ABSTRACT
Objectives. In Erdheim-Chester disease (ECD), the empirical single dose (SD, 100 mg/day) anakinra sometimes induces only partial responses. Since SD is usually well tolerated, doubling the dose might improve response while maintaining an acceptable safety profile.
Methods. A retrospective analysis was performed of outcomes under double-dose (DD) of anakinra in 4 ECD patients who did not exhibit a complete response (CR) under SD treatment. Bone, retroperitoneal, neurologic/orbital, peritoneal, pericardial, right atrium, and pleural involvements were recorded. CR, partial response (PR), stable disease, progressive disease (PD) and tolerance of DD were assessed.
Results. SD treatment was a second or third line treatment in three patients after interferon-therapy failure. Two patients, including one with a BRAF mutation, achieved a CR and one patient with a NRAS mutation achieved a PR with DD treatment. The fourth patient, wild-type for both genes, did not respond to a first DD treatment, but then achieved CR under SD associated with a reduced dose of vemurafenib (960 mg/d). Bone and retroperitoneal lesions partially improved on imaging with SD in all patients, but were further improved under DD with two patients achieving CR. With SD treatment, two patients with right atrial masses showed sustained CR. Under DD treatment, two patients with massive serositis refractory to SD, showed PR.
Conclusion. DD improved the response to anakinra and lead to two CRs and a PR in three out of four ECD patients, with minor and comparable side-effects to those of SD, while failures were essentially related to massive serositis.
Introduction
Erdheim-Chester disease (ECD) is a rare non-Langerhans histiocytic disorder caused by tissue infiltration by foamy macrophages exhibiting positive CD68 and negative CD1a immunostaining. The fibro-inflammatory infiltration can lead to several organic damages, including retroperitoneal fibrosis with hydronephrosis, peri-aortitis, cardiac injuries and pleuropericardial effusions, insipidus diabetes, central nervous system involvement, bone pain, exophthalmia, and constitutional symptoms.Citation1-Citation4
Based on the demonstrated involvement of IL(Interleukin)-1 and IL-6 pathways in the pathophysiology of the disease, anakinra (recombinant IL-1 receptor antagonist) showed interesting results and good tolerance at the daily subcutaneous (SC) dose of 100 mg in 2 patients exhibiting partial responses and unacceptable side-effects, or contra-indication, with the use of interferon-alpha therapy.Citation5 This posology of anakinra was empirically chosen to correspond to the same protocol used in rheumatoid arthritis (100 mg/d SC), for which this drug was initially engineered. However, further cases of complete response (CR) and partial response (PR) or failure are observed with this posology in ECD.Citation6-Citation11 Tocilizumab, a monoclonal antibody blocking the IL-6 receptor, has also been tried with success in a few patients.Citation12 More recently, treatments targeting the RAS/RAF/MEK/ERK tumourigenic mutational network provided several cases of dramatic CR in ECD.Citation13-Citation15 However, several concerns persist with these drugs, including cases of failure, PR, or poor tolerance, and unacceptable side effects, including cutaneous malignancies in ECD, as in melanoma, in which they were first and largely used.Citation14-Citation17 Secondary medium/long term loss of efficacy of these drugs, related to secondary induction of downstream neo-mutational defects, is also described in melanoma.Citation18 Moreover, targetable mutations have not been detected in a significant proportion (almost 40%) of ECD patients. Therefore, increased doses and a combination of anakinra with some selected drugs are possible interesting strategies to improve the outcomes in terms of efficacy and treatment tolerance in ECD.
From this perspective, we report a series of 4 ECD cases, exhibiting better responses with a well-tolerated double dose of anakinra, or its combination with lowered doses of vemurafenib. In parallel, we performed sensitive molecular biology tests in these patients to understand the outcomes under anakinra with regards to the presence of certain molecular defects, and we discuss the hypothesis of a common pathophysiogenic mechanism between IL-1 and the RAS/RAF/MEK/ERK network in ECD.
Results
The main clinical, biologic and genetic characteristics of the four patients (3 men, 1 woman; mean age (range) at diagnosis: 59.8 (52–68) years old) and treatment history before anakinra are shown in . Molecular screening found no defect in one patient (Patient 4), a BRAF V600E mutation in two others (Patients 2 and 3) and a NRAS mutation in the last (Patient 1), which have been previously reported.Citation19 SD was the first-line treatment in only one patient (Patient 2) and the second- or third-line treatment in the others, always after high dose interferon failure. Before SD treatment, Patients 1, 3 and 4 exhibited PR, stable disease or PD and/or poor treatment tolerance with previous lines of treatment described in .
Table 1. Patients' characteristics, treatment courses and outcomes.
At initiation of SD treatment, all patients exhibited multi-systemic active ECD with constitutional symptoms, including episodic febricula (n = 2) and moderate (n = 2, with weight loss ≤10 kg) or severe (n = 2) impaired general status. An analysis of treatment outcomes under SD versus DD is summarized in and detailed as follows, considering global and specific organ/symptom responses.
In terms of global responses with SD, three patients exhibited PR (Patients 1, 3 and 4), and one patient progressed (Patient 2). Under DD, the patients achieved CR (Patients 3 and 4), sustained improved PR (Patient 1, with a decrease of more than 75% of ECD symptoms) and PD after a temporary PR (Patient 2). Therefore, DD was pursued in the three patients with CR or PR. In one of the two patients with CR (Patient 3), SD was eventually resumed, and CR was maintained for the seven following months. DD was stopped in the patient with failure. This patient showed no improvement under canakinumab or lenalidomide plus corticosteroids, but a good response under vemurafenib (960 mg b.i.d orally) associated with SD treatment, although the BRAF mutant clone was minor. This association was initiated as a rescue treatment because of recurring pericarditis responsible for cardiogenic shock and because BRAF V600E mutation was found in a minority of tumour cells and at only one of the two biopsy sites. After two months of treatment, vemurafenib-related painful palmoplantar erythrodysesthesia led to a vemurafenib dose decrease and SD withdrawal. At rapid disease relapse, half-dose vemurafenib and SD treatment were resumed, leading to CR, sustained for 18 months.
Regarding cardiac and pleural involvements, all three patients with right atrial masses exhibited sustained (Patient 3 and 4) or newly acquired CR (Patient 2) under DD, even though this was only transient for Patient 2. Patients 1 and 2 achieved only PR and PD, respectively, on massive precardiac and pleuro-pericardiac serositis under SD. With DD treatment, they achieved a sustained PR and a transient PR, respectively, for the serositis.
Xanthelasma was present in two patients for which SD led to CR and PR, respectively, followed by CR in both patients on DD. Retro-orbital/neurologic active involvement was present only in Patient 2, as an active bilateral exophthalmos. She achieved stable disease and PR with SD treatment and PR and CR with DD treatment, for each eye respectively. Patients 2 and 3 had insipidus diabetes that was not modified by treatments; this involvement was considered to be from sequelae.
Notably, two patients (Patients 1 and 3) exhibited unpublished intraperitoneal involvements of the disease, with the added clinical features of subacute appendicitis/peritonitis and sub-occlusive syndrome. CT-scan imaging and surgical procedures found mesenteric panniculitis, peritoneal nodules and serositis in both patients, associated with pseudo-tumoural appendicitis images for Patient 3 ( and ). For both patients, extemporaneous histopathologic findings confirmed specific ECD involvement (). The two patients showed PR and CR with SD, then CR with DD, respectively, for this involvement.
Figure 1. Abdominal CT-scan findings in Erdheim-Chester disease. (A) - Appearance of acute uncomplicated appendicitis with a marked inflammatory thickening of the whole appendix (up to 14 mm; dotted line and perpendicular measuring bar), in the usual anatomical localization, without stercolithis. Significant infiltration of adjacent mesenteric fat, without collection, is noted. (B) - Left pararenal lymphadenopathy (12–13 mm; dotted line and perpendicular measuring bar). Atrophied right kidney (not shown) and hypertrophic left kidney (15 × 9 × 9 cm).
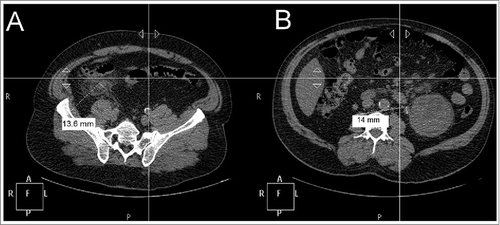
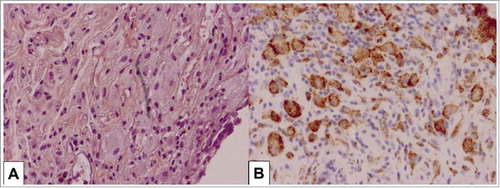
Figure 2. Pathologic features of appendicular/mesenteric tissues in Erdheim-Chester disease involvement. Tissue infiltration by pathologic histiocytes with foamy cytoplasm: H&E x 400 (A) and CD68 staining x400 (B).
Bone and retroperitoneal imaging showed improvements with both doses, the DD regimen producing a CR in terms of functional imaging (no more metabolic activity on 18FDG/PET-scan, despite persistent inactive fibrosis on CT-scan) in both locations in the two patients (Patients 3 and 4).
All patients had initial moderate renal insufficiency that improved under SD treatment and then with DD treatment (). Both doses exhibited identical minor side-effects, consisting mainly of transient reactions at injection sites and/or an episodic cutaneous rash in all. Patients 3 and 4 presented with mild and transient neutropenia under DD treatment. At last follow-up, all patients had good and stable global outcomes under DD treatment for Patient 1 (PR) and Patient 4 (CR), SD treatment for Patient 3 (CR), and SD treatment with half-dose vemurafenib for Patient 2 (CR).
Discussion
Doubling the dose improved response to anakinra in 3 of 4 ECD patients who were previously refractory and/or partial responders to interferon therapy or to anakinra SD treatment. Indeed, two CRs and one long term PR response were observed with anakinra DD treatment, with the same good tolerance profile compared to conventional SD treatment. Failures of DD treatment were essentially related to cardiac and pleuritic massive serositis, whereas both SD and DD treatments were effective in unpublished mesenteric and appendicular locations of the disease. In this small series, these outcomes do not seem to be correlated with the molecular status of the disease, including BRAF V600E or NRAS mutations.
The efficacy of anakinra SD treatment appeared variable; both efficacy and failure are reported in sparse cases of cardiac, neurological and pleural ECD involvements.Citation7,Citation9,Citation20 The variability of the anakinra response in ECD should depend on the organ involved and might be related to at least two phenomena. First, beside the blockade of the inflammatory paracrine action of circulating IL-1β, the efficacy of anakinra in ECD is also probably related to the blockade of juxtacrine, i.e., direct intercellular contact and interactions between pathologic histiocytic cells via membranous IL-1α overexpression.Citation5 This is highlighted by the case of Patient 2, who failed to respond to canakinumab, a selective inhibitor of IL-1β, but exhibited a partial response under anakinra, which blocks the activity of both IL-1β and IL-1α.Citation21 Therefore, the systemic and local actions supporting the efficacy of anakinra might be variable according to the amount of the drug needed to neutralize both circulating IL-1β and membranous IL-1α. Serum dosage and peripheral blood mononuclear cell surface flow cytometry have shown significant differences in the amount of IL-1β and IL-1α, respectively, in ECD patients.Citation5 Second, in addition to patient's weight, the bioavailability of the drug may be impaired by the difficulty it has in reaching the pathologic cells, which are drowned in abundant serositis, as observed in the massive pericarditis and pleuritis of our patients. Interestingly, Diamond et al. reported 2 cases of intracranial ECD with dramatic radiologic and clinical responses to an initial treatment with anakinra SD and postulated that the ineffectiveness of anakinra in certain localizations of ECD (brain and heart) may be explained by intrinsic refractory manifestations of the disease in those cases, rather than by the types of organs involved. Therefore, they concluded that anakinra could be an alternative first-line therapy for severe forms of ECD, regardless of mutational status,Citation22 as was also found herein.
The tolerance to anakinra is often good in long term use, as reported in ECDCitation5,Citation8,Citation23 and in several auto-inflammatory and rheumatic diseases.Citation24-Citation28 Therefore, increasing the dose of anakinra to obtain the best possible efficacy without a significant increase in safety risk appears to be a credible therapeutic alternative. Indeed, the high specificity of the natural IL-Ra (or its recombinant form) for its receptor, which is essentially expressed by monocytic/macrophagic cells, explains the lack of systemic or tissue toxicities with this treatment.
The results of this short series show a constant effectiveness, even though sometimes partial, of anakinra in ECD, regardless of the observed underlying molecular defect. However, the exact role and history of these mutations in ECD are not yet fully understood. Indeed, BRAF mutation is widely observed in several tumoural diseases. Hence, it appears to be non-specific and is not the unique molecular event in ECD pathogeny. BRAF mutation is found in less than 60% of biopsies performed in the short series of ECD patients. The robust local and systemic response in ECD may be explained by BRAF V600E mutation, which activates oncogene-induced senescence pathways, likely attracting inflammatory cells and inducing a senescent pro-inflammatory phenotype in both mutated and bystander infiltrating cells..Citation29,Citation30 Other pathogenic molecular defects, including those of NRAS, KRAS or MEK-ERK pathway genes, are also reported in ECD.Citation14,Citation31 On the other hand, studies in melanoma demonstrated a link between pathogenic mechanisms of both BRAF mutation and interleukin-1. These studies showed that the inflammatory process in this disease is produced by an overproduction of this proinflammatory cytokine by the specific and environmental fibroblastic cells of tumours that exhibit BRAF mutation.Citation18,Citation32 Moreover, Murakami et al. showed in a model of Langerhans cell histiocytosis (LCH) that BRAF mutation leads to IL-1 overproduction and therefore indicates that both BRAF mutations and IL-1 form a loop in regulation as potential therapeutic targets.Citation33 Taken together, these findings allow us and others to suggest that the increased dose of anti-IL-1 drugs or their combination with anti-BRAF drugs may offer a synergic and optimal treatment option in refractory ECD.Citation21,Citation32,Citation34 BRAF and/or MEK inhibitors have or will likely proved their frequent efficacy in patients with corresponding mutations in prospective studies.Citation15 Therefore, the strategic place of anti-cytokine therapy such as anakinra, which exhibits the most effect in ECD,Citation11 remains to be specified considering the toxicity profiles of these targeted therapies and patients' history of cutaneous neoplasm other than melanoma.
Patients and methods
Patient selection
In our cohort of 10 ECD patients treated with daily subcutaneous single dose (SD) anakinra treatment of 100 mg (SD), six patients achieved a CR. In the 4 remaining patients, we retrospectively collected the systematic analysis of global and organ-specific outcomes following double dose (DD) anakinra treatment. For all patients, the diagnosis was based on histology and the presence of clinical and radiological pictures suggestive of ECD. This study received the Institutional Review Board (CPP Nord-Ouest III, ref. CHU: A14-D62-VOL.23) agreement.
Genetic analyses
The detailed, highly sensitive genetic techniques used herein were previously reported, including the related results for two patients (Patients 1 and 2).Citation19,Citation21 Briefly, genomic DNA was extracted from formalin-fixed, paraffin-embedded samples after histologic review and enrichment by macrodissection to ≥ 10% histiocytes. All samples were obtained from patients before any therapy. Detection of BRAF V600 and NRASQ61 mutations was performed by pyrosequencing for all patients. For Patient 2, in which the BRAF V600 mutation was not initially detected, further analysis for BRAF mutations with multiplex picodroplet digital polymerase chain reaction (PCR)Citation19 (Raindance Technologies) finally identified this genetic defect in 20% of pathologic histiocytic cells. Screening for mutations in other genes was performed with Sequenom mass spectrometric-based genotyping assays, as previously described (NRAS, KRAS, PIK3CA, and AKT1 hotspot mutations).Citation35
Treatment and assessment of outcomes
DD corresponded to a subcutaneous dose of anakinra of 100 mg b.i.d. Clinical outcomes were assessed as complete response (CR; with complete resolution of ECD symptoms), partial response (PR; partial resolution of ECD symptoms), stable disease (no change in ECD symptoms), or progressive disease (PD; worsening of ECD symptoms). Early and long-term tolerances, including renal function, were also studied. Radiological response was assessed based on RECIST criteria 1.1: CR was defined as the disappearance of a given lesion; PR as a decline of at least 30% in the sum of the longest lesion diameters; PD as an increase of at least 20% in the sum of lesion diameters or appearance of new lesions; and stable disease was defined as neither PR nor PD. Most patients did not undergo positron emission tomography, and response was therefore evaluated based on alternative imaging studies available.
Disclosure of potential conflicts of interest
AA received financial support from SOBI for a study on anakinra in giant cell arteritis. All other authors have declared that there are no conflicts of interest regarding the publication of this paper.
References
- Haroche J, Arnaud L, Amoura Z. Erdheim-chester disease. Curr Opin Rheumatol. 2012;24:53–9. doi:10.1097/BOR.0b013e32834d861d. PMID:22089098.
- Mazor RD, Manevich-Mazor M, Shoenfeld Y. Erdheim-chester disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. doi:10.1186/1750-1172-8-137. PMID:24011030.
- Cives M, Simone V, Rizzo FM, Dicuonzo F, Cristallo Lacalamita M, Ingravallo G, Silvestris F, Dammacco F. Erdheim-Chester disease: a systematic review. Crit Rev Oncol Hematol. 2015;95:1–11. doi:10.1016/j.critrevonc.2015.02.004. PMID:25744785.
- Arnaud L, Hervier B, Néel A, Hamidou MA, Kahn J-E, Wechsler B, Pérez-Pastor G, Blomberg B, Fuzibet J-G, Dubourguet F, et al. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117:2778–82. doi:10.1182/blood-2010-06-294108. PMID:21239701.
- Aouba A, Georgin-Lavialle S, Pagnoux C, Martin Silva N, Renand A, Galateau-Salle F, Le Toquin S, Bensadoun H, Larousserie F, Silvera S, et al. Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease. Blood. 2010;116:4070–6. doi:10.1182/blood-2010-04-279240. PMID:20724540.
- Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–92. doi:10.1182/blood-2014-03-561381. PMID:24850756.
- Darstein F, Kirschey S, Heckl S, Rahman F, Schwarting A, Schuchmann M, Galle PR, Zimmermann T. Successful treatment of erdheim-chester disease with combination of interleukin-1-targeting drugs and high-dose glucocorticoids. Intern Med J. 2014;44:90–2. doi:10.1111/imj.12329. PMID:24450524.
- Courcoul A, Vignot E, Chapurlat R. Successful treatment of Erdheim-Chester disease by interleukin-1 receptor antagonist protein. Joint Bone Spine. 2014;81:175–7. doi:10.1016/j.jbspin.2013.06.013. PMID:23953221.
- Cohen-Aubart F, Maksud P, Saadoun D, Drier A, Charlotte F, Cluzel P, Amoura Z, Haroche J. Variability in the efficacy of the IL1 receptor antagonist anakinra for treating Erdheim-Chester disease. Blood. 2016;127:1509–12. doi:10.1182/blood-2015-09-672667. PMID:26847247.
- Podestà MA, Graziani G, Reggiani F, Buemi M, Badalamenti S, Ponticelli C. Improvement of Erdheim-Chester disease-related renal failure after treatment with anakinra. Kidney Res Clin Pract. 2014;33:165–7. doi:10.1016/j.krcp.2014.07.007. PMID:26877969.
- Goyal G, Shah MV, Call TG, Litzow MR, Wolanskyj-Spinner AP, Koster MJ, Tobin WO, Vassallo R, Ryu JH, Hook CC, et al. Efficacy of biological agents in the treatment of Erdheim-Chester disease. Br J Haematol. 2017; doi:10.1111/bjh.14997.
- Berti A, Cavalli G, Guglielmi B, Biavasco R, Campochiaro C, Tomelleri A, Nicoletti R, Panzacchi A, Ferrarini M, Dagna L. Tocilizumab in patients with multisystem Erdheim-Chester disease. Oncoimmunology. 2017;6:e1318237. doi:10.1080/2162402X.2017.1318237. PMID:28680751.
- Haroche J, Cohen-Aubart F, Emile J-F, Arnaud L, Maksud P, Charlotte F, Cluzel P, Drier A, Hervier B, Benameur N, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory erdheim-chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–500. doi:10.1182/blood-2012-07-446286. PMID:23258922.
- Cohen Aubart F, Emile J-F, Maksud P, Galanaud D, Cluzel P, Benameur N, Aumaitre O, Amoura Z, Haroche J. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180:150–3. doi:10.1111/bjh.14284. PMID:27711968.
- Diamond EL, Subbiah V, Lockhart AC, Blay J-Y, Puzanov I, Chau I, Raje NS, Wolf J, Erinjeri JP, Torrisi J, et al. Vemurafenib for BRAF V600-mutant erdheim-chester disease and langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-basket study. JAMA Oncol. 2018;4:384–8.
- Estrada-Veras JI, O'Brien KJ, Boyd LC, Dave RH, Durham B, Xi L, Malayeri AA, Chen MY, Gardner PJ, Alvarado-Enriquez JR, et al. The clinical spectrum of erdheim-chester disease: an observational cohort study. Blood Adv. 2017;1:357–66. doi:10.1182/bloodadvances.2016001784. PMID:28553668.
- Gianfreda D, Musetti C, Nicastro M, Maritati F, Cobelli R, Corradi D, Vaglio A. Erdheim-chester disease as a mimic of igg4-related disease: a case report and a review of a single-center cohort. Medicine (Baltimore). 2016;95:e3625. doi:10.1097/MD.0000000000003625. PMID:27227923.
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee M-K, Attar N, Sazegar H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi:10.1038/nature09626. PMID:21107323.
- Emile J-F, Diamond EL, Hélias-Rodzewicz Z, Cohen-Aubart F, Charlotte F, Hyman DM, Kim E, Rampal R, Patel M, Ganzel C, et al. Recurrent RAS and PIK3CA mutations in erdheim-chester disease. Blood. 2014;124:3016–9. doi:10.1182/blood-2014-04-570937. PMID:25150293.
- Killu AM, Liang JJ, Jaffe AS. Erdheim-chester disease with cardiac involvement successfully treated with anakinra. Int J Cardiol. 2013;167:e115–117. doi:10.1016/j.ijcard.2013.04.057. PMID:23659884.
- Franconieri F, Martin-Silva N, de Boysson H, Galateau-Salle F, Emile J-F, Bienvenu B, Aouba A. Superior efficacy and tolerance of reduced doses of vemurafenib plus anakinra in erdheim-chester disease: Towards the paradigm of combined targeting and immune therapies. Acta Oncol. 2016;55:930–2. doi:10.3109/0284186X.2015.1120885. PMID:27031008.
- Diamond EL, Abdel-Wahab O, Durham BH, Dogan A, Ozkaya N, Brody L, Arcila M, Bowers C, Fluchel M. Anakinra as efficacious therapy for 2 cases of intracranial erdheim-chester disease. Blood. 2016;128:1896–8. doi:10.1182/blood-2016-06-725143. PMID:27535996.
- Tran TA, Pariente D, Lecron JC, Delwail A, Taoufik Y, Meinzer U. Treatment of pediatric erdheim-chester disease with interleukin-1-targeting drugs. Arthritis Rheum. 2011;63:4031–2. doi:10.1002/art.30638. PMID:21898344.
- Emmi G, Silvestri E, Squatrito D, Vitale A, Bacherini D, Vannozzi L, Emmi L, D'Elios MM, Cantarini L, Prisco D. Long-term efficacy and safety of anakinra in a patient with Behçet's disease and concomitant tuberculosis infection. Int J Dermatol. 2017;56:218–20. doi:10.1111/ijd.13337. PMID:27336860.
- Chang Z, Spong CY, Jesus AA, Davis MA, Plass N, Stone DL, Chapelle D, Hoffmann P, Kastner DL, Barron K, et al. Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS). Arthritis & Rheumatology (Hoboken, NJ). 2014;66:3227–32. doi:10.1002/art.38811.
- Kullenberg T, Löfqvist M, Leinonen M, Goldbach-Mansky R, Olivecrona H. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology (Oxford). 2016;55:1499–506. doi:10.1093/rheumatology/kew208. PMID:27143789.
- Lopalco G, Vitale A, Iannone F, Cantarini L. Anakinra long-term efficacy and safety in the management of Schnitzler's syndrome and latent tuberculosis infection. Clin Exp Rheumatol. 2016;34:353. PMID:26940429.
- Cavalli G, Franchini S, Aiello P, Guglielmi B, Berti A, Campochiaro C, Sabbadini MG, Baldissera E, Dagna L. Efficacy and safety of biological agents in adult-onset Still's disease. Scand J Rheumatol. 2015;44:309–14. doi:10.3109/03009742.2014.992949. PMID:25656459.
- Cangi MG, Biavasco R, Cavalli G, Grassini G, Dal-Cin E, Campochiaro C, Guglielmi B, Berti A, Lampasona V, von Deimling A, et al. BRAFV600E-mutation is invariably present and associated to oncogene-induced senescence in Erdheim-Chester disease. Ann Rheum Dis. 2015;74:1596–602. doi:10.1136/annrheumdis-2013-204924. PMID:24671772.
- Cavalli G, Biavasco R, Borgiani B, Dagna L. Oncogene-induced senescence as a new mechanism of disease: the paradigm of erdheim-chester disease. Front Immunol. 2014;5:281. doi:10.3389/fimmu.2014.00281. PMID:24982657.
- Aouba A. KRASG12D, pulmonary LCH, and atorvastatin. Blood. 2017;130:391–2. doi:10.1182/blood-2017-06-788455. PMID:28751357.
- Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT, Li Y, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–40. doi:10.1158/1078-0432.CCR-12-1632. PMID:22850568.
- Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, Nagata K, Horie Y, Hayashi K, Imamura T, Morimoto A, et al. Interleukin-1 loop model for pathogenesis of langerhans cell histiocytosis. Cell Commun Signal. 2015;13:13. doi:10.1186/s12964-015-0092-z. PMID:25889448.
- Cavalli G, De Luca G, Dagna L. Advances in potential targeted therapies for erdheim-chester disease. Expert Opin Orphan Drugs. 2017;5:253–60. doi:10.1080/21678707.2017.1285226.
- Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, Fu S, Luthra R, Garrido-Laguna I, Kurzrock R. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS ONE. 2011;6:e22769. doi:10.1371/journal.pone.0022769. PMID:21829508.
