ABSTRACT
Purpose: Mucosal immunization is suggested to be crucial for controlling tumors in the mucosal region; however, therapeutic DNA vaccination with electroporation in various mucosal sites has yet to become clinically adaptable. Since tumor-draining lymph nodes (tdLNs) have been suggested as immune-educated sites that can be utilized to mount a potent antitumor immune response, we examined whether intramuscular DNA vaccination with electroporation at sites that target the mucosal tdLNs could elicit mucosal immune response to restrict tumor growth.
Experimental Design: The efficacy and mechanism of intramuscular administration of a therapeutic DNA vaccine with electroporation at different sites was examined by lymphocyte analysis, tumor growth, mouse survival, as well as integrin expression, in mice bearing orthotopic HPV16 E6/E7+ syngeneic TC-1 tumors in various mucosal areas.
Results: While provoking comparable systemic CD8+ T cell responses, intramuscular hind leg vaccination generated stronger responses in cervicovaginal-draining LNs to control cervicovaginal tumors, whereas intramuscular front leg vaccination generated stronger responses in oral-draining LNs to control buccal tumors. Surgical removal of tdLNs abolished the antitumor effects of therapeutic vaccination. Mucosal-tdLN-targeted intramuscular vaccination induced the expression of mucosal-homing integrins LPAM-1 and CD49a by tumor-specific CD8+ T cells in the tdLNs. Inhibition of these integrins abolished the therapeutic effects of vaccination and the infiltration of tumor-specific CD8+ T cells into mucosal tumors.
Conclusions: Our findings demonstrate that tumor draining lymph nodes targeted intramuscular immunization can effectively control mucosal tumors, which represents a readily adaptable strategy for treating mucosal cancers in humans.
Introduction
Increasing evidence has highlighted the importance of infiltrating, local CD8+ T cells in the treatment of cancers (for review see Citation1-Citation3). Particularly, studies in patients have demonstrated that the regression of cancer is highly correlated with an observed local CD8+ T cell response in the tumor microenvironment (TME).Citation4 As such, recent studies have focused on strategies to enhance the generation of tumor infiltrating lymphocytes (TILs) following immunotherapy, such as therapeutic vaccination.Citation5-Citation7
Sandoval et al. have previously reported that therapeutic vaccination through the mucosal route (i.e. intranasal administration) is more effective at generating a local antitumor immune response against mucosal tumors, such as lung and oral tumors, compared to systemic vaccination.Citation8 Similarly, we have previously demonstrated that intravaginal vaccination or intramuscular (IM) priming vaccination with intravaginal booster vaccination can result in the generation of a stronger, local antigen-specific antitumor immune response against cervicovaginal tumors.Citation9,Citation10 However, many intratumoral or localized mucosal vaccinations, including intravaginal vaccination, are invasive in nature and are often associated with low participation in clinical settings.Citation11,Citation12 Thus, there is a need to explore alternative methods that can be more easily adopted in clinical settings, while still generating strong, local antitumor responses against tumors located in the mucosal regions.
The role of tumor-draining lymph nodes (tdLNs) in tumor progression as well as antitumor immunity has been a hot topic of debate. Recognized as the first site for tumor metastasis, tdLNs were shown to be under direct influence of immunosuppressive cytokines, growth factors, and immunosuppressive cell populations that originate from the TME (for review see).Citation13,Citation14 At the same time, due to the draining of tumor-released antigens and endogenous danger signals from decaying tumor cells into tdLNs, as well as the migration of tumor antigen presenting dendritic cells (DCs) from TME to tdLNs, tdLNs have been suggested as immune-educated sites and exert crucial functions in the generation of antitumor immune responses (for review see).Citation15,Citation16 Importantly, it has been demonstrated that despite the potentially immunosuppressive environment harbored by tdLNs, therapeutic immunization utilizing lymph node (LN)-targeting nanoparticles can effectively stimulate the generation of potent tumor infiltrating, antigen-specific CD8+ T cell responses for the control of tumors.Citation17
It has been observed that IM administration of prophylactic vaccines in the deltoid muscle of the arm often leads to increased F-18 fluorodeoxyglucose uptake as well as elevated activities in the axillary LNs.Citation18,Citation19 It has also been shown that IM administration of drugs or vaccines in the quadriceps muscles of the thigh leads to accumulation of the molecules and enhancement of immune responses in the inguinal LNs.Citation20,Citation21 Since axillary and inguinal LNs have been identified as the draining LNs for oral and cervical cancers, respectively,Citation22-Citation24 we hypothesize that selecting the IM vaccination sites that target the various mucosal tdLNs can lead to the generation of potent infiltrating antigen-specific CD8+ T cell responses for the control of mucosal tumors.
Thus, in the current study, we establish a preclinical orthotopic model of human papillomavirus (HPV)-mediated cervicovaginal cancer and vaccinated mice intramuscularly with a clinical grade therapeutic HPV DNA vaccine, pNGVL4a-CRT/E7(detox),Citation25 in the front leg or the hind leg. We showed that hind leg IM vaccination mounted a strong antigen-specific CD8+ T cell response in the inguinal and iliac cervicovaginal draining LNs as well as a potent antitumor effect against cervicovaginal tumors by inducing the infiltration of antigen-specific CD8+ T cells into the tumor tissues. Furthermore, we demonstrated that these tdLNs, as well as the expression of mucosal integrins LPAM-1 and CD49a by antigen-specific CD8+ T cells in these tdLNs, are crucial for the observed antitumor effects generated by IM hind leg vaccination against cervicovaginal tumors. This result was further supported by our observation that the surgical removal of tdLNs or the blockade of LPAM-1 or CD49a hindered cervicovaginal CD8+ T cell infiltration and antitumor efficacy following immunization.
Results
Intramuscular vaccinations at various sites induces differential localization of antigen-specific CD8+ T cell response
We first sought to examine the localization of protein antigens following IM DNA vaccine injection with electroporation at various sites. Using luciferase-encoded DNA plasmids, pcDNA3-Luciferase, as a model of vaccine administration (Fig. S1A), we observed that IM administration of pcDNA3-Luciferase in the front leg resulted in the accumulation of luminescence signals in the upper body of C57BL/6 mice, while the localization of luminescence signals was observed in the lower body of mice vaccinated IM with pcDNA3-Luciferase in the hind leg (Fig. S1B). No significant differences among the intensities of luminescence signals were observed (Fig. S1C). Together, these data suggest that IM administration of DNA plasmids at different sites do not affect the resultant expression levels of encoded protein antigens, but do influence the localization of the expressed antigens.
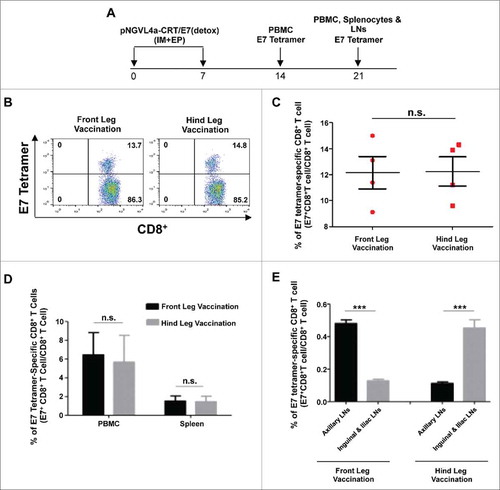
We next evaluated how the localization of the antigen at various sites affects the generation of antigen-specific CD8+ T cell responses following IM DNA vaccination. To evaluate this, we chose to use a clinical grade therapeutic HPV16 DNA vaccine that we previously developed, pNGVL4a-CRT/E7(detox),Citation25 because it has been demonstrated to induce potent HPV16-E7-specific CD8+ T cell responses against HPV16-E7 expressing tumors in vivo,Citation26 and is currently being evaluated in clinical trialsCitation27 (NCT00988559). IM administration of pNGVL4a-CRT/E7(detox) in the front or hind leg elicited similar magnitudes of E7-specific CD8+ T cell responses in the blood at one week after the last vaccination (), as well as similar amounts of E7-specific CD8+ T cells in the blood and the spleen at two weeks after the last vaccination (). However, when examining the E7-specific CD8+ T cell responses in various LNs, a stronger response was observed in the axillary LNs of mice vaccinated in the front leg, while a stronger response was observed in the inguinal and iliac LNs of mice vaccinated in the hind leg (). Thus, consistent with the current literature, these data support our hypothesis that the site of IM immunization determines the localization of elicited antigen-specific immune responses.
Figure 1. Local and systemic immune responses produced by vaccination at different sites in naïve mice. A. Schema of the experiment. Briefly, female C57BL/6 mice (five to eight weeks old, four/group) were vaccinated with 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA via IM injection, followed by electroporation in front leg or hind leg twice at seven-day intervals. Peripheral blood lymphocytes were prepared one week after the final vaccination. Peripheral blood lymphocytes, splenocytes, and lymph node (LN) cells were prepared 14 days after the final vaccination. Cells were stained with anti-mouse CD8+ antibody, H2-Db/E7 tetramer, and 7-AAD. B. Representative flow cytometry images of E7-specific CD8+ T cells in peripheral blood one week after the final vaccination. C. Summary bar graph showing E7-specific CD8+ T cells in peripheral blood one week after the final vaccination. D. Summary bar graph showing E7-specific CD8+ T cells in peripheral blood and splenocytes two weeks after the final vaccination. E. Summary bar graph showing both front leg and hind leg vaccination induced E7-specific CD8+ T cells in related draining lymph nodes. Data are presented as mean ± SD. (***p < 0.001, n.s. not significant).
Location of intramuscular immunization impacts therapeutic efficacy of DNA vaccine against tumors at various mucosal sites
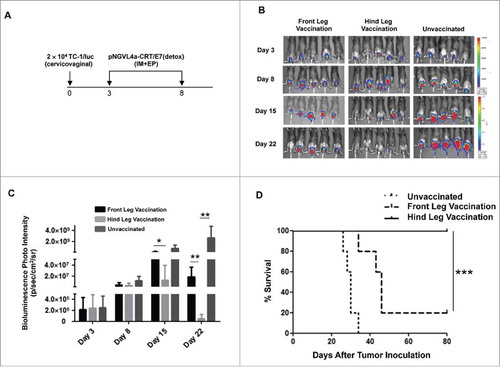
To evaluate how the location of IM therapeutic DNA vaccination affects its ability to control mucosal tumors, we set up an orthotopic model of cervical cancer by inoculating C57BL/6 mice in the cervicovaginal tract with luciferase and HPV16-E7 expressing the TC-1 tumor cell line (TC-1/luc).Citation28 Mice were then vaccinated IM with pNGVL4a-CRT/E7(detox) either in the front or hind leg (). Tumor growth was monitored by bioluminescence imaging based on the expression of luciferase by TC-1/luc cells. We observed the cervicovaginal tumor volume of mice vaccinated with pNGVL4a-CRT/E7(detox) in the hind leg was significantly reduced compared to untreated mice or mice immunized in the front leg (). Furthermore, all of the mice vaccinated in the hind leg survived for more than 80 days after cervicovaginal tumor inoculation, whereas only 20% of mice vaccinated in the front leg were still alive (). This serves as a clinically relevant model because HPV16 is responsible for causing 50% of all cervical cancers in humans (for review see Citation29).
Figure 2. Characterization of therapeutic antitumor effects of HPV vaccine administered intramuscularly at different sites in a cervicovaginal tumor model. A. Schema of the experiment. Briefly, female C57BL/6 mice (five to eight weeks old, five/group) were injected with 2 × 104 TC-1/luc cells at the intravaginal cavity on day 0. The mice were vaccinated with or without 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA vaccine via intramuscular (IM) injection, followed by electroporation at different sites on day 3, and subsequently boosted once five days later. The growth of cervicovaginal tumors was monitored with bioluminescence imaging. B. Bioluminescence images of the cervicovaginal TC-1/luc tumor-bearing mice. C. Bar graph depicting the mean luminescence intensity of cervicovaginal TC-1/luc tumor-bearing mice. D. Kaplan-Meier survival of the TC-1/luc tumor-bearing mice. Data are presented as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001).
In addition to cervical cancer, we also evaluated the therapeutic importance of IM vaccination site in an orthotopic model of head and neck cancer by inoculating C57BL/6 mice in the cheek with TC-1/luc cells (around 30% of head and neck cancer express HPV16 Citation30). Mice were then vaccinated IM with pNGVL4a-CRT/E7(detox) in the front or hind leg, and the tumor growth was monitored by bioluminescence imaging (Fig. S2A). Contrary to the findings observed in the cervicovaginal model, front leg vaccination resulted in significantly better control of buccal TC-1/luc tumors in mice as compared to no vaccination or vaccination in the hind leg (Fig. S2B-C).
Together, these data suggest that IM vaccination in the hind leg results in better therapeutic efficacy against tumors in cervicovaginal mucosa, while front leg immunization provided better therapeutic effects against tumors in oral mucosa.
Hind leg intramuscular vaccination preferentially induced CD8+ T cells in cervicovaginal draining lymph nodes and in the microenvironment of cervicovaginal tumors compared to front leg vaccination
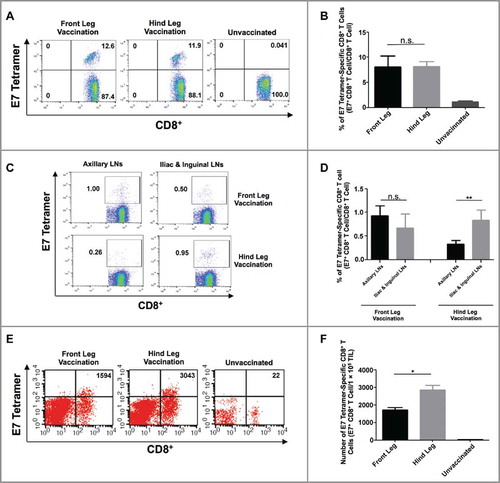
To link the control of mucosal tumors with the differential localization of antigen-specific CD8+ T cell responses resulting from targeted IM vaccination, we analyzed the generation and localization of E7-specific CD8+ T cells following IM pNGVL4a-CRT/E7(detox) injection with electroporation at various sites in orthotopic cervicovaginal TC-1/luc tumor-bearing mice. Following cervicovaginal tumor challenge and IM immunization, we analyzed the E7-specific CD8+ T cell response in the blood, LNs, and cervicovaginal tumor tissues. Similar to the results observed in naïve mice (), front or hind leg vaccination generated similar amounts of systemic E7-specific CD8+ T cells (). In cervicovaginal tumor-bearing mice vaccinated in the front leg, no significant differences between the E7-specific CD8+ T cells in axillary LNs versus the iliac and inguinal LNs were observed. Conversely, we observed that hind leg vaccination induced a significantly stronger E7-specific CD8+ T cell response in the iliac and inguinal LNs compared to that in axillary LNs in cervicovaginal tumor-bearing mice (). The site of IM vaccination does not appear to affect the immune suppressive cell populations in the LNs, as no significant differences in regulatory T cell (Tregs) and myeloid-derived suppressor cell (MDSC) populations were observed among the different LNs of front or hind leg vaccinated, cervicovaginal tumor-bearing mice (Fig. S3A-B). Importantly, a greater infiltration of E7-specific CD8+ T cells into the cervicovaginal tumors were observed in mice vaccinated in the hind leg compared to those immunized in the front leg (). These data suggest that hind leg IM DNA vaccination leads to the generation of enhanced antigen-specific CD8+ T cell responses in cervicovaginal tdLNs, resulting in preferential recruitment of these CD8+ T cells into cervicovaginal tumors, contributing to better tumor control.
Figure 3. Analysis of HPV16/E7-specific systemic and local immune responses produced by intramuscular vaccination at different sites in TC-1 tumor-bearing mice. (A-B) Female C57BL/6 mice (five to eight weeks old, five/group) were injected with 2 × 104 TC-1/luc cells at the intravaginal cavity on day 0. The mice were vaccinated with or without 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA vaccine via IM injection, followed by electroporation at different sites on day 3, and subsequently boosted again five days later. Peripheral blood lymphocytes were prepared one week after the final vaccination. The cells were stained with anti-mouse CD8 antibody, H2-Db/E7 tetramer, and 7-AAD. A. Representative flow cytometry images of E7-specific CD8+ T cells in peripheral blood. B. Summary of E7-specific CD8+ T cells in peripheral blood. (C-D) Female C57BL/6 mice (five to eight weeks old, five/group) were injected with 5 × 104 TC-1/luc cells at the intravaginal cavity on day 0. The mice were vaccinated with 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA vaccine via IM injection, followed by electroporation at different sites on day 4 and boosted once four days later. LNs were harvested seven days after the final vaccination and stained for HPV16/E7-specific CD8+ T cells. C. Representative flow cytometry images of E7-specific CD8+ T cells in LNs. D. Summary of E7-specific CD8+ T cells in LNs. (E-F) one week after the final vaccination, tumor tissues from mice described in subfigures A-B were harvested, and the tumor infiltrating lymphocytes were prepared and stained with anti-mouse CD8 antibody, H2-Db/E7 tetramer, and 7-AAD. E. Representative flow cytometry images of E7-specific CD8+ T cells in TILs. F. Summary of E7-specific CD8+ T cells in TILs. Data are presented as mean ± SD. (* p < 0.05, ** p < 0.01, n.s. not significant).
Resection of cervicovaginal draining lymph nodes hindered the recruitment of antigen-specific CD8+ T cells into cervicovaginal tumors and abolished the antitumor effects elicited by hind leg intramuscular vaccination
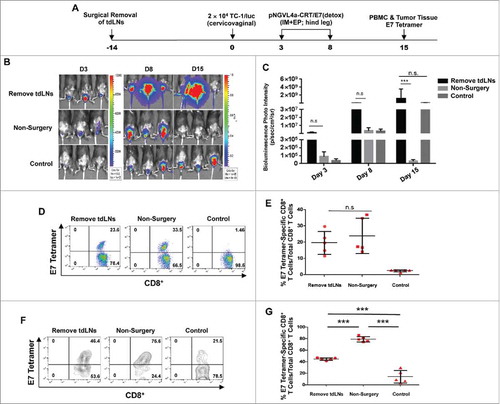
Previously, it has been suggested that antigens in the TME can flow into tdLNs or be taken up by the DCs that migrate to tdLNs (for review see Citation15,Citation16). Therefore, we hypothesize that tdLNs are immune-educated sites primed with tumor antigen-specific CD8+ T cells, and IM DNA vaccination targeting tdLNs serves as a boost for these responses, resulting in the observed enhanced antitumor immunity. To confirm the importance of mucosal tdLNs in the control of mucosal tumors following targeted IM vaccination, the iliac and inguinal cervicovaginal draining LNs were surgically removed in C57BL/6 mice prior to cervicovaginal TC-1/luc tumor cell inoculation and hind leg IM pNGVL4a-CRT/E7(detox) vaccination (). The tumor growth following inoculation was monitored by bioluminescence imaging. Resection of iliac and inguinal LNs abolished the ability of hind leg pNGVL4a-CRT/E7(detox) vaccination to control cervicovaginal TC-1/luc tumors (). When examining the generation of E7-sepcific CD8+ T cell responses in vaccinated, cervicovaginal tumor-bearing mice, resection of iliac and inguinal LNs did not alter the magnitude of systemic E7-specific CD8+ T cell response in circulation (). However, the infiltration of E7-specific CD8+ T cells into cervicovaginal tumors following hind leg vaccination is significantly reduced in the absence of iliac and inguinal LNs (). Together, these experiments demonstrate the importance of mucosal tdLNs in facilitating the infiltration of antigen-specific CD8+ T cells into mucosal tumors and the therapeutic effect of targeted IM therapeutic immunization.
Figure 4. Draining LNs are critical for generating antitumor immune response in tumor-bearing mice. A. Schematic illustration of the experiment. Draining iliac and inguinal LNs of female C57BL/6 mice (five to eight weeks old, five/group) were removed by surgery. Two weeks later, these mice, along with the controls, were inoculated intravaginally with 2 × 104 TC-1/luc cells on day 0. The mice were vaccinated with pNGVL4a-CRT/E7(detox) DNA vaccine via IM administration, followed by electroporation on days 3 and 8. Tumor growth was monitored through bioluminescence imaging. On day 15, peripheral blood lymphocytes and TILs were prepared in order to detect E7-specific CD8+ T cells with E7 tetramer staining. B. Bioluminescence images of the cervicovaginal TC-1/luc tumor-bearing mice. C. Bar graph depicting the mean luminescence intensity of cervicovaginal TC-1/luc tumor-bearing mice. D. Representative flow cytometry images of HPV16/E7-specific CD8+ T cells in peripheral blood. E. Summary of HPV16/E7-specific CD8+ T cells in peripheral blood. F. Representative flow cytometry images of HPV16/E7-specific CD8+ T cells in TILs. G. Summary of HPV16/E7-specific CD8+ T cells in TILs. Data are presented as mean ± SD. (*** p < 0.001, n.s. not significant).
Hind leg intramuscular vaccination increased the number of mucosal integrins LPAM-1 and CD49a expressing antigen-specific CD8+ T cells in the tumor-draining lymph nodes of cervicovaginal tumor-bearing mice
To identify possible mechanisms contributing to the preferential recruitment of E7-specific CD8+ T cells from the tdLNs into cervicovaginal tumors following hind leg IM pNGVL4a-CRT/E7(detox) vaccination, we characterized the phenotype of E7-specific CD8+ T cells in the various LNs and in the tumor tissues of hind leg vaccinated, cervicovaginal tumor-bearing mice (). Analysis of integrin expression revealed that IM hind leg immunization increased the population of integrins LPAM-1 (α4β7)- or CD49a (α1β1)-expressing E7-specific CD8+ T cells and decreased the population of CD103 (αEβ7)-expressing E7-specific CD8+ T cells in the cervicovaginal tdLNs (iliac and inguinal LNs) compared to those in the non-tdLNs (axillary LNs) (). A similar frequency of LPAM-1-expressing E7-specific CD8+ T cells was observed in cervicovaginal tumors as in non-tdLNs (ntdLNs). Further, a lower frequency of CD103-expressing E7-specific CD8+ T cells was detected in the cervicovaginal tumors than those in both tdLNs and ntdLNs, while a higher frequency of CD49a-expressing E7-specific CD8+ T cells was observed in the cervicovaginal tumors than those in both tdLNs and ntdLNs. No differences in the frequency of CD49d (α4β1)-expressing E7-specific CD8+ T cells were observed in different regions (). These results suggested that increase in LPAM-1-expressing T cell population and CD49a-expressing T cell population, as well as a decrease of CD103-expressing T cell populations, may be important for the enhanced infiltration of E7-specific CD8+ T cells into cervicovaginal tumors following hind leg IM vaccination.
Figure 5. Expressions of LPAM-1 (α4β7), CD49a (α1β1), CD103 (αEβ7), and CD49d (α4β1) by E7-specific CD8+ T cells in the lymph nodes and cervicovaginal tumor after hind leg pNGVL4a-CRT/E7(detox) vaccination in cervicovaginal TC-1/luc tumor-bearing or naïve mice. (A-B) Tumor-bearing female C57BL/6 mice (five to eight weeks old, five/group) were established by inoculation with 2 × 104 TC-1/luc cells at the intravaginal cavity on day 0. The mice were then vaccinated with 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA vaccine via IM injection, followed by electroporation in the hind leg on days 3 and 8. Seven days after the final vaccination, E7-specific CD8+ T cells were detected in the iliac, inguinal and axillary LNs and vaginal tumor tissue. A. Schematic diagram of experiment. B. Expressions of LPAM-1, CD49a CD103, and CD49d were analyzed after gating on E7 tetramer-positive CD8+ T cells. (C-D) Naïve female C57BL/6 mice (five to eight weeks old, five/group) were vaccinated with 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA vaccine via IM injection, followed by electroporation in the hind leg on days 0 and 5. Seven days after the final vaccination, E7-specific CD8+ T cells were detected in the iliac, inguinal and axillary LNs. C. Schematic diagram of experiment. D. Expressions of LPAM-1, CD49a CD103, and CD49d were analyzed after gating on E7 tetramer-positive CD8+ T cells. The experiments were reproduced twice with a pool of 5∼8 mice/experiment. Data are presented as mean ± SD. (* p < 0.05, **p < 0.01, *** p < 0.001, n.s. not significant).
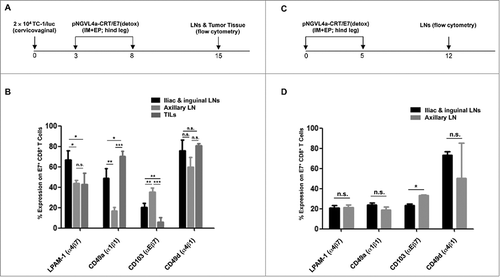
To test the hypothesis that the differential integrin expression is due to the pre-exposure of E7-antigens by the immune cells in the tdLNs, we characterized the integrin expressions in various LNs of naïve, tumor-free mice treated with IM pNGVL4a-CRT/E7(detox) vaccination in the hind leg (). Under naïve setting, we observed similar frequencies of LPAM-1-, CD49a-, or CD49d-expressing E7-specific CD8+ T cells, as well as lower frequencies of CD103-expressing E7-specific CD8+ T cells, in cervicovaginal draining iliac and inguinal LNs compared to those in non-cervicovaginal draining axillary LNs (). Particularly, we observed different frequencies of LPAM-1- and CD49a-expressing E7-specific CD8+ T cell populations in the iliac and inguinal LNs of hind leg-vaccinated naïve versus tumor-bearing mice ( and ). Similarly, hind leg vaccination with a DNA vaccine encoding an irrelevant antigen, Ovalbumin (OVA), in cervicovaginal TC-1/luc tumor-bearing mice did not increase the frequencies of LPAM-1- or CD49a-expressing OVA-specific CD8+ T cells in the iliac and inguinal tdLNs compared to those in the axillary ntdLNs (Fig. S4). Together, these data suggest that hind leg vaccination with cervicovaginal tumor antigens stimulates the expression of integrins LPAM-1 and CD49a by tumor antigen-specific CD8+ T cells in the cervicovaginal tdLNs.
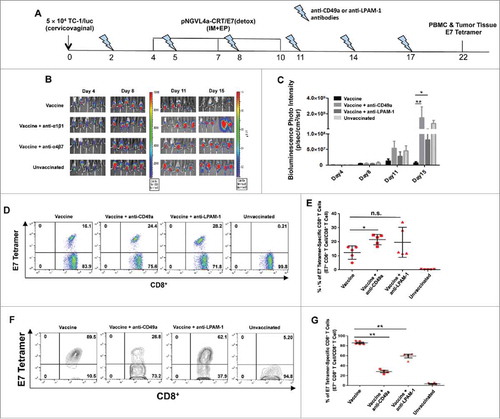
Neutralization of LPAM-1 and CD49a dampened the antitumor efficacy of hind leg intramuscular vaccination by hindering the recruitment of antigen-specific CD8+ T cells into cervicovaginal tumors
To address the role of LPAM-1 and CD49a in the infiltration of CD8+ T cells and the therapeutic effect of hind leg IM vaccination against cervicovaginal tumors, we administered neutralizing antibodies against these molecules into cervicovaginal tumor-bearing and hind leg pNGVL4a-CRT/E7(detox)-vaccinated mice (). The ability of hind leg vaccination to control the growth of cervicovaginal tumors significantly decreased when the mice were also treated with anti-CD49a or anti-LPAM-1 antibodies (). Compared to cervicovaginal tumor-bearing and hind leg-vaccinated mice without antibody administration, an increase in circulating E7-specific CD8+ T cells was observed in those treated with anti-CD49a but not in those treated with anti-LPAM-1 (). Most importantly, both anti-CD49a or anti-LPAM-1 antibody treatments significantly reduced the amount of cervicovaginal tumors infiltrating E7-specific CD8+ T cells following hind leg pNGVL4a-CRT/E7(detox) vaccination (). Together, these data suggest that both CD49a and LPAM-1 contributed to the recruitment of E7-specific CD8+ T cells to the cervicovaginal tumors following hind leg IM immunization.
Figure 6. Blockade of LPAM-1 (α4β7) and CD49a (α1β1) inhibits E7-specific CD8+ T cell infiltration and cancer vaccine activity on intravaginally implanted TC-1 tumors. A. Schematic illustration of the experiment. Female C57BL/6 mice (five to eight weeks old, five/group) were grafted with 5 × 104 TC-1/luc cells in the cervicovaginal tract on day 0. The mice were then vaccinated three times with 10 μg/mouse of pNGVL4a-CRT/E7(detox) DNA vaccine via IM injection, followed by electroporation in the hind legs on days 4, 7 and 10. They were also treated with either anti-LPAM-1 (500 μg/dose) or anti-CD49a (150 μg/dose) antibodies two days before the first vaccination, on day 2, and repeated every three days. Tumor growth was monitored using bioluminescence imaging. On day 22, peripheral blood lymphocytes and TILs were prepared and stained with anti-mouse CD8 antibody, H2-Db/E7 tetramer, and 7-AAD. B. Summary of bioluminescence images of the cervicovaginal TC-1/luc tumor-bearing mice. C. Bar graph depicting the mean luminescence intensity of cervicovaginal TC-1/luc tumor-bearing mice (* p < 0.05, ** p < 0.01). D. Representative flow cytometry images of E7-specific CD8+ T cells in peripheral blood. E. Summary of E7-specific CD8+ T cells in peripheral blood. F. Representative flow cytometry images of E7-specific CD8+ T cells in TILs. G. Summary of E7-specific CD8+ T cells in TILs. Data are presented as mean ± SD. (* p < 0.05, ** p < 0.01, n.s. not significant).
Discussion
This study highlights the ability to elicit potent antitumor responses against mucosal cancers through targeted intramuscular DNA immunization. Specifically, we showed that IM injection of a DNA cancer vaccine with electroporation in the quadriceps muscle of the hind leg could effectively control tumors located in the cervicovaginal mucosa (). Likewise, IM vaccination in the deltoid muscle of the front leg could effectively control tumors located in the oral mucosa (Fig.–S2).
Our results further support the need to elicit a potent antitumor immune response within TME for mucosal tumor control. Many studies advocate the necessity of mucosal immunization to induce effective mucosal immunities,Citation8,Citation31,Citation32 while several others argue for the ability of IM administration of vaccines to generate potent immune responses in mucosal tracts.Citation33-Citation35 Regardless of administration route, it is agreed that the induction of local mucosal CD8+ T cell responses is critical for the control of mucosal diseases.Citation36,Citation37 Here, we demonstrated the ability of targeted IM cancer DNA vaccine injection with electroporation to elicit an antitumor response for the control of mucosal tumors ( and S2), supporting the potential of generating mucosal immunity without the need for mucosal immunization. Particularly, Sandoval et al. has demonstrated that IM administration of a cancer vaccine fails to control tumors located in the lung or the oral cavity in comparison to administration through the intranasal route.Citation8 Our data agrees with their finding in that the typical (hind leg) in vivo IM vaccination is ineffective at controlling the growth of tumors located in the cheek (Fig. S2). However, by identifying the ideal site of IM vaccination, we demonstrated that IM vaccination in the front leg can induce potent therapeutic antitumor effects against an oral tumor model (Fig. S2). Similarly, we demonstrated that while IM front or hind leg administration of a cancer vaccine elicited a similar magnitude of systemic antigen-specific CD8+ T cell response, vaccination in the hind leg enhanced the recruitment of these T cells into the cervicovaginal TME and resulted in better cervicovaginal tumor control ( and ).
Particularly, we demonstrated the preferential recruitment of CD8+ T cells into cervicovaginal tumors after IM hind leg vaccination is associated with enhanced antigen-specific CD8+ T cell responses in the iliac and inguinal cervicovaginal tdLNs (). When compared to naïve LNs, tdLNs appear to be immune suppressed, with lower frequencies of both total and matured DCs (Fig. S5). This observation is consistent with existing literature. Previous publications have demonstrated that although tdLNs are immune suppressed environments with fewer CD8± CD11b- cross-presenting DCs as compared to ntdLNs, the tdLNs contain a higher number of tumor antigen-specific CD8+ T cells that can be re-stimulated ex vivo to secret IFN-γ, TNF-α, and IL-2, demonstrating that albeit immune suppressed tdLNs are also tumor antigen primed, thereby allowing for the generation of strong immune response following tdLNs targeted immunization.17 Similarly, it has been reported that despite the gradual decrease of overall antigen-presenting cell population in the tdLNs throughout tumor development, there is a significant increase in migratory, tumor antigen expressing, CD103± DC population in the tdLNs, as compared to that in ntdLNs, which facilitated the priming of tumor-specific T cells in the tdLNs.Citation38 Similarly, we have demonstrated that IM DNA vaccination targeting the tdLNs resulted in the generation of stronger tumor infiltrating CTL responses and better tumor control and prolonged survival as compared to vaccination targeting ntdLNs (). Furthermore, surgical removal of tdLNs reduced the infiltration of E7-specific CD8+ T cells to cervicovaginal tumors and abolished the ability of therapeutic immunization to control tumor growth (). Thus, our data further support the concept that even though tdLNs are under the immune suppressive influence of tumor, they are also immune-educated sites primed with the ability to elicit potent antitumor immune responses upon boosting with cancer vaccines (as reviewed in Citation15,Citation16).
At the molecular level, we showed that IM hind leg vaccination preferentially induced the expression of integrins LPAM-1 and CD49a by these antigen-specific CD8+ T cells in the cervicovaginal tdLNs (). It has been suggested that antigen-presenting DCs originated from the site of disease play an important role in imprinting the T cells in the regional LNs with appropriate homing signals,Citation39,Citation40 and that, in the absence of tumors, tumor antigen-specific immunization failed to induce significant proliferation of antigen-specific CD8+ T cells.Citation41 In line with these findings, we have shown that tumor antigen-specific vaccination in naïve mice, as well as immunization with a vaccine encoding non-tumor antigens in cervicovaginal tumor-bearing mice, failed to induce LPAM-1 and CD49a integrin expressions by the respective CD8+ T cells in the cervicovaginal draining LNs ( and S4). Importance of homing integrins in mediating mucosal immunity has been well recognized (for review see Citation42). The role of CD49a in mediating T cell homing to mucosal regions, particularly to the buccal area and the lung, has been reported in both preclinicalCitation8,Citation43 and humanCitation8 settings. CD49a has also been identified to be highly expressed by CD8+ T cells residing in the skin epithelial as well as gut and cervical mucosal tissue samples of human subjects.Citation44 Likewise, LPAM-1 has been shown to facilitate the homing of T cells to gut mucosaCitation45 as well as the cervicovaginal tractCitation9,Citation10 in preclinical settings, which is further supported by the clinical finding that LPAM-1 was expressed on the surface of nearly all T cells isolated from the cervical mucosa of healthy human subjects or patients with cervical intraepithelial neoplasia 2/3 (CIN2/3).Citation46 This information further supports the ability of hind leg IM pNGVL4a-CRT/E7(detox) vaccination to generate potent cervicovaginal antitumor immunity by inducing the expressions of LPAM-1 or CD49a on E7-specific CD8+ T cells (). We have observed a similar integrin expression profile for buccal tumor TILs as that of cervicovaginal tumor TILs, with high CD49a and CD49d and low CD103 expression (Fig. S6). Although buccal tumor TILs do not express high levels of LPAM-1 as observed for cervicovaginal tumor TILs, this is expected because LPAM-1 is not a known homing marker for the buccal mucosa. Importantly, administration of anti-LPAM-1 or anti-CD49a neutralizing antibodies reduced the infiltration of E7-specific CD8+ T cells to cervicovaginal tumors and abolished the ability of therapeutic immunization to control the growth of tumors (), further highlighting the crucial role of homing integrins in controlling mucosal cancers.
The therapeutic vaccine used in the current study, pNGVL4a-CRT/E7(detox), has been tested in a phase I clinical trial in 32 patients with CIN2/3 lesions.Citation27 In the study, the vaccine was administered via 1) gene gun delivery on the thigh, 2) IM injection in the deltoid muscle without electroporation, or 3) cervical intralesional injection without electroporation. While the vaccine was well-tolerated by patients, only 8 of 27 patients who received all vaccinations experienced histologic regression of disease. Furthermore, the increase in intraepithelial CD8+ T cell infiltration was only observed in patients that received intralesional injection. We have previously demonstrated in a preclinical study that IM injection of pNGVL4a-CRT/E7(detox) with electroporation is more efficacious at generating E7-specific CD8+ T cell-mediated antitumor responses compared to IM injection only or delivery via gene gun.Citation26 In our current study, we further demonstrated that IM injection of the vaccine with electroporation in the hind leg is more effective at generating E7-specific immunity against diseases located in the cervicovaginal tract, while administration of the vaccine in the front leg via similar methods resulted in better control against diseases located in the oral cavity ( and S2). These observations provide direct rationales for the design of future clinical investigations of pNGVL4a-CRT/E7(detox) in various human disease settings.
While our results argued for the ability to generate mucosal immunity through targeted IM DNA immunization instead of the need for mucosal vaccination, we recognize the limitation that no comparisons between the efficacy of targeted IM immunization versus intravaginal immunization (i.e. hind leg versus intravaginal vaccination or front leg versus buccal vaccination) against mucosal tumors were made in the current study. We have previously showed that intravaginal injection of a DNA vaccine with electroporation generated stronger therapeutic antitumor effects in a preclinical cervicovaginal tumor model compared to IM injection with electroporation,Citation9 perhaps through the inflammatory responses induced at the mucosal area by intravaginal electroporation.Citation47,Citation48 Nonetheless, at least in the context of therapeutic DNA vaccine, vaccine injection with electroporation in the cervicovaginal tract has yet to become a clinically applicable treatment strategy (as discussed in Citation9,Citation49). Thus, the ability to enhance the therapeutic efficacy of IM injection of a DNA vaccine with electroporation against mucosal tumors still holds strong translational relevance.
Of note, in the current study, we defined axillary lymph nodes as the buccal draining lymph nodes due to the proximity of axillary lymph nodes to the site of front leg vaccination, and characterized the CD8+ T cells responses in the axillary lymph nodes, as compared to cervicovaginal draining iliac and inguinal lymph nodes, when the vaccination is administered in the front or hind leg. It should be pointed out that the primary sentinel lymph nodes for head and neck cancers are the cervical lymph nodes rather than axillary lymph nodes, as established by previous publications (reviewed inCitation50). However, while less typical, axillary lymph node metastasis has been observed in multiple occasions in patients with cancers of oral origin,Citation51-Citation54 suggesting the potential draining from cancers of the head and neck to the axillary lymph nodes. Similarly, we have demonstrated that front leg vaccination generated a significantly stronger antigen-specific CD8+ T cells response in the axillary lymph nodes of naïve mice as compared to that in the iliac and inguinal lymph nodes, which exerts potent antitumor effects against tumors located in the buccal area ( and S2), supporting the translational relevance of front leg vaccination for improved oral cancer control. Future studies on the therapeutic mechanisms of targeted intramuscular DNA vaccination in the oral cancer setting, including the potential cross talk between axillary and cervical lymph nodes, are warranted.
This study reports the importance of targeting the immune response elicited by intramuscular DNA vaccination to mucosal tumor draining lymph nodes for the generation of antitumor CD8+ T cell responses capable of trafficking into the mucosal tumors. As intramuscular injection with electroporation represents a common method for the administration of therapeutic DNA vaccines in human patients, the current findings may lead to readily adaptable modifications of medical practice for the potential treatment of mucosal tumors.
Materials and methods
Mice
Female C57BL/6 mice (five- to eight-weeks old) were purchased from the Charles River Laboratories (Frederick, MD). All mice were maintained at the Johns Hopkins University School of Medicine Oncology Animal Facility (Baltimore, MD) under specific pathogen-free conditions. All experiments were performed according to protocols approved by the Johns Hopkins Institutional Animal Care and Use Committee and in accordance with recommendations for the proper use and care of laboratory animals.
Antibodies and other reagents
FITC-conjugated anti-mouse CD8a (clone 53.6.7), purified anti-mouse CD16/32 (clone 2.4 G2), and purified anti-mouse CD49a (clone Ha31/8) antibodies were purchased from BD Pharmingen (San Diego, CA). 7-AAD was also purchased from BD Pharmingen. Zombie Aqua, BV421-conjugated anti-mouse CD11c (clone N418), PE-Cy7-conjugated anti-mouse I-Ab (clone AF6-120.1), BV785-conjugated anti-mouse CD86 (clone GL-1), Percp.Cy5.5-conjugated anti-mouse CD103 (clone 2E7) and anti-mouse CD49d (clone R1-2), as well as APC-conjugated anti-mouse CD29 (clone HMβ1-1) antibodies, were purchased from BioLegend (San Diego, CA). PE-conjugated anti-mouse CD40 (clone 1C10), FITC-conjugated rat anti-mouse CD4 (clone RM4-5) and anti-mouse Gr-1 (clone RB6-8C5), PE-conjugated anti-mouse Foxp3 (clone FJK-16s) and anti-mouse CD11b (clone M1/70), APC-conjugated anti-mouse CD25 (clone PC61.5) and anti-mouse LPAM-1 (clone DATK32), as well as Percp.Cy5.5-conjugated anti-mouse CD49a (cloneHa31/8) antibodies, were purchased from eBioscience (San Diego, CA). Purified anti-mouse LPAM-1 (clone DATK32) antibodies were purchased from BioXcell (West Lebanon, NH). PE-conjugated, HPV16/E7aa49-57 peptide-loaded H2-Db tetramers were obtained from the National Institute of Allergy and Infectious Diseases Tetramer Facility (Atlanta, GA). PE-conjugated, ovalbumin (OVA)aa257-264 peptide-loaded H2-Kb tetramer was purchased from MBL International Corporation (Woburn, MA).
Cell line
The establishment of the HPV16 E6/E7 and firefly luciferase-expressing TC-1 tumor cell line (TC-1/luc) have been previously described.Citation28 The cells were maintained in RPMI-1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, 400 μg/mL G418 disulfate salt, and 10 % fetal bovine serum (FBS).
DNA plasmid and administration
The generations of the pNGVL4a-CRT/E7(detox),Citation25 pcDNA3-OVA,Citation55 and pcDNA3-LuciferaseCitation56 plasmids have been previously described. DNA plasmids were prepared using endotoxin-free kits (Qiagen). For DNA vaccination, 50 µL of DNA was prepared and administered in the front (deltoid) or hind (quadriceps) legs of mice via intramuscular (IM) injection, followed by electroporation with an ECM830 Square Wave Electroporation System (BTX Harvard Apparatus company, Holliston, MA, USA). The mice were boosted as indicated.
In Vivo bioluminescence imaging of luciferase expression
Luciferase expressions by either DNA plasmid injection or TC-1/luc cells were monitored by bioluminescence using a Xenogen imaging system (Xenogen). Briefly, D-Luciferin was dissolved in 7.8 mg/mL in PBS, filter sterilized, and stored at -80°C. Mice were given D-Luciferin by intraperitoneal (IP) injection (200 μL/mouse, 75 mg/kg) and anesthetized with isoflurane. In vivo bioluminescence imaging for luciferase expression was conducted on a cryogenically-cooled IVIS system using the Living Image acquisition and analysis software (Xenogen). Mice were placed onto the pre-warmed stage inside the light-tight camera box with continuous exposure to 1%-2% isoflurane. Images were acquired 10 mins after D-luciferin administration and imaged for 2 mins. The levels of light from the bioluminescent cells were detected using the IVIS imager, and subsequently integrated and digitized. The region of interest from displayed images was designated around the vagina and quantified as total photon counts using the Living Image 2.50 software (Xenogen).
In Vivo tumor treatment experiment
To test the antitumor effect of pNGVL4a-CRT/E7(detox) DNA vaccine when vaccinated at different sites, an orthotopic cervicovaginal tumor model and an oral cavity tumor model were used. In orthotopic cervicovaginal tumor models, five- to eight-week old female C57BL/6 mice (five/group) were injected with 2 × 104 of TC-1/luc cells at the intravaginal cavity on day 0. The mice were vaccinated with 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA via intramuscular injection, followed by electroporation at different sites on day 4 and boosted once four days later. In oral cavity tumor models, five- to eight-week old female C57BL/6 mice were injected with 2 × 104 of TC-1/luc cells in the cheeks on day 0. The mice were vaccinated with 20 μg/mouse of pNGVL4a-CRT/E7(detox) DNA via intramuscular injection, followed by electroporation at different sites on day 4 and boosted once four days later. For antibody blockade experiments in orthotopic cervicovaginal tumor models, five- to eight-week old female C57BL/6 mice (five/group) were grafted with 2 × 104 of TC-1/luc cells in the cervicovaginal tract on day 0. The mice were then vaccinated twice with 10 μg/mouse pNGVL4a-CRT/E7(detox) DNA vaccine via intramuscular injection, followed by electroporation on days 4 and 8 in the hind legs. These mice were also treated with either anti-LPAM-1 (500μg/mouse/dose) or anti-CD49a (150μg/mouse/dose) neutralizing monoclonal antibodies via intraperitoneal injection one day after the first vaccination and repeated every three days. The growth of cervicovaginal tumor was monitored with bioluminescence imaging as described above at indicated time points.
Preparation of single-cell suspensions from lymph nodes and TC-1 tumors
At the indicated time points after vaccination, various lymph nodes and TC-1/luc tumor tissues were surgically excised using sterile techniques, placed in RPMI-1640 medium containing 100 U/mL penicillin and 100 μg/mL streptomycin, and washed with PBS. The tissues were then minced into 1∼2-mm pieces and immersed in serum-free RPMI-1640 medium containing 0.05 mg/mL collagenase I, 0.05 mg/mL collagenase IV, 0.025 mg/mL hyaluronidase IV, 0.25 mg/mL DNase I, 100 U/ml penicillin, and 100 μg/ml streptomycin, and incubated at 37°C with periodic agitation. The tissue digest was then filtered through a 70-μm nylon filter mesh to remove undigested tissue fragments. The resultant single cell suspensions were washed twice with PBS, and viable cells were then determined using trypan blue dye exclusion.
Flow cytometry analysis
For tetramer staining, peripheral blood mononuclear cells (PBMCs), single cells of lymph nodes, spleen, and tumors from the mice were stained with purified anti-mouse CD16/32 first, and then with anti-mouse CD8-FITC. Finally, they are stained with either the PE-conjugated H-2Db tetramer loaded with HPV16 E7aa49–57 peptide, or with the PE-conjugated, OVAaa257-264 peptide loaded with H2-Kb tetramer at 4°C. After washing, the cells were stained with 7-AAD before flow cytometry analyses to exclude dead cells. T regulatory (Treg) cells from mouse lymph nodes were detected through FITC-conjugated anti-mouse CD4 and APC-conjugated anti-mouse CD25 antibody staining. Cells were then permeabilized and fixed per manufacturer's instructions (eBioscience, San Diego, CA), followed by intracellular staining of Foxp3. To detect CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs), lymph node cells were stained with FITC-conjugated anti-mouse Gr-1 and PE-conjugated anti-mouse CD11b antibodies. To detect LPAM-1 (α4β7), CD103 (αEβ7), CD49a (α1β1), and CD49d (α4β1) expression on HPV16 E7 or OVA-specific CD8+ T cells, cells from lymph nodes and tumors were stained with anti-mouse CD8, LPAM-1, CD103, CD29, CD49a, CD49d, and either HPV16 E7aa49–57 peptide-loaded H-2Db or OVAaa257-264 peptide-loaded H2-Kb tetramer. The cells were acquired using a FACSCalibur flow cytometer and analyzed with the CellQuest Pro software (BD biosciences, Mountain View, CA). To detect the dendritic cell population and phenotype, lymph node cells were stained with anti-mouse CD11c, H2-IAb, CD86, and CD40. The cells were acquired with CytoFlex S flow cytometer (Beckman Coulter, Miami, FL) and analyzed with FlowJo software (BD biosciences, Mountain View, CA).
Statistical analyses
All data are expressed as mean ± standard deviation (S.D.) and are representative of at least two separate experiments. Comparisons between individual data points were made using Student's t-tests. The non-parametric Mann-Whitney test was used for comparing two different groups. Survival distributions for mice in different groups were compared through Kaplan-Meier survival curves, and by use of the long-rank tests. Of note, *, ** and *** indicate P values less than 0.05, 0.01, and 0.001, respectively; n.s., not significant.
Disclosure of interest
T.-C. Wu is a co-founder of and has an equity ownership interest in Papivax LLC. Also, he owns Papivax Biotech Inc. stock options and is a member of Papivax Biotech Inc.'s Scientific Advisory Board. Additionally, under a licensing agreement between Papivax Biotech Inc. and the Johns Hopkins University, Dr. Wu and Dr. Hung are entitled to royalties on an invention described in this article. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Other co-authors have declared that no conflicts of interest exist.
Grant support
This work was supported by the United States National Institutes of Health (NIH) Cervical Cancer Specialized Program of Research Excellence (SPORE) (P50 CA098252), 5R21 grant (AI109259), 1R21 grant (CA194896), and 2R01 (CA114425).
Authors' contributions
Conception and design: J. Qiu, S. Peng, C.F. Hung, T.C. Wu
Development of methodology: J. Qiu, S. Peng, C.F. Hung, T.C. Wu
Acquisition of data (provided animals, acquired and manages patients, provided facilities, etc.): J. Qiu, S. Peng, Y. Ma, L. Han, C.F. Hung, T.C. Wu
Analysis and interpretation of data (e.g., statistical analysis, computational analysis): J. Qiu, S. Peng, A. Yang, Y. Ma, L. Han, C.F. Hung, T.C. Wu
Writing, review, and/or revision of the manuscript: S. Peng, A. Yang, M.A. Cheng, E. Farmer, C.F. Hung, T.C. Wu
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J. Qiu, S. Peng, A. Yang, C.F. Hung, T.C. Wu
Study supervision: C.F. Hung, T.C. Wu
KONI_A_1463946.zip
Download Zip (2.4 MB)Additional information
Funding
References
- Schatton T, Scolyer RA, Thompson JF, Mihm MC, Jr. Tumor-infiltrating lymphocytes and their significance in melanoma prognosis. Methods Mol Biol. 2014;1102:287–324.
- Santoiemma PP, Powell DJ, Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16:807–20.
- Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, Darcy PK, Loi S. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. doi:10.1186/s12916-015-0431-3. PMID:26300242.
- Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5. doi:10.1158/0008-5472.CAN-11-1316. PMID:21846822.
- Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, Alvarez RD, Roden RB, Pardoll D, Hung CF. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013;73:2493–504. doi:10.1158/0008-5472.CAN-12-4241. PMID:23418322.
- Jin C, Liu Y, Zhu J, Xia T, Zhang B, Fei Y, Ma B, Ye J, Chen W. Recombinant Salmonella-based CEACAM6 and 4-1BBL vaccine enhances T-cell immunity and inhibits the development of colorectal cancer in rats: In vivo effects of vaccine containing 4-1BBL and CEACAM6. Oncol Rep. 2015;33:2837–44. doi:10.3892/or.2015.3901. PMID:25872647.
- Gordy JT, Luo K, Zhang H, Biragyn A, Markham RB. Fusion of the dendritic cell-targeting chemokine MIP3alpha to melanoma antigen Gp100 in a therapeutic DNA vaccine significantly enhances immunogenicity and survival in a mouse melanoma model. J Immunother Cancer. 2016;4:96.
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5:172ra20. doi:10.1126/scitranslmed.3004888. PMID:23408053.
- Sun Y, Peng S, Qiu J, Miao J, Yang B, Jeang J, Hung CF, Wu TC. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene Ther. 2015;22:528–35. doi:10.1038/gt.2015.17. PMID:25786869.
- Sun YY, Peng S, Han L, Qiu J, Song L, Tsai Y, Yang B, Roden RB, Trimble CL, Hung CF. Local HPV recombinant Vaccinia Boost following priming with an HPV DNA Vaccine enhances Local HPV-Specific CD8+ T-cell-Mediated Tumor control in the Genital Tract. Clin Cancer Res. 2016;22:657–69. doi:10.1158/1078-0432.CCR-15-0234. PMID:26420854.
- Pialoux G, Hocini H, Perusat S, Silberman B, Salmon-Ceron D, Slama L, Journot V, Mathieu E, Gaillard C, Petitprez K. Phase I study of a candidate vaccine based on recombinant HIV-1 gp160 (MN/LAI) administered by the mucosal route to HIV-seronegative volunteers: the ANRS VAC14 study. Vaccine. 2008;26:2657–66.
- Meque I, Dube K, Bierhuizen L, Zango A, Veldhuijzen N, Cumbe F, Feldblum PJ, van de Wijgert J. Willingness to participate in future HIV prevention trials in Beira, Mozambique. Afr J AIDS Res. 2014;13:393–8. doi:10.2989/16085906.2014.985239. PMID:25555105.
- Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res. 2014;2:701–7. doi:10.1158/2326-6066.CIR-14-0115. PMID:25092811.
- Stachura J, Wachowska M, Kilarski WW, Guc E, Golab J, Muchowicz A. The dual role of tumor lymphatic vessels in dissemination of metastases and immune response development. Oncoimmunology. 2016;5:e1182278. doi:10.1080/2162402X.2016.1182278. PMID:27622039.
- Mehta NK, Moynihan KD, Irvine DJ. Engineering new approaches to Cancer Vaccines. Cancer Immunol Res. 2015;3:836–43. doi:10.1158/2326-6066.CIR-15-0112. PMID:26156157.
- Gardner A, Ruffell B. Dendritic cells and Cancer immunity. Trends Immunol. 2016;37:855–65. doi:10.1016/j.it.2016.09.006. PMID:27793569.
- Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, Hubbell JA, Swartz MA. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2:436–47. doi:10.1158/2326-6066.CIR-14-0019-T. PMID:24795356.
- Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, Imai T, Inoue M, Kitano S, Kichikawa K. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26:248–52. doi:10.1007/s12149-011-0568-x. PMID:22271546.
- Coates EE, Costner PJ, Nason MC, Herrin DM, Conant S, Herscovitch P, Sarwar UN, Holman L, Mitchell J, Yamshchikov G. Lymph Node activation by PET/CT following Vaccination with licensed Vaccines for Human Papillomaviruses. Clin Nucl Med. 2017;42:329–34. doi:10.1097/RLU.0000000000001603. PMID:28288041.
- Yoshikawa H, Seebach S. Lymphotropic delivery of cyclosporin A by intramuscular injection of biodegradable microspheres in mice. Biol Pharm Bull. 1996;19:1527–9.
- Cantisani R, Pezzicoli A, Cioncada R, Malzone C, De Gregorio E DU, Piccioli D. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J Immunol. 2015;194:1717–25.
- Raj LSM, Boaz K, Natarajan S. Prognostic significance of Lymph Node pattern in Oral Squamous Cell Carcinoma (OSCC). J Clin Diagn Res. 2014;8:232–5. doi:10.7860/JCDR/2014/7365.3974. PMID:24596783.
- Thakare E, Gawande M, Chaudhary M, Seralathan M, Kannan K. Detection of micrometastasis in lymph nodes of oral squamous cell carcinoma: A comparative study. J Oral Maxillofac Pathol. 2013;17:374–80. doi:10.4103/0973-029X.125202. PMID:24574655.
- Li X, Yin Y, Sheng X, Han X, Sun L, Lu C, Wang X. Distribution pattern of lymph node metastases and its implication in individualized radiotherapeutic clinical target volume delineation of regional lymph nodes in patients with stage IA to IIA cervical cancer. Radiat Oncol. 2015;10:40. doi:10.1186/s13014-015-0352-5. PMID:25886535.
- Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–78.
- Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, Wu TC, Pai SI. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–9. doi:10.1016/j.vaccine.2009.07.005. PMID:19622402.
- Alvarez RD, Huh WK, Bae S, Lamb LS, Jr., Conner MG, Boyer J, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol. 2016;140:245–52.
- Huang B, Mao CP, Peng S, He L, Hung CF, Wu TC. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine. 2007;25:7824–31.
- Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, et al. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15:989–1007.
- Badoual C, Sandoval F, Pere H, Hans S, Gey A, Merillon N, Van Ryswick C Q-CF, Bruneval P, Brasnu D. Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head Neck. 2010;32:946–58.
- Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122:4606–20. doi:10.1172/JCI63287. PMID:23143305.
- Macedo R, Rochefort J, Guillot-Delost M, Tanaka K, Le Moignic A NC, Baillou C, Mateo V, Carpentier AF, Tartour E. Intra-cheek immunization as a novel vaccination route for therapeutic vaccines of head and neck squamous cell carcinomas using plasmo virus-like particles. Oncoimmunology. 2016;5:e1164363.
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi:10.1038/nature07469. PMID:18997770.
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–64. doi:10.1084/jem.20090858. PMID:20156972.
- Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. 2014;6:221ra13. doi:10.1126/scitranslmed.3007323. PMID:24477000.
- Belyakov IM, Ahlers JD, Brandwein BY, Earl P, Kelsall BL, Moss B, Strober W, Berzofsky JA. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J Clin Invest. 1998;102:2072–81. doi:10.1172/JCI5102. PMID:9854042.
- Trimble CL, Peng S, Thoburn C, Kos F, Wu TC. Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3. Cancer Immunol Immunother. 2010;59:799–803.
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. Critical role for CD103(+)/CD141(+) Dendritic cells bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell immunity in Melanoma. Cancer Cell. 2016;30:324–36. doi:10.1016/j.ccell.2016.06.003. PMID:27424807.
- Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–43. doi:10.1016/j.it.2006.03.007. PMID:16580261.
- Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–49. doi:10.1016/j.immuni.2006.01.015. PMID:16618602.
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Rüegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–84. doi:10.1016/j.immuni.2004.12.008. PMID:15723806.
- Brinkman CC, Peske JD, Engelhard VH. Peripheral tissue homing receptor control of naive, effector, and memory CD8 T cell localization in lymphoid and non-lymphoid tissues. Front Immunol. 2013;4:241. doi:10.3389/fimmu.2013.00241. PMID:23966998.
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–79. PMID:14975239.
- Cheuk S, Schlums H, Gallais Serezal I, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M. CD49a expression defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic function in Human skin. Immunity. 2017;46:287–300. doi:10.1016/j.immuni.2017.01.009. PMID:28214226.
- De Calisto J, Villablanca EJ, Wang S, Bono MR, Rosemblatt M, Mora JR. T-cell homing to the gut mucosa: general concepts and methodological considerations. Methods Mol Biol. 2012;757:411–34. doi:10.1007/978-1-61779-166-6_24. PMID:21909925.
- Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, Kos F, Teague J, Jiang Y, Barat NC. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi:10.4049/jimmunol.1002756. PMID:21037100.
- Ahlen G, Soderholm J, Tjelle T, Kjeken R, Frelin L, Hoglund U, Blomberg P, Fons M, Mathiesen I, Sällberg M. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–53. PMID:17878373.
- Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol. 2008;82:5643–9. doi:10.1128/JVI.02564-07. PMID:18353952.
- Sun Y, Peng S, Yang A, Farmer E, Wu TC, Hung CF. Coinjection of IL2 DNA enhances E7-specific antitumor immunity elicited by intravaginal therapeutic HPV DNA vaccination with electroporation. Gene Ther. 2017;24:408–15. doi:10.1038/gt.2017.38. PMID:28492521.
- Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. PMID:12139232.
- Rayatt SS, Dancey AL, Fagan J, Srivastava S. Axillary metastases from recurrent oral carcinoma. Br J Oral Maxillofac Surg. 2004;42:264–6. doi:10.1016/j.bjoms.2003.12.004. PMID:15121278.
- Koga-Yamakawa E, Dovedi SJ, Murata M, Matsui H, Leishman AJ, Bell J, Ferguson D, Heaton SP, Oki T, Tomizawa H. Intratracheal and oral administration of SM-276001: a selective TLR7 agonist, leads to antitumor efficacy in primary and metastatic models of cancer. Int J Cancer. 2013;132:580–90. doi:10.1002/ijc.27691. PMID:22733292.
- Trosman SJ, Koyfman SA, Ward MC, Al-Khudari S, Nwizu T, Greskovich JF, Lamarre ED, Scharpf J, Khan MJ, Lorenz RR. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA Otolaryngol Head Neck Surg. 2015;141:457–62. doi:10.1001/jamaoto.2015.136. PMID:25742025.
- Kang S. Unsuspected axillary lymph node metastasis of nasopharyngeal and cervical cancer on 18FDG PET/CT: a case report. Nucl Med Rev Cent East Eur. 2016;19:20–1. doi:10.5603/NMR.2016.0032. PMID:27900757.
- Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, Kumar S, Wu TC. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–17. doi:10.1172/JCI17293. PMID:12840065.
- Huang B, Mao CP, Peng S, Hung CF, Wu TC. RNA interference-mediated in vivo silencing of fas ligand as a strategy for the enhancement of DNA vaccine potency. Hum Gene Ther. 2008;19:763–73. doi:10.1089/hum.2007.059. PMID:18627219.
