ABSTRACT
Interleukin (IL)-27 is a multifunctional cytokine that belongs to the IL-6/IL-12 family and has potent antitumor activity through various mechanisms. Our novel findings indicate that IL-27 directly acts on hematopoietic stem cells and promotes their expansion and differentiation into myeloid progenitors to control infection and to eradicate tumors.
Hematopoiesis is tightly orchestrated by hematopoietic stem cells (HSCs) in the bone marrow (BM) niche to ultimately produce mature myeloid and lymphoid blood cells. During infection, HSCs, which are predominantly dormant, briefly expand, proliferate, and differentiate in response to inflammatory signals such as pro-inflammatory cytokines. This process is called emergency myelopoiesis and plays a critical role in counterbalancing the loss of myeloid cells and replenishing them to control the infection, because myeloid cells generally have very low proliferative activity.
Macrophages are characterized by high plasticity and diversity, with two major phenotypes. M1 macrophages can augment helper T (Th)1 immune responses, resulting in strong microbicidal and tumoricidal activity, and M2 macrophages have an immunoregulatory function that helps to promote tissue remodeling and tumor progression. Under various pathogenic conditions, immature myeloid cells designated as myeloid-derived suppressor cells emerge and exert strong immunosuppressive functions. These cells are generated as a normal physiological response and are developed by stimulation with several tumor- or infection-derived cytokines, such as granulocyte macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and macrophage colony-stimulating factor, but blocked from differentiating. Generally, tumor-bearing hosts and cancer patients have increased infiltration of immunosuppressive myeloid cells, such as M2 macrophages and myeloid-derived suppressor cells.
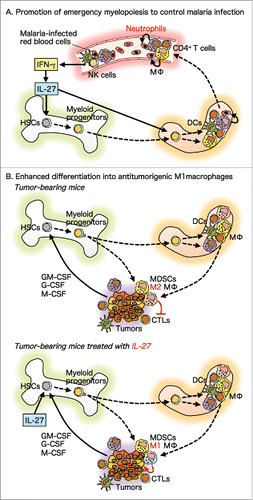
Interleukin (IL)-27, a member of the IL-6/IL-12 family of cytokines, is a multifunctional cytokine with both pro-inflammatory and anti-inflammatory properties. It promotes the early induction of Th1 differentiation and generation of cytotoxic T lymphocytes, but it inhibits the differentiation of naive CD4+ T cells into Th2 and Th17 cells and suppresses the production of pro-inflammatory cytokines.Citation1,Citation2 IL-27 is composed of Epstein-Barr virus-induced gene 3 and p28, and its receptor (R) consists of IL-27Rα and glycoprotein130, a common receptor subunit for the IL-6 family of cytokines.Citation1,Citation2 We first demonstrated the antitumor effects of IL-27 in 2004 using a mouse transplantable tumor model.Citation3 Accumulating evidence obtained using several preclinical mouse and human tumor models indicates that IL-27 has potent antitumor activity against various types of tumors without apparent adverse effects; IL-27 acts via multiple mechanisms including cytotoxic T lymphocytes and natural killer cells, depending on the characteristics of individual tumors.Citation4,Citation5 In addition, we first clarified its promoting effects on the expansion and differentiation of HSCs into myeloid progenitors by establishing IL-27-overexpressing transgenic mice.Citation6 We also recently elucidated the protective effects of IL-27 on a mouse model of blood-stage malaria infection by promoting emergency myelopoiesis (),Citation7 as well as its antitumor effects through enhanced differentiation into antitumorigenic M1 macrophages ().Citation8
Figure 1. Protective effects against tumors and infection by IL-27 through promotion of expansion and differentiation of HSCs into myeloid progenitors. (A) The mouse model of blood-stage malaria infection enhances IL-27 expression through IFN-γ production in the BM and spleen, and IL-27 then promotes the expansion and differentiation of HSCs into myeloid progenitors, enhancing the production of neutrophils to control the infection. Thus, IL-27 is a novel cytokine that directly acts on HSCs and promotes emergency myelopoiesis. (B) Tumor-bearing mice generally have increased infiltration of immunosuppressive myeloid cells such as M2 macrophages and myeloid-derived suppressor cells. In mouse transplantable tumor models, however, IL-27 directly acts on HSCs in the BM and promotes their expansion and differentiation into myeloid progenitors, which have an enhanced ability to further differentiate into antitumorigenic M1 macrophages. These M1 macrophages then infiltrate into tumors and eradicate them mainly in a nitric oxide–dependent manner. CTL, cytotoxic T lymphocyte; DC, dendritic cell; GM-CSF, granulocyte macrophage colony-stimulating factor; MΦ, macrophage; MDSC, myeloid-derived suppressor cell; NK, natural killer.
The IL-27-transgenic mice show enhanced myelopoiesis with splenomegaly and extramedullary hematopoiesis in the spleen.Citation6 Consistent with this, IL-27 together with stem cell factor synergistically and vigorously expands mouse BM cells for a long period.Citation7 Among the various types of HSCs and progenitors in the BM, IL-27 and stem cell factor predominately act on long-term repopulating HSCs and promote their differentiation into myeloid progenitors, which have a unique potential to differentiate into migratory dendritic cells, neutrophils, and mast cells and less so into macrophages and basophils, but not into plasmacytoid dendritic cells, conventional dendritic cells, T cells, or B cells. Among the cytokines, IL-27 in synergy with stem cell factor has the strongest ability to augment the expansion of lineage−Sca-1+c-Kit+ (LSK) cells, which is a population highly enriched in HSCs, and their differentiation into myeloid progenitors retaining the same LSK phenotype.Citation7
In the mouse model of blood-stage malaria infection, interferon (IFN)-γ production induced by IL-12 and phagocytic cells in the spleen play critical roles in controlling parasitemia to remove infected red blood cells.Citation9 IL-27Rα-deficient mice show a greater increase in parasitemia during the early infection.Citation7 The infection enhances IL-27 expression through IFN-γ production in the BM and spleen, and IL-27 then promotes the expansion and differentiation of LSK cells into myeloid progenitors, enhancing the production of neutrophils to control the infection ().Citation7 Thus, IL-27 is one of the few cytokines that directly acts on HSCs and promotes emergency myelopoiesis.
In tumor-bearing mice, IL-27 was revealed to augment the infiltration of myeloid cells into tumors. Intriguingly, however, deletion experiments of these myeloid cells and admixture experiments of them with parental tumors show that the IL-27-mediated tumor-infiltrating myeloid cells have reduced immunosuppressive activity and potent antitumor activity, rather than protumor activity as usually observed in non-treated tumor-bearing mice ().Citation8 The myeloid cells express a higher level of inducible nitric oxide synthase and directly kill tumors, mainly in a nitric oxide–dependent manner. In the BM of tumor-bearing mice, IL-27 increases the LSK cell population, which has an enhanced ability to differentiate into antitumorigenic M1 macrophages.Citation8 These M1 macrophages then infiltrate into tumors and eradicate them.
In analogy with the effects of IL-27 on HSCs, some researchers have proposed the possibility of IL-27 inducing the expansion and differentiation of leukemic stem cells. Aberrant IL-27Rα signaling or IL-27 stimulation of leukemic stem cells that contain proliferative mutations like BCR/ABL may enhance myeloid cell growth, potentially leading to myeloproliferative neoplasms. However, IL-27-overexpressing transgenic mice never show any spontaneous development of leukemia during the entire life span under normal conditions.Citation10 Moreover, supporting the effect of IL-27 on hematopoiesis in the BM, the p28 subunit of IL-27 is unique in having a polyglutamic acid domain, which is the acidic domain with hydroxyapatite-binding ability and bone tropism to bone sialoprotein. Therefore, therapeutic applications of IL-27 targeting hematologic tumor and solid tumor metastasis with bone tropism could prove beneficial.
Abbreviations
| BM | = | bone marrow |
| HSC | = | hematopoietic stem cell |
| IFN | = | interferon |
| IL | = | interleukin |
| LSK | = | lineage−Sca-1+c-Kit+ |
| R | = | receptor |
| Th | = | helper T |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Mizoguchi I, Higuchi K, Mitobe K, Tsunoda R, Mizuguchi J, Yoshimoto T. Interleukin-27: regulation of immune responses and disease development by a pleiotropic cytokine with pro- and anti-inflammatory properties. In: Yoshimoto T, Yoshimoto T, eds. Cytokine frontiers: regulation of immune responses in health and disease. Tokyo: Springer; 2013. p. 353–375.
- Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi:10.1146/annurev-immunol-032414-112134.
- Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi:10.1158/0008-5472.CAN-03-2084.
- Mizoguchi I, Chiba Y, Furusawa JI, Xu M, Tsunoda R, Higuchi K, Yoshimoto T. Therapeutic potential of interleukin-27 against cancers in preclinical mouse models. Oncoimmunology. 2015;4:e1042200. doi:10.1080/2162402X.2015.1042200.
- Yoshimoto T, Chiba Y, Furusawa J, Xu M, Tsunoda R, Higuchi K, Mizoguchi I. Potential clinical application of interleukin-27 as an antitumor agent. Cancer Sci. 2015;106:1103–1110. doi:10.1111/cas.12731.
- Seita J, Asakawa M, Ooehara J, Takayanagi S, Morita Y, Watanabe N, Fujita K, Kudo M, Mizuguchi J, Ema H, et al. Interleukin-27 directly induces differentiation in hematopoietic stem cells. Blood. 2008;111:1903–1912. doi:10.1182/blood-2007-06-093328.
- Furusawa J, Mizoguchi I, Chiba Y, Hisada M, Kobayashi F, Yoshida H, Nakae S, Tsuchida A, Matsumoto T, Ema H, et al. Promotion of expansion and differentiation of hematopoietic stem cells by interleukin-27 into myeloid progenitors to control infection in emergency myelopoiesis. PLoS Pathog. 2016;12:e1005507. doi:10.1371/journal.ppat.1005507.
- Chiba Y, Mizoguchi I, Furusawa J, Hasegawa H, Ohashi M, Xu M, Owaki T, Yoshimoto T. Interleukin-27 exerts its antitumor effects by promoting differentiation of hematopoietic stem cells to M1 macrophages. Cancer Res. 2017; Nov 1:pii: canres.0960.2017. doi:10.1158/0008-5472.CAN-17-0960.
- Yoneto T, Waki S, Takai T, Tagawa Y, Iwakura Y, Mizuguchi J, Nariuchi H, Yoshimoto T. A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J Immunol. 2001;166:6236–6241. doi:10.4049/jimmunol.166.10.6236.
- Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol 2007;179:4415–4423. doi:10.4049/jimmunol.179.7.4415.
