLBCL patients cause direct impairment of CD8 T cell proliferation. In contrast, monocytes from healthy donors did not display immunosuppressive activity.
By investigation of circulating myeloid populations in Eµ-myc lymphoma-bearing mice we were able to draw parallels with the findings in DLBCL patients. For example, there was a strong correlation between circulating CD11b+CD115+ monocytes and tumor burden, which was observed in both spontaneous and transplantable Eµ-myc tumor settings. In addition, Ly6Clo monocytes, which expressed higher levels of Cd163, became the dominant monocyte population in tumor-bearing mice. Furthermore, these cells displayed an immunosuppressive genetic profile, evidenced by elevated expression of Cd274 and Pdcd1lg2, Arg1 and Ido1, and suppressed PD-1 negative CD8 T cells in an arginase / IDO dependent manner. Together, these data show that mouse Ly6Clo monocytes that accumulate in the circulation during Eµ-myc lymphoma development have similar characteristics to the elevated CD14+HLA-DRlo monocyte population observed in DLBCL patients.Citation9,32,36
The significance of Gr-MDSC in prognosis of B-NHLs is less clear than for Mo-MDSC cells, largely because some studies have shown that high neutrophil numbers at diagnosis represents a poor prognostic factor for clinical outcome in patients with DLBCL,Citation40,41 whilst others show no such association.Citation26 Although we observed significant granulocytosis in Eu-myc tumor-bearing mice, depletion of these cells did not impact on tumor growth in this setting.
The accumulation of Ly6Clo monocytes in circulation may be a consequence of increased Ly6Chi to Ly6Clo conversion, preferential expansion/survival of the Ly6Clo subset, or altered migration of monocyte subsets and differentiation into macrophages. Monocyte adoptive transfer experiments revealed that in tumor-bearing hosts the recovery of transferred Ly6Clo monocytes from blood was higher than for Ly6Chi cells, whereas in healthy hosts the recovery of subsets was equivalent. This implies that the tumor microenvironment preferentially supports the survival of Ly6Clo cells, or alternatively promotes migration of Ly6Chi cells out of circulation. Furthermore, the majority of recovered monocytes (>80%) in tumor-bearing hosts were of the Ly6Clo phenotype, regardless of their phenotype upon transfer. Conversely, in healthy mice, the initial Ly6C expression phenotype was maintained upon recovery. This strongly suggests that signals from the tumor environment leads to increased conversion of Ly6Chi to Ly6Clo monocytes. Recently it has been shown that signals from endothelial cells convert Ly6Chi to Ly6Clo monocytes, in a Notch ligand-dependent manner.Citation42 Previous reports have described pro-angiogenic behavior in aggressive B cell lymphoma which is associated with a poor clinical outcome.Citation43,44 Further studies are required to determine whether dysregulated angiogenesis and an increase in endothelial cells in Eµ-myc lymphoma promotes conversion of mobilized Ly6Chi monocytes to long-lived Ly6Clo monocytes. Identifying whether circulating immunosuppressive monocytes in B cell lymphoma hosts are precursors cells or a reservoir for equivalent populations in the malignant lymphoid tissues, will allow us to potentially target these cells with systemic treatment.
Macrophages and certain monocyte subsets are inherently phagocytic, making them ideal targets for nanomedicine. It was recently shown that Ly6Clo monocytes have greater phagocytic activity.Citation19 This provided the impetus to selectively target tumor-evoked Ly6Clo monocytes with liposome-based approaches. We demonstrated that delivery of bisphosphate-encapsulated liposomes, used extensively for in vivo depletion of macrophages,Citation45,46 effectively removed Ly6Clo monocytes and to a greater extent than Ly6Chi monocytes in tumor-bearing hosts. Importantly, this monocyte/macrophage depletion alone resulted in transient suppression of Eµ-myc tumor growth. Liposomes had no direct effect on the viability of Eµ-myc tumors cells, lymphocyte numbers were not affected by this treatment and no evidence of treatment-related toxicity was observed at the dose tested. Although a trend existed, there was no statistically significant survival advantage for Clod-Lipo treated mice, indicating that additional immune stimulation coinciding with monocyte depletion may be required to prevent tumor outgrowth.
Liposomal doxorubicin (e.g. Doxil®) is approved for treatment of ovarian cancer, multiple myeloma and sarcoma. For treatment of B cell lymphoma, liposomal doxorubicin has been shown to reduce incidence of cardiotoxicity and other side effects when compared to conventional doxorubicin, whilst retaining efficacy.Citation47,48 In the Eµ-myc model we demonstrate that Doxil treatment leads to a selective, albeit transient, reduction in the numbers of circulating Ly6Clo monocytes, which was associated with an early suppression or tumor growth. The extent to which the anti-tumor effect of Doxil is associated with reduction of immunosuppressive monocytes versus direct cytotoxic effects on tumor cells remains to be elucidated. Regardless, targeted liposomal delivery of cytotoxic agents to myeloid cells may represent a novel and safe strategy to alleviate immunosuppression and boost responses to immunotherapeutic vaccines.
We predict that exclusively inhibiting immunosuppressive pathways utilized by monocytes/macrophages may be suboptimal in eliciting potent and persistent anti-tumor immunity. We rationalize that providing immune stimulation in parallel with removing immunosuppression will result in superior generation/restoration of T cell function and elimination of established tumors. We have supportive evidence to show that administration of an agonistic antibody which targets the T cell co-stimulatory molecule 4-1BB (CD137), leads to effective generation, expansion and survival of anti-lymphoma CD8 T cells.Citation31 When we utilized anti-4-1BB mAb agonism to drive T cell stimulation following Doxil treatment, we observed enhanced, but short-lived anti-tumor protection. Further development and optimization of this and other combination immunotherapy approaches that provide the dual benefit of alleviating immunosuppression together with unleashing anti-tumor T cell activity is warranted. Nivolumab (anti-PD-1 mAb) is approved for second-line treatment of Hodgkin's Lymphoma and is in phase II/III trials for non-Hodgkin's B lymphoma. Anti-4-1BB antibodies are also currently being tested in clinical trials against a variety of lymphomas. We predict that this two-pronged immunotherapuetic strategy could be implemented as consolidation treatment after front-line treatment with Rituximab-chemotherapy based regimes, where modulation of the immune microenvironment and engaging anti-tumor immunity might be most effective at preventing tumor relapse. Finally, although the focus here is B cell lymphomas, the implications of these findings may be applicable to multiple other cancer types where myeloid cell immunosuppression has been reported.
Methods
Mice and lymphoma model
Inbred C57BL/6 and SJL/B6.ptprca mice were obtained from the Animal Resources Centre (Perth, Australia). Mice were aged between 6 and 10 weeks, sex matched and housed under specific pathogen-free conditions. Eµ-myc transgenic (Tg) mice on the C57BL/6 background were bred and maintained onsite at the Translational Research Institute Biological Research Facility (TRI-BRF) (Brisbane, Australia). All experiments were performed in accordance with the animal ethics guidelines provided by the National Health and Medical Research Council of Australia and approved by the University of Queensland Health Sciences Animal Ethics Committee (approval 295/15). Transplantable murine lymphomas were derived from Eμ-myc Tg mice, which spontaneously develop B-cell lymphoma owing to constitutive expression of the c-myc oncogene under the immunoglobulin H promoter.Citation25 The Eμ-myc clone 4242 used for transplantation was a freshly isolated lymphoma from the lymph nodes of an Eμ-myc transgenic mouse and stably transduced with green fluorescent protein (GFP) by retroviral transduction as previously described.Citation49 Mice in tumor-bearing groups were inoculated intravenously with 1 × 105 tumor cells. Tumor growth was monitored by flow cytometry, measuring CD19+GFP+ events in peripheral blood.
Patient eligibility and blood sampling
Patient samples were obtained from a planned prospective laboratory sub-study sponsored by the ALLG within the NHL21 clinical trial.Citation50 Patients had CD20+ diffuse large B cell lymphoma (DLBCL) with international prognostic index (IPI) 2–5 or IPI 0–1 with bulky tumor (≥7.5 cm). Clinical parameters were source verified and IPI centrally reviewed. Thirty millilitres of blood was collected pre-therapy. Blood was also taken from healthy laboratory volunteers without prior malignant or autoimmune disorders. All participants gave written informed consent and participating Hospital/Research Institute Ethics Committees approved the study.
Flow cytometry
Fluorochrome-conjugated monoclonal antibodies (mAbs) against mouse CD43 (1B11), CD115 (AFS98), CD3 (145-2C11), CD4 (RM4-5), CD8 (YTS156.7.7), CD19 (1D3), Ly6G (1A8), CX3CR1 (SA011F11), Ly6C (HK1.4), PDL-1 (10F.9G2), F4/80 (BM8), CD45.1 (A20) and CD11b (M1/70) were used to label cells and purchased from Biolegend (San Diego, CA, USA) and BD Pharmingen (Franklin Lakes, New Jersey, USA). Cells were labelled at optimal concentrations of mAb for 25 min at 4°C in PBS containing 2% newborn calf serum and 2 mM EDTA. Flow-count fluorospheres (Beckman Coulter, Brea, California, USA) were added to the samples to calculate cell numbers upon acquisition. Labelled cells were acquired on Gallios (Beckman Coulter, Brea, California, USA) Astrios or Aria flow cytometers and analyzed using the Kaluza version 1.2 (Beckman Coulter, Brea, California, USA) software.
RNA extraction, purification and cDNA synthesis
Total RNA was extracted and purified from sorted Ly6Chi and Ly6Clo cells using the ISOLATE II RNA micro kit (Bioline, NSW, Australia) according to the manufacturers' instructions. cDNA was synthesized from 400 ng of total RNA using Superscript III Reverse Transcriptase (Invitrogen, Thermo Fisher Scientific) and random hexameric primers according to the manufacturer's instructions.
Quantitative real-time PCR (qPCR), and primers
Relative expression of genes was determined using the Applied Biosystems™ ViiA™ 7 Real-Time PCR System (ABI, Singapore) Relative gene expression levels were analyzed by qPCR using Mus musculus primer sequences designed for use with Applied Biosystems™ SYBR green master mix. Standard qPCR cycles was performed in all cases; polymerase activation step at 95°C for 10 min, followed by 40 cycles of two-step thermal cyclic PCR at 95°C for 15 sec and 60°C for 1 min. Primers were synthesized and obtained from Integrated DNA Technologies (IDT, NSW, Australia) and they include the following:Arg1, ACAAGACAGGGCTCCTTTCAG and GGCTTATGGTTACCCTCCCG, Cd163, GTGCTGGATCTCCTGGTTGTA and GGAGCGTTAGTGACAGCAGA, Cd274 (PD-L1), TCACTTGCTACGGGCGTTTAC and CACCACTAACGCAAGCAGG, Ido1, CAAAGCAATCCCCACTGTATCC and CTATGTCGTGCAGTGCCTTTTC, Pdcd1lg2 (PD-L2), GCCTCAGCCTAGCAGAAACT and TTTGGGTTCCATCCGACTCAG. Primer sequences for the endogenous controls used are as follow: Rpl5, GTACATCGGAAGCACATCATGG and CTCCATCATGTCTGGAGTTACG, Gusb, CAGTTGTGTGGGTGAATGGGA and CACTCTGGACCAGCTTGCTA, and Hprt, CCAGCGTCGTGATTAGCGAT and GCAAGTCTTTCAGTCCTGTCC. Raw Ct values from the qPCR experiments were imported and processed using the ReadqPCR and NormqPCR Bioconductor R packages.Citation51 The GeNorm methodCitation52 embedded within NormqPCR was used to identify the two most robust housekeeping genes (Rpl5 and Gusb) and gene expression data was normalized to the endogenous controls using the delta Ct method. The resulting delta Ct values are used for differential testing using an unpaired two-tailed Student's t-test for each gene where P<0.05 was considered statistically significant.
Nanostring™ nCounter RNA quantification
RNA was extracted from freshly sorted monocyte populations harvested from blood of day 12 Eµ-myc 4242 transplanted mice (10 week old wildtype mice), 11 week old Eµ-myc transgenic mice and age-matched healthy mice, and RNA quantified using Qubit RNA HS Assay (Thermo Fisher). Genes were quantified using the nCounter platform (Nanostring™ Technologies, Seattle, WA, USA). Immunosuppression-related genes were analyzed using a customized nCounter mouse immunology panel. Hybridizations were carried out according to the NanoString Gene Expression Assay Manual. Five microliter of each RNA sample (100ng) was mixed with 20μl of nCounter Reporter probes in hybridization buffer and 5μl of nCounter Capture probes for a total reaction volume of 30μl. The hybridizations were incubated at 65°C for 16–20 hours. Raw data was imported and analysed in the NanoString® data analysis tool nSolver. For normalization, gene expression data was internally controlled to the mean of the positive control probes to account for inter-assay variability. (Rounded) expression data was then normalized using DESeqCitation53 to obtained normalized count data and visualized as a heat map.
Monocyte adoptive transfer
Monocytes were isolated from the peripheral blood of Eµ-myc 4242 tumor-bearing SJL/B6.ptprca mice at day 12. Blood was collected into heparinized PBS, lysed with ACK lysis buffer then spun 300xg 4°C 5 minutes. CD19+ cells were depleted using EasySep™ Mouse CD19 Positive Selection Kit II (StemCell Technologies, Vancouver, Canada) as per manufacturer's instructions. CD19-depleted cell fraction was labelled at optimal concentrations of mAb for 25 min at 4°C in PBS containing 2% newborn calf serum and 2 mM EDTA. Cells were sorted into Ly6Chi and Ly6Clo populations on the Astrios (Beckman Coulter, Brea, California, USA) or Aria (BD, Franklin Lakes, New Jersey, USA) to a purity of greater than 95%. Sorted cells were injected intravenously at 2 × 105 into healthy or Eµ-myc 4242 tumor-bearing C57Bl/6 hosts. Adoptively transferred monocytes were monitored in the blood via flow cytometry and identified as CD45.1+ within the CD45.2+ host. Single cell suspensions were lysed with ACK lysis buffer. Cells were labelled as per the flow cytometry protocol.
Monoclonal antibody and liposome treatments for cell depletions or activation in vivo
Granulocytes were depleted in tumor-bearing mice by administration of 100 µg anti-Ly6G mAb – clone 1A8, or isotype-matched control (BioXCell, NH, USA) on days 5, 8, 11 and 14 after tumor inoculation. Clodronate liposomes (clodronateliposomes.com, Haarlem, The Netherlands) and Doxil® (Doxorubicin hydrochloride liposomes) (Jannsen, Horsham, PA, USA), we used to deplete monocyte and macrophage populations. Liposomes were administered at 1mg/kg intravenously on days 2 and 9 post-tumor inoculation. Anti-4-1BB mAb – clone 3H3 (BioXCell, NH, USA) was administered at 100 µg doses on days 5 and 12 to stimulate T cells. All antibodies were given via intraperitoneal route.
Murine T cell suppression assays
Monocytes were isolated from the peripheral blood of C57Bl/6 mice bearing day 12 Eµ-myc 4242 tumours. Blood was collected into heparinized PBS, lysed with ACK lysis buffer then spun 300xg 4°C 5 minutes. CD19+ cells were depleted using EasySep™ Mouse CD19 Positive Selection Kit II (StemCell Technologies, Vancouver, Canada) as per manufacturer's instructions. The CD19-depleted cell fraction was labelled at optimal concentrations of mAb for 25 min at 4°C in PBS containing 2% newborn calf serum and 2 mM EDTA. Cells were sorted into Ly6Chi and Ly6Clo populations on the Astrios (Beckman Coulter, Brea, California, USA) or Aria (BD). CD8+ T cells from healthy mice were isolated using EasySep™ Mouse CD8+ T Cell Isolation Kit (StemCell Technologies, Vancouver, Canada) as per manufacturer's instructions. Enriched CD8+ T cells were labelled with 5uM CellTrace Violet (Life Technologies, Carlsbad, California, United States) to monitor proliferation. CTV-labelled CD8+ T cells and sorted monocytes were suspended at 1.5 × 106 cells/ml in RMPI + NaPyr + 2ME + P/S/G + 10% FCS + 1ng/ml hIL-2. Dynabeads® Mouse T-Activator CD3/CD28 for T Cell Expansion and Activation (Thermo Fisher Scientific, Waltham, Massachusetts, USA) were added to the cultures at a 1:1 ratio of beads : CD8+ T cells. Cultures were incubated at 37°C for 4 days. Proliferation of T cells was measured by CTV dilution using the Gallios (Beckman Coulter, Brea, California, USA) and analyzed using the Kaluza version 1.2 (Beckman Coulter, Brea, California, USA) software. Division index was calculated as total number of divisions / number of cells in the start of the culture. Percentage cells dividing was calculated as the number of original cells which proliferated / total number of starting cells. Inhibition of arginase was achieved with the addition of 300 µM of N-hydroxy-nor-Arginine (nor-NOHA) (Cayman Chemical, An Arbor, Michigan, USA). Inhibition of indoleamine was achieved with the addition of 100 µM of 1-methyl-DL-tryptophan (1-MT) (Sigma-Aldrich, St Louis, Missouri, USA). HTS Transwell® 96 well plates with 3 µm pore size (Corning, Lowell, MA, USA) were used in some experiments for physical separation of monocytes and T cells.
Human T cell suppression assays
CFSE stained mononuclear cells, either monocyte replete or depleted, were seeded in 96-well round-bottom plates at 2–5 × 105 cells/well in RMPI 1640 + 10% FBS + 1x penicillin/streptomycin + 10U/ml IL-2. Monocytes were depleted using an immuno-magnetic CD14 positive selection procedure (EasySep, StemCell Technologies) and depletion efficacy ranged from 70% – 96% (median: 85.2%). Remaining cells were stimulated with anti-CD3/CD28/4-1BB beads (Invitrogen, Carlsbad, California, USA) at a ratio of 1:10 bead:cell. Cells were cultured for 96 hours and proliferation assessed by flow cytometry (LSRII flow cytometer, BD BioSciences). For monocyte – T cell co-cultures, monocytes were FACS sorted based on CD14+HLA-DRlo expression and responder CD3+ T cells was purified by negative selection from autologous PBMC using a negative Pan-T cell isolation protocol (Miltenyi Biotec). After immuno-magnetic selection T cell purity was greater than 90%. Purified T cells were stained with CFSE and 5 × 104 were plated into 96 well round bottom plates with 200U/ml IL-2. Graded numbers of monocytes (5 × 104, 2.5 × 104, and 1.25 × 104) were added to the T cells. Polyclonal T cell expansion was achieved by addition of anti-CD3/CD28/4-1BB beads at a ratio of 1:10 bead:cell and proliferation was assessed by CFSE dilution on flow cytometry.
Detection of IFN-γ
IFNγ levels in mouse sera were detected using an ELISA kit from R&D Systems (MN, USA) as per manufacturer's instructions. Acquisition and analysis was performed on a Multiskan FC Photometer (Thermo Scientific, MA, USA).
Statistical analysis
Results are expressed as the mean ± SEM. Univariate analysis was carried out using either Student's t-test for data which were normally distributed (passed D'Agnostino-Pearson omnibus normality test) or Mann-Whitney test for those which were not normally distributed. Grouped data were analysed by one or two-way ANOVA. Kaplan-Meier plots in conjunction with a log-rank test were used to assess mouse survival (GraphPad Prism 6 software, San Diego, CA). Significance was accepted at P values < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authorship contributions
SJM designed and performed experiments, data analysis and assisted with writing of the manuscript. ZKT, TK, BLD, MSFS, MN, PYL, CK, FV and DM assisted with experimentation, data analysis and provided experimental feedback. RM, GRL and MKG provided critical input throughout the study and critical review of the manuscript. SRM conceived the study, designed and supervised the experiments, assisted with data analysis and wrote the manuscript.
2017ONCOIMM0745R-s02.docx
Download MS Word (454.7 KB)Acknowledgments
The authors thank Bradley Buchan, Rebecca West, Ben Harvie, Kendall Hepple and Kamil Sokolowski, (Biological Resources Facility) for mouse husbandry and technical assistance, and David Sester, Dalia Khalil and Yitian Ding (Flow Cytometry Facility) for technical assistance. Prof Ricky Johnstone (Peter MacCallum Cancer Centre) for originally providing the Eµ-myc transgenic mice and the Australasian Leukaemia Lymphoma Group for access to the NHL21 samples. This work was supported by Project Grant (1044355) from the National Health and Medical Research Council (NHMRC) of Australia. ZKT is supported by an Advance Queensland Early Career Fellowship (07416). PYL is supported by a University of Queensland International Scholarship. TK and MDN are supported by an Australian Government Research Training Program (RTP) Scholarship. CK is supported by an NHMRC Early Career Fellowship (1120171). MKG is supported by the Leukaemia Foundation of Australia. SRM is supported by an NHMRC Career Development Fellowship (1061429).
References
- Sun HL, Pan YQ, He BS, Nie ZL, Lin K, Peng HX, Cho WC, Wang SK. Prognostic performance of lymphocyte-to-monocyte ratio in diffuse large B-cell lymphoma: an updated meta-analysis of eleven reports. Onco Targets Ther. 2016;9:3017–23. PMID:27284252.
- Tadmor T, Bari A, Sacchi S, Marcheselli L, Liardo EV, Avivi I, Benyamini N, Attias D, Pozzi S, Cox MC, et al. Monocyte count at diagnosis is a prognostic parameter in diffuse large B-cell lymphoma: results from a large multicenter study involving 1191 patients in the pre- and post-rituximab era. Haematologica. 2014;99:125–30. doi:10.3324/haematol.2013.088161. PMID:23935023.
- Li ZM, Huang JJ, Xia Y, Sun J, Huang Y, Wang Y, Zhu YJ, Li YJ, Zhao W, Wei WX, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One. 2012;7:e41658. doi:10.1371/journal.pone.0041658. PMID:22911837.
- Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi:10.1016/j.jacc.2013.09.019. PMID:24140662.
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi:10.1038/nrc2444. PMID:18633355.
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi:10.1038/nature10138. PMID:21654748.
- Allaoui R, Bergenfelz C, Mohlin S, Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirström K, Påhlman S, et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun. 2016;7:13050. doi:10.1038/ncomms13050. PMID:27725631.
- Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, Xu X, Zhang H, Santin AD, Lou G, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126:4157–73. doi:10.1172/JCI87252. PMID:27721235.
- Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–81. doi:10.1182/blood-2010-05-283820. PMID:21063024.
- Xiu B, Lin Y, Grote DM, Ziesmer SC, Gustafson MP, Maas ML, Zhang Z, Dietz AB, Porrata LF, Novak AJ, et al. IL-10 induces the development of immunosuppressive CD14(+)HLA-DR(low/-) monocytes in B-cell non-Hodgkin lymphoma. Blood Cancer J. 2015;5:e328. doi:10.1038/bcj.2015.56. PMID:26230952.
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–70. doi:10.1126/science.1142883. PMID:17673663.
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14:392–404. doi:10.1038/nri3671. PMID:24854589.
- Feng AL, Zhu JK, Sun JT, Yang MX, Neckenig MR, Wang XW, Shao QQ, Song BF, Yang QF, Kong BH, et al. CD16+ monocytes in breast cancer patients: expanded by monocyte chemoattractant protein-1 and may be useful for early diagnosis. Clin Exp Immunol. 2011;164:57–65. doi:10.1111/j.1365-2249.2011.04321.x. PMID:21361908.
- Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15. doi:10.1158/1078-0432.CCR-13-0525. PMID:23653148.
- Andersen MN, Abildgaard N, Maniecki MB, Moller HJ, Andersen NF. Monocyte/macrophage-derived soluble CD163: a novel biomarker in multiple myeloma. Eur J Haematol. 2014;93:41–7. doi:10.1111/ejh.12296. PMID:24612259.
- Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, Gottlieb D, Ritchie D, Gill D, Gandhi MK. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013; 19:731–42. doi:10.1158/1078-0432.CCR-12-2693. PMID:23224400.
- Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang LC, Heitjan D, Snyder LA, et al. CCL2 Blockade Augments Cancer Immunotherapy. Cancer Res. 2010;70:109–18. doi:10.1158/0008-5472.CAN-09-2326. PMID:20028856.
- Hanna BS, McClanahan F, Yazdanparast H, Zaborsky N, Kalter V, Rossner PM, Benner A, Dürr C, Egle A, Gribben JG, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2016;30:570–9. doi:10.1038/leu.2015.305. PMID:26522085.
- Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–90. doi:10.1126/science.aac9407. PMID:26494174.
- Lapuc I, Bolkun L, Eljaszewicz A, Rusak M, Luksza E, Singh P, Miklasz P, Piszcz J, Ptaszynska-Kopczynska K, Jasiewicz M, et al. Circulating classical CD14++CD16- monocytes predict shorter time to initial treatment in chronic lymphocytic leukemia patients: Differential effects of immune chemotherapy on monocyte-related membrane and soluble forms of CD163. Oncol Rep. 2015;34:1269–78. doi:10.3892/or.2015.4088. PMID:26135617.
- Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res 2014;20:1601–9. doi:10.1158/1078-0432.CCR-13-2508. PMID:24323899.
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419–30. doi:10.1007/s00262-011-1028-0. PMID:21644036.
- Wu C, Wu X, Zhang X, Chai Y, Guo Q, Li L, Yue L, Bai J, Wang Z, Zhang L. Prognostic significance of peripheral monocytic myeloid-derived suppressor cells and monocytes in patients newly diagnosed with diffuse large b-cell lymphoma. Int J Clin Exp Med. 2015;8:15173–81. PMID:26629001.
- Giallongo C, Parrinello NL, Tibullo D, La Cava P, Romano A, Chiarenza A, et al. Monocytic Myeloid Derived Suppressor CELLS (M-MDSC) As Prognostic Factor in Chronic Myeloid Leukemia Patients Treated with Dasatinib. Blood. 2015;126:2767.
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985;318:533–8. doi:10.1038/318533a0. PMID:3906410.
- Azzaoui I, Uhel F, Rossille D, Pangault C, Dulong J, Le Priol J, Lamy T, Houot R, Le Gouill S, Cartron G, et al. T-cell defect in diffuse large B-cell lymphomas involves expansion of myeloid-derived suppressor cells. Blood. 2016;128:1081–92. doi:10.1182/blood-2015-08-662783. PMID:27338100.
- Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017;17:349–62. doi:10.1038/nri.2017.28. PMID:28436425.
- Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5:e1247135. doi:10.1080/2162402X.2016.1247135. PMID:28123883.
- McKee SJ, Doff BL, Soon MS, Mattarollo SR. Therapeutic Efficacy of 4-1BB Costimulation Is Abrogated by PD-1 Blockade in a Model of Spontaneous B-cell Lymphoma. Cancer Immunol Res. 2017;5:191–7. doi:10.1158/2326-6066.CIR-16-0249. PMID:28115358.
- Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, Zhao W, Leow CC, Hollingsworth R. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 2015;17:661–70. doi:10.1016/j.neo.2015.08.004. PMID:26408258.
- Kobayashi T, Doff BL, Rearden RC, Leggatt GR, Mattarollo SR. NKT cell-targeted vaccination plus anti-4-1BB antibody generates persistent CD8 T cell immunity against B cell lymphoma. Oncoimmunology. 2015;4:e990793. doi:10.4161/2162402X.2014.990793. PMID:25949907.
- Khalifa KA, Badawy HM, Radwan WM, Shehata MA, Bassuoni MA. CD14(+) HLA-DR low/(-) monocytes as indicator of disease aggressiveness in B-cell non-Hodgkin lymphoma. Int J Lab Hematol. 2014;36:650–5. doi:10.1111/ijlh.12203. PMID:24636145.
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi:10.1038/nri3789. PMID:25614318.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi:10.1038/nri2506. PMID:19197294.
- De Veirman K, Van Valckenborgh E, Lahmar Q, Geeraerts X, De Bruyne E, Menu E, Van Riet I, Vanderkerken K, Van Ginderachter JA. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol. 2014;4:349. doi:10.3389/fonc.2014.00349. PMID:25538893.
- Keane C, Vari F, Hertzberg M, Cao KA, Green MR, Han E, Seymour JF, Hicks RJ, Gill D, Crooks P. Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: a population-based study. Lancet Haematol. 2015;2:e445–55. doi:10.1016/S2352-3026(15)00150-7. PMID:26686046.
- Marchesi F, Cirillo M, Bianchi A, Gately M, Olimpieri OM, Cerchiara E, Renzi D, Micera A, Balzamino BO, Bonini S, et al. High density of CD68+/CD163+ tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematological oncology. 2015;33:110–2. doi:10.1002/hon.2142. PMID:24711044.
- Harris JA, Jain S, Ren Q, Zarineh A, Liu C, Ibrahim S. CD163 versus CD68 in tumor associated macrophages of classical Hodgkin lymphoma. Diagnostic pathology. 2012;7:12. doi:10.1186/1746-1596-7-12. PMID:22289504.
- Klein JL, Nguyen TT, Bien-Willner GA, Chen L, Foyil KV, Bartlett NL, Duncavage EJ, Hassan A, Frater JL, Kreisel F. CD163 immunohistochemistry is superior to CD68 in predicting outcome in classical Hodgkin lymphoma. Am J Clin Pathol. 2014;141:381–7. doi:10.1309/AJCP61TLMXLSLJYS. PMID:24515766.
- Keam B, Ha H, Kim TM, Jeon YK, Lee SH, Kim DW, Kim CW, Heo DS. Neutrophil to lymphocyte ratio improves prognostic prediction of International Prognostic Index for patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2015;56:2032–8. doi:10.3109/10428194.2014.982642. PMID:25382617.
- Troppan K, Deutsch A, Gerger A, Stojakovic T, Beham-Schmid C, Wenzl K, Neumeister P, Pichler M. The derived neutrophil to lymphocyte ratio is an independent prognostic factor in patients with diffuse large B-cell lymphoma. Br J Cancer. 2014;110:369–74. doi:10.1038/bjc.2013.763. PMID:24357794.
- Gamrekelashvili J, Giagnorio R, Jussofie J, Soehnlein O, Duchene J, Briseno CG, Ramasamy SK, Krishnasamy K, Limbourg A, Kapanadze T, et al. Regulation of monocyte cell fate by blood vessels mediated by Notch signalling. Nat Commun. 2016;7:12597. doi:10.1038/ncomms12597. PMID:27576369.
- Ribatti D, Nico B, Ranieri G, Specchia G, Vacca A. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia. 2013;15:231–8. doi:10.1593/neo.121962. PMID:23479502.
- Suhasini AN, Wang L, Holder KN, Lin AP, Bhatnagar H, Kim SW, Moritz AW, Aguiar RCT. A phosphodiesterase 4B-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia. 2016;30:617–26. doi:10.1038/leu.2015.302. PMID:26503641.
- Barrera P, Blom A, van Lent PL, van Bloois L, Beijnen JH, van Rooijen N, de Waal Malefijt MC, van de Putte LB, Storm G, van den Berg WB. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43:1951–9. doi:10.1002/1529-0131(200009)43:9%3c1951::AID-ANR5%3e3.0.CO;2-K. PMID:11014344.
- Nakamura T, Abu-Dahab R, Menger MD, Schafer U, Vollmar B, Wada H, Lehr CM, Schäfers HJ. Depletion of alveolar macrophages by clodronate-liposomes aggravates ischemia-reperfusion injury of the lung. J Heart Lung Transplant. 2005;24:38–45. doi:10.1016/j.healun.2003.10.007. PMID:15653377.
- Oki Y, Ewer MS, Lenihan DJ, Fisch MJ, Hagemeister FB, Fanale M, Romaguera J, Pro B, Fowler N, Younes A, et al. Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: an open label, single arm, phase II trial. Clin Lymphoma Myeloma Leuk. 2015;15:152–8. doi:10.1016/j.clml.2014.09.001. PMID:25445468.
- Zhou D, Li L, Bao C, Zhu J, Zhu L, Yang X, Zheng Y, Zhou M, Luo X, Xie W, et al. Replacement of conventional doxorubicin by pegylated liposomal doxorubicin in standard RCHOP chemotherapy for elderly diffuse large B-Cell lymphoma: a retrospective study in China. Int J Clin Exp Med 2015; 8:22497–502. PMID:26885233.
- Lindemann RK, Newbold A, Whitecross KF, Cluse LA, Frew AJ, Ellis L, Williams S, Wiegmans AP, Dear AE, Scott CL, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci U S A. 2007;104:8071–6. doi:10.1073/pnas.0702294104. PMID:17470784.
- Hertzberg M, Gandhi MK, Trotman J, Butcher B, Taper J, Johnston A, Gill D, Ho SJ, Cull G, Fay K, et al. Early treatment intensification with R-ICE and 90Y-ibritumomab tiuxetan (Zevalin)-BEAM stem cell transplantation in patients with high-risk diffuse large B-cell lymphoma patients and positive interim PET after 4 cycles of R-CHOP-14. Haematologica. 2017;102:356–63. doi:10.3324/haematol.2016.154039. PMID:28143954.
- Perkins JR, Dawes JM, McMahon SB, Bennett DL, Orengo C, Kohl M. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genomics. 2012;13:296. doi:10.1186/1471-2164-13-296. PMID:22748112.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi:10.1186/gb-2002-3-7-research0034. PMID:12184808.
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi:10.1186/gb-2010-11-10-r106. PMID:20979621.
