ABSTRACT
Purpose: While recent studies described the role of IKZF1/3 proteins in multiple myeloma (MM) cells, few have highlighted the significance of IKZF1/3 expression in T-cells. In this study we examine the prognostic and predictive value of IKZF1/3 expression in T-cells in patients with MM stage III.
Experimental design: We analysed the IKZF1/3 expression levels in T-cells from 45 MM stage I (MMI) and 50 newly diagnosed MM stage III (MMIII) patients, according to Durie-Salmon staging system, by flow cytometry to examine their prognostic and predictive value. We also combined in vivo observations with in vitro assays to determine the effect of IKZF1/3 expression on the T-cell immunophenotype and anti-tumour T-cell response in 162 MMIII patients.
Results: We found that high IKZF3, but not IKZF1, expression in T-cells correlates with superior overall survival in MMIII patients treated with immunomodulatory drugs (thalidomide, lenalidomide and pomalidomide). Moreover, we show that higher IKZF3 expression in T-cells inhibits myeloma-specific T-cell response in vitro and that the immunophenotype of patients with high IKZF3 expression shows features that are contrary to the changes induced by immunomodulatory drugs. Although we observed higher IKZF3 expression levels in T-cells from patients with MMIII compared to MMI, IKZF3 expression was unaffected by the tumour microenvironment.
Conclusion: In conclusion, IKZF3 expression in T-cells is a predictive value for clinical outcome in MMIII patients treated with immunomodulatory drugs due to its profound modulation of T-cell functionality.
Introduction
Although MM has been acknowledged as an incurable disease, recent achievements have challenged this paradigm. In particular, the introduction of immunomodulatory drugs, such as lenalidomide and pomalidomide, has expanded the therapeutic options for patients with MM. In addition to their known anti-MM effects,Citation1,Citation2 they modulate the immune system and enhance T-cell function.
IKZF1/3 proteins are transcriptional factors that play a major role in lymphocytes differentiation, IKZF1 is a well-known regulator of Notch target genes.Citation3,Citation4 IKZF1/3 regulate chromatin remodelling of their target genes either by directly binding to them and subsequent formation of heterochromatin, or by recruiting other proteins such as HDAC1.Citation5,Citation6
The post-transcriptional regulation of IKZF1/3 is poorly understood, heterodimerization with other proteins is thought to be one of the mechanisms by which IKZF1/3 functions are regulated.Citation7-Citation9 Spleen tyrosine kinase (SYK) and Bruton’s Tyrosine Kinase (BTK) are found to phosphorylate unique sites in the zinc finger domain of IKZF1 and therefore increase its nuclear localization and DNA binding activity.Citation7,Citation10 Moreover, the phosphorylation of IKZF1 by Casein Kinase II (CK2) at its C-terminal region regulates its ability to control G1/S cell cycle progression.Citation11
Recent data have highlighted the role of the CRL4CRBN ubiquitin ligase complex and the IKZF1/3 in the mode of action of immunomodulatory drugs. Krönke et al. showed that the binding of lenalidomide to the CRBN-CRL4 ubiquitin ligase complex augments the binding affinity of CRBN to IKZF1/3 and that this specific binding leads to the subsequent ubiquitination and degradation of IKZF1/3, which are essential for the survival of MM cells.Citation12
The role of IKZF1/3 on T-cell functionality was first reported by Thomas et al. and Bandyopadhyay et al. They described the influence of IKZF1 on the regulation of IL-2 in T-cells.Citation13,Citation14 Similarly, Krönke et al. showed by downregulating IKZF1/3 in human CD3+ T-cells that IKZF1/3 suppress autocrine IL-2 secretion from T-cells and that downregulation of IKZF1/3 enhances IL-2 secretion from T-cells.Citation12
Aside from these in vitro observations, recent data have highlighted the prognostic value of CRBN-dependent proteins expression not only in MM cellsCitation15-Citation18 but also in immune cells for the outcome of patients with MM treated with immunomodulatory drugs.Citation19 In addition to the correlation of the expression of CRBN and its associated genes, such as IKZF1, IRF4, MCT-1 and CD147, with overall survival (OS), the authors also described the expression of IKZF1/3 protein in T- and B-cells in the bone marrow (BM) of a small cohort of patients with MM. They found that higher IKZF1 expression correlates with increased progression-free (PFS) and overall survival as well as an improved response after lenalidomide-based therapy.
The aim was to confirm recent findings regarding the prognostic and predictive value of IKZF1/3 protein expression levels in T-cells in a large cohort and to clarify the significance of IKZF1/3 expression in T-cell functionality in patients with MM.
Results
Flow cytometry analyses reveal marked differences in the frequency of T-cells expressing IKZF1/3 proteins in healthy donors and patients with plasma cell diseases
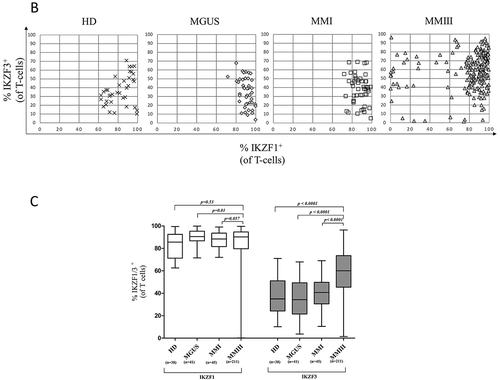
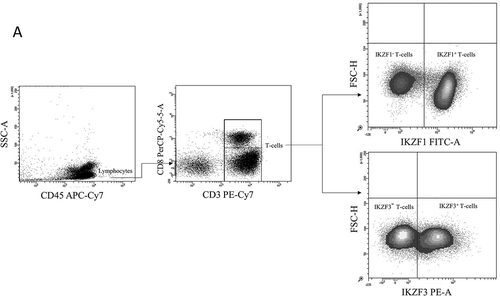
We compared the expression levels of IKZF1/3 protein in T-cells from 38 healthy donors (HD), 43 Monoclonal Gammopathy of Undetermined Significance (MGUS), 45 MMI and 211 MMIII patients, by flow cytometry analyses. T-cells were gated as SSClow CD45+ CD3+, and the protein expression level was measured as the percentage of T-cells expressing the proteins ().
Figure 1. Flow cytometry analysis of IKZF1/3 expression in T-cells from healthy donors and plasma cell disease patients.
Representative flow cytometry plots showing the gating strategy used to analyse IKZF1/3 levels in CD3+ T-cells.
XY scatter plots for IKZF1 expression (on the X-axis) versus IKZF3 expression (on the Y-axis) showing the average distribution in HD (n = 38), MGUS (n = 43), MMI (n = 49) and MMIII (n = 211) patients; every point represents one individual.
The differences in IKZF1/3+ T-cells frequencies in HD and patients with different plasma cell diseases.
By comparing individuals in each group, we found that all HD, MGUS patients and MMI patients displayed an IKZF1 expression level between 62.5 and 99.8%, whereas MMIII patients displayed an expression level between 0.1 and 99.6%, with approximately 13% of the patients exhibiting expression levels lower than 60%. All HD, MGUS patients and MMI patients exhibited an IKZF3 expression level between 3.7 and 71%, whereas MMIII patients exhibited an expression level between 1.4 and 96.4%, with approximately 30% of the patients exhibiting IKZF3 expression levels higher than 71% ().
By comparing the different groups, we found that patients with MMIII displayed a significantly higher IKZF3 protein expression level compared to HD (p < 0.0001), MGUS patients (p < 0.0001) and MMI patients (p < 0.0001). A significant difference was not observed in the IKZF1 expression level between MMIII patients and HD (p = 0.53); however, MMIII patients displayed a slight decrease in the expression level of IKZF1 compared to MGUS patients (p = 0.01) and a trend compared to MMI patients (p = 0.057) ().
High IKZF3 expression in T-cells correlates with superior OS in symptomatic MMIII patients treated with immunomodulatory drugs
Given that most of the patients displayed different IKZF1/3 expression levels in their T-cells, we examined the role of IKZF1/3 expression level in the progression to symptomatic MMIII as well as the response to immunomodulatory drugs. We analysed the IKZF1/3 protein expression level in 42 samples collected from patients diagnosed with MMI. Based on the IKZF1/3 expression level in T-cells, the patients were subdivided into the IKZF1/3high or IKZF1/3low groups; the subdivision was based on the median protein expression level. PFS was measured as described in the method section; Kaplan-Meier analysis was performed to analyse the results.
No significant difference, in terms of PFS, was observed between IKZF1high patients and IKZF1low patients (p = 0.77, hazard ratio (HR) = 1.1); or, IKZF3high patients and IKZF3low patients (p = 0.59, HR = 1.28) ().
Figure 2. Correlation of the IKZF1/3 expression level in T-cells with PFS in untreated asymptomatic MMI patients and OS in MMIII patients treated with immunomodulatory drugs.
Kaplan-Meier plot illustrating the influence of IKZF1 (left) or IKZF3 (right) on PFS in patients with asymptomatic MMI (n = 45).
Kaplan-Meier plot illustrating the influence of IKZF1 (left) or IKZF3 (right) on overall survival in patients with symptomatic MMIII treated with immunomodulatory drugs (n = 50); the data were analysed using the log-rank (Mantel-Cox) test.
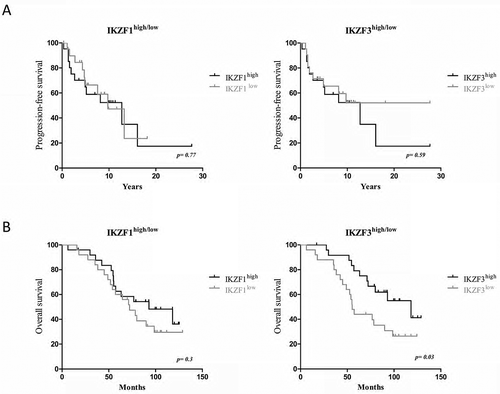
To further study the effect of IKZF1/3 expression on T-cells in patients with MMIII, we analysed IKZF1/3 expression in pretreatment samples from 50 MMIII patients. All MMIII patients were treated with immunomodulatory drugs; lenalidomide, thalidomide or pomalidomide (n = 38, 11 and 1, respectively). Kaplan-Meier analysis was performed to analyse the difference in the OS between patients’ groups. We found that IKZF3high patients demonstrate a significantly higher OS rate compared to IKZF3low patients (p = 0.03, HR = 0.44), however, a significant difference was not found between the IKZF1high and IKZF1low patients (p = 0.35, HR = 0.68). ().
Independent analyses have been performed to assess the possible interfering factors between the patients with IKZF1/3high and patients with IKZF1/3low, in terms of risk group (high or standard-risk) and age. No significant difference has been observed between both groups (Figure S4), this highlights the predictive value of IKZF3 as an independent marker.
IKZF3high MM patients exhibit features of immunodeficiency before the treatment but exhibit increased T-cell response to lenalidomide compared to IKZF3low patients
Since the IKZF3high patients were found to have superior OS compared to IKZF3low patients treated with immunomodulatory drugs, we sought to examine the influence of high IKZF3 expression on T-cells during an immune response on a cellular level. We examined the CD8+ antigen-specific T-cell response in 13 pretreatment samples from MMIII patients with different IKZF3 expression levels using our MART-1aa26-35*A27L model as described in the method section (). Interestingly, we found that patients without a specific T-cell response (negative ELISPOT, n = 9) displayed significantly higher IKZF3 expression levels compared to patients with a specific T-cell response (positive ELISPOT, n = 4, p = 0.01) ().
Figure 3. The effect of IKZF3 expression on immune functionality and response to lenalidomide.
Representative ELISPOTs from two patients with MMIII with a positive specific T-cell response against the MART-1aa26-35*A27L peptide (above) or with a negative T-cell response (below).
Percentages of IKZF3+ T-cells from patients with positive or negative ELISPOTs.
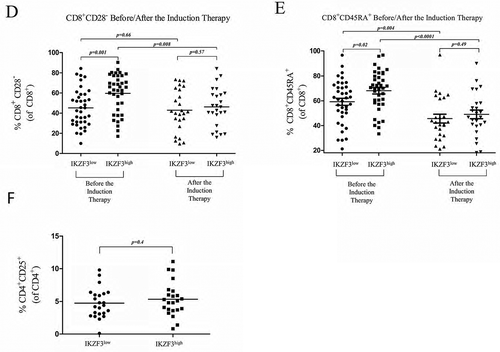
IL-2 concentrations measured by ELISA using T-cells collected from 14 MMIII patients who were previously categorized into the IKZF3high and IKZF3low subgroups; the cells were incubated with CD3/CD28 activation beads and 10 µM lenalidomide or DMSO as a control. D, E) Flow cytometry analysis of the percentage of CD8+CD28− regulatory T-cells and CD8+CD45RA+ T-cells (compared to total CD8+ T-cells) from IKZF3high or IKZF3low MMIII patients before and after lenalidomide-based induction therapy.F) Flow cytometry analysis of the percentage of CD4+CD25+ regulatory T-cells in IKZF3high or IKZF3low MMIII patients.
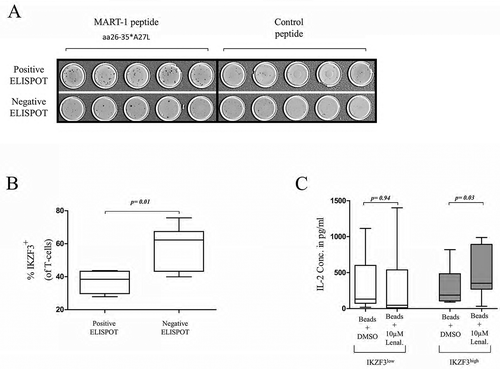
As Krönke et al. showed that the immunomodulatory effect of lenalidomide is achieved by the inhibition of the IKZF3-mediated repression of IL-2 gene expression,Citation12 we further examined how the IKZF3 expression level in T-cells affects lenalidomide-induced IL-2 excretion. CD3+ T-cells isolated from the peripheral blood (PB) of 14 MMIII patients were incubated with antiCD3/CD28 activation beads at a cell:bead ratio of 1:4 and either 10 µM lenalidomide or DMSO as a control. Seven of these 14 patients exhibited high IKZF3 expression, and seven patients exhibited low IKZF3 expression; the cells were then incubated for 24 h, and the supernatants were collected and used to measure IL-2 concentrations by ELISA. IL-2 concentrations in the IKZF3low patients of the control and lenalidomide groups were not significantly different (p = 0.94); however, IKZF3high patients exhibited significantly higher IL-2 excretion levels in the lenalidomide group than those in the control group (p = 0.03) ().
Based on the previous findings showing that lenalidomide boost the immune response by upregulating functional markers, such as CD28, and downregulating CD45RA on CD8+ T-cells, we examined the expression of these markers in newly diagnosed patients with MMIII before and after 4 cycles of lenalidomide-based induction therapy.
Pre-treatment, IKZF3high patients displayed significantly lower expression levels of CD28 surface markers on CD8+ T-cells than IKZF3low patients (p = 0.001), thus reflecting the presence of more CD8+CD28− regulatory T-cells in IKZF3high patients; however, a significant difference was not observed between the two groups after induction therapy (p = 0.57), showing that lenalidomide-based induction therapy upregulated CD28 expression in IKZF3high patients only (). By comparing CD45RA expression levels between the two groups, we found that IKZF3high patients expressed significantly higher levels of CD45RA before treatment (p = 0.02). Then, we compared CD45RA expression after the induction therapy in IKZF3high/low patients, and found that in both groups, the expression of CD45RA was downregulated; however, IKZF3high patients displayed a greater decrease in CD45RA expression compared to IKZF3low patients ().
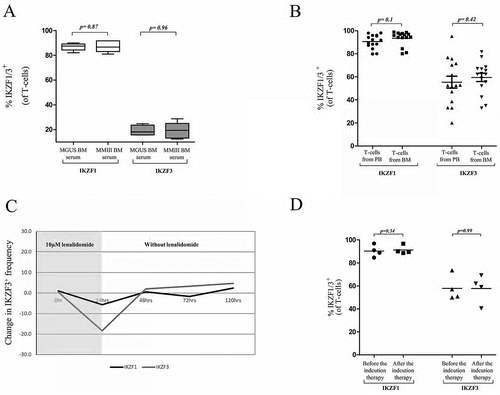
Figure 4. The modulation of IKTF1/3 expression in vivo and in vitro.
Changes in IKZF1/3+ T-cell frequency after incubation with MGUS BM serum or MMIII BM serum.
IKZF1/3 expression levels in T-cells from the peripheral blood or bone marrow of 15 MMIII patients.
The expression levels of IKZF1/3 in the DMSO-treated and lenalidomide-treated T-cells; after 24 h, the lenalidomide and DMSO were washed from the cells, and the changes were monitored every 24 h (n = 3).
IKZF1/3+ T-cell frequency in 4 patients with MMIII before and after induction therapy (4xVRD).
We then analysed the frequency of CD4+CD25+ regulatory T-cells in samples collected from 46 MMIII patients in the IKZF3high and IKZF3low groups, but a significant difference was not observed between the two groups (p = 0.4) ().
The modulation of IKZF1/3 in vivo and in vitro
We performed different experiments to examine whether there is a basal level of IKZF1/3 protein expression or whether the tumour-induced soluble factors can modulate their expression in T-cells. First, we performed an in vitro assay to test the effect of the tumour microenvironment. CD3+ T-cells isolated from the PB from 5 HD were incubated in RPMI medium containing 10% MGUS BM serum, as a BM control, or 10% newly diagnosed MMIII BM serum for 48 h. The cells were then collected, and the IKZF1/3 expression level was analysed using flow cytometry. We did not observe differences in IKZF1 or IKZF3 expression between the MGUS and MMIII BM serum (p = 0.87, p = 0.96 respectively) (). IKZF1 gene expression level has been measured using RT-PCR, but also no significant difference was observed in the expression level between the different conditions (Figure S5).
To further check the possible influence of the tumour microenvironment in modulating the level of IKZF1/3 in vivo, we examined the IKZF1/3 expression levels in T-cells collected from the BM and PB of 15 newly diagnosed MMIII patients, and then compared the expression levels of IKZF1/3 between both samples. No significant differences were observed in the expression levels of IKZF1 and IKZF3 between both conditions, the results that confirmed the findings from the in vitro experiments, which showed that the tumour microenvironment could not influence IKZF1/3 expression levels ().
We then tested whether the T-cells could restore the basal expression level of IKZF1/3 protein after the lenalidomide-induced degradation. T-cells from 3 different HD were incubated with either DMSO or 10 µM lenalidomide for 24 h, after 24 h the cells were washed and incubated in fresh medium for 72 h. Cells were tested for the IKZF1/3 expression level during the experiment every 24 h. We found that the IKZF1/3 expression level could be restored to normal baseline levels 24 h after the washing of lenalidomide ().
Then, we tested whether the baseline expression level of IKZF1/3 could also be restored after the lenalidomide-induced degradation of IKZF1/3 in vivo. We measured the IKZF1/IKZF3 expression levels in T-cells from 4 MMIII patients before and after lenalidomide-based induction therapy (4xVRD). Interestingly we did not observe differences in IKZF1/3 expression levels before and after the induction therapy (). Together with the previous in vitro results, we showed that IKZF1/3 expression can be restored after lenalidomide degradation.
Discussion
As immunomodulatory drugs are an essential part of the current therapeutic strategies for MM, predictive factors regarding the efficiency of those drugs are urgently required. Recent reports about the predictive value of IKZF1/3 expression on OS of MM patients after lenalidomide-based therapy demonstrated conflicting results.Citation15,Citation17-Citation19 While Bolomsky et al. reported that the expression of IKZF1 in T-cells was predictive for OS in MM patients; we found that only IKZF3 expression has a predictive value.
Our analysis focused on the different effects of high and low IKZF1/IKZF3 expression in T-cells on T-cell function and immunophenotype. Few data described the impact of IKZF1/IKZF3 on T-cells. Krönke et al. showed that the autocrine IL-2 secretion of T-cells is decreased by IKZF3.Citation12 This observation is in line with the finding that lenalidomide enhances IL-2-driven T-cell activationCitation20 and with our finding that CD3+ T-cells from IKZF3high patients exhibited features of immunodeficiency and significantly upregulated IL-2 secretion when treated with lenalidomide. In our study, we showed that the expression of IKZF3 in CD8+ T-cells was associated with decreased expression of CD28, a surface marker that is downregulated on CD8+ regulatory T-cells, and increased expression of CD45RA, whereas the opposite effects were described as the impact of lenalidomide on the immunophenotype of T-cells.Citation21
Most notably, our results demonstrate that high IKZF3 levels predict better OS in symptomatic MMIII patients treated with immunomodulatory drugs, whereas controversially high IKZF3 expression impairs T-cells by disrupting the IL-2 loop. A possible explanation might be that patients with high IKZF3 expression levels benefit more from the treatment with immunomodulatory drugs and that immunomodulatory drugs are able to better boost suppressed T-cell responses in patients with high IKZF3 expression levels compared to patients with low IKZF3 expression levels. Another alternative is that MM patients with T-cells that express low IKZF3 expression levels may possess a more aggressive MM clone that emerged despite the presence of highly functional T-cells.
An important topic is the basis of the variable IKZF3 expression levels in T-cells. Although we showed the downregulation of IKZF3 following incubation with immunomodulatory drugs in vitro, the IKZF3 levels in the T-cells from MM patients were not significantly affected by a lenalidomide-based induction therapy in vivo; these results were confirmed with our in vitro assay. Additionally, BM serum was not able to influence the IKZF1/3 expression level in our in vitro assay. However, we cannot rule out whether a longer incubation time between T-cell, in the context of long lasting exposure, and malignant plasma cells may induce robust changes in IKZF1/3 expression.
One can speculate that IKZF1/3 expression levels might be patient-specific. On the other hand, the decreased expression levels of IKZF3 in early MMI – compared to advanced plasma cell disease (MMIII) – might suggest that the expression of IKZF3 in T-cells is upregulated by the presence of the malignant plasma cell clone as a tumour escape mechanism. Another alternative suggestion is that patients with higher expression of IKZF3 in their T-cells are more susceptible to developing symptomatic MM. Therefore, impaired T-cell functionality increases the risk of malignant plasma cell clone progression.
Materials and methods
Patients samples and ethics statement
To analyse the expression of IKZF1/3 proteins in T-cells, Mononuclear cells (MNC) freshly isolated from the PB/BM using ficoll-hypaque density gradient centrifugation (Biochrom) were frozen in freezing medium (90% heat inactivated fetal calf serum (FCS) from PAA Laboratories, Germany, and 10% Dimethyl sulfoxide (DMSO) from SERVA Electrophoresis GmbH) in cryogenic vials. Vials were first placed in isopropanol-filled chamber at −80°C overnight, then transported into a liquid nitrogen storage tank. In order to thaw the samples, vials were removed from the liquid nitrogen tank and placed immediately into 37°C water bath for less than one minute, then transferred gently into prewarmed complete medium (20% FCS + 80% RPMI 1640 cell culture medium from PAA Laboratories) and centrifuged at 400x g for 5 min. Cells were then counted and resuspended in appropriate volume of phosphate-buffered saline (PBS) (from Sigma-Aldrich) for further analyses. Buffy coats from HD (Institute for Immunology/IKTZ, University of Heidelberg, Germany) were used as control samples. In accordance with the Declaration of Helsinki, all human studies were performed after obtaining written informed consent, and based on institutional guidelines, all human studies were approved by the Ethics Committee of the Medical Faculty at the University of Heidelberg. Data safety management was performed according to the data protection regulations of the University Hospital Heidelberg.
Patients
A total of 298 patients have been enrolled in this study. Of them, 212 were newly diagnosed with MMIII, while 45 were diagnosed with MMI (according to Durie-Salmon staging system) and 41 patients were diagnosed with MGUS (). 38 HD have been used as a control.
Table 1. Clinical characteristics of the patients enrolled in the study.
All MMIII patients received treatment including thalidomide, lenalidomide or pomalidomide for the Induction therapy. For the OS trial, 50 MMIII have been included. The inclusion of those patients was based on the availability of frozen MNC isolated before the beginning of any treatment, the administration of thalidomide, lenalidomide or pomalidomide for the treatment and the availability of clinical follow-up data. For the PFS trial, 42 MMI have been included. The inclusion of those patients was based on the availability of frozen MNC isolated before the beginning of any treatment and the availability of clinical follow-up data until the first progression to MMIII.
The rest of the patients have been included in the NCT02495922 trial. Details about inclusion and exclusion criteria can be found elsewhere.Citation22
Molecular cytogenetic testing
Molecular cytogenetic testing was performed using a previously described method.Citation23 Briefly, CD138+ BM plasma cells (PCs) were purified using auto-magnetic-activated cell sorting with anti-CD138 immunobeads as published.Citation24 For interphase fluorescence in situ hybridization (iFISH) analyses, a panel of two-color probe sets was used to detect numerical changes at the chromosomal loci 1q21/13q14, 5p15/5q35, 8p21/19q13, 9q34/15q22, and 11q22.3/17p13 as well as the IgH-translocations t(11;14)(q13;q32), t(4;14)(p16;q32), t(14;16)(q32;q23), or any other IgH-rearrangement. Hybridization was performed according to the manufacturer’s instructions (Cytocell) and a minimum of 100 interphase nuclei per probe were evaluated using an automated spot counting system (Applied Spectral Imaging). Hybridization efficiency was validated using interphase nuclei obtained from the BM of a HD, and the thresholds for gains, deletions, and translocations were set at 10%. High-risk cytogenetic definition was considered as per Krönke et al. – the presence of deletion 17p and/or t(4;14) or t(14;16).Citation15
Assay method
The measurement of IKZF1/3 protein level in T-cells has been done using flow cytometry analyses. Briefly, 1 million MNC were resuspended in 100 µl PBS and incubated with fluorochrome-labelled antibodies against CD3, CD8, CD25, CD28, CD45, and CD45RA (all from BD Bioscience). The intranuclear staining of IKZF1/3 was performed using transcription factor buffer set (BD Bioscience) according to manufacturer’s protocol, and fluorochrome-labelled antibodies against IKZF1/3 (BD Bioscience). Flow cytometry measurements were performed using a BD FACSCanto flow cytometer and analysed with BD FACSDiva software.
Western blotting
Western blotting has been performed to test the expression of IKZF1/IKZF3 in T cells. 30 µg of protein lysate were run on 10% standard polyacrylamide sodium dodecyl sulfate-polyacrylamide (SDS) gels. Proteins were then transferred from the gels to PVDF membranes (Invitrogen) by semi-dry electroblotting. The blot was blocked with tris-buffered saline (TBS) supplemented with 0.05% Tween 20 (9127, Carl Roth) and 5% Bovine Serum Albumin (BSA, Sigma-Aldrich). After blocking, the membranes were incubated with anti-IKZF1, anti-IKZF3 or anti-Actin (ab26083, ab139408, ab8227, Abcam) antibodies at 4°C overnight. The membranes were then incubated with secondary Goat Anti-Rabbit IgG H&L (ab205718, Abcam) for 2 h at room temperature, protein bands were visualized by Pierce ECL Western Blotting Substrate kit (32106, ThermoFischer) according to the manufacturer’s instructions, followed by semi-automatic development of photo by Amersham Imager 600.
Assay quality control
The percentage of T-cells expressing the target (IKZF1/3) has been used as a quantification method. Gating was assigned using control antibodies to subtract unspecific binding.
To test the sensitivity and accuracy of the method, we performed pomalidomide based experiments. As pomalidomide causes ubiquitination and degradation of IKZF1, we incubated T-cells with different concentrations of pomalidomide (0.1µM, 0.5µM, 1µM and 5µM) for 16 h, then performed flow cytometry analyses to measure the percentage of IKZF1+ T-cells. The percentage of T-cells expressing IKZF1 was inversely proportional to the concentration of pomalidomide (Figure S1).
We also performed Western Blotting assay to verify the IKZF1 and IKZF3 expression in T-cells of healthy donors with and without Pomalidomide. 10 million Peripheral blood mononuclear cells (PBMCs) were incubated with 1µM or 5µM Lenalidomide or DMSO as a control for 16 h. Then protein lysate has been used for western blotting detection of IKZF1/IKZF3 level in T cells (Figure S2).
In order to test the reproducibility and the effect of freezing-thawing cycle, levels of IKZF1/3 were measured freshly and after one full cycle of freezing-thawing in ten samples of MNC isolated from the peripheral blood of HD. The measurements were coefficient correlated with no observed effect of the freezing on the percentage of positive cells (Figure S3).
Study design
42 MMI patients have been enrolled in the PFS study and 50 MMIII patients have been enrolled in the OS study. Both of the studies have been conducted retrospectively. As patients have been classified into IKZF1/3 high or low groups, stratification analyses have been performed to analyse the difference between the two groups in terms of cytogenetic risk group (high or standard risk) and age (Figure S4). The samples assays have been stained and measured blinded to all clinical data including therapy, progression and survival using an internal number for each sample and correlated with the clinical data.
Peptides
The melanoma antigen recognized by T-cells 1 (MART-1)aa26-35*A27L peptide (ELAGIGILTV) and the human leukocyte antigen (HLA)-A2-restricted irrelevant control peptide (LLIIVILGV; a control for unspecific T-cell activation) were synthesized by the peptide-synthesis-facility of the German Cancer Research-Center Heidelberg using standard procedures.
Isolation of t-cells
The isolation of CD3+ T-cells from MNCs was achieved by immunomagnetic cell sorting (MACS System, Miltenyi Biotec) according to the manufacturer’s protocol.
Expansion of MART-1aa26-35*a27l-specific T-cells and IFN-γ ELISPOT assay
PBMCs from HLA-A*02+ HD were used to generate MART-1aa26-35*A27L-specific T-cells. T-cells specific for this antigen show cross-reactivity for HM1.24, an antigen highly-expressed on MM cells, and are able to lyse autologous MM cells.Citation25 Immature DCs were obtained by culturing plastic-adherent PBMCs for 5 days in RPMI 1640 medium containing GM-CSF (800 U/ml, Sargramostim, Bayer), IL-4 (500 U/ml, R&D Systems) and 5% heat-inactivated human serum. The maturation of immature DCs was then induced by supplementing TNF-α (10 ng/ml, R&D Systems), IL-6 (1000 U/ml, PromoCell) and prostaglandin E2 (1 µg/ml, Biomol/Enzo Lifesciences) for 2 days in the presence of the MART-1aa26-35*A27L peptide (10 µg/ml) to load the DCs. Afterwards, autologous PBMCs were incubated for 7 days together with mature DCs loaded with MART-1aa26-35*A27L peptide in T-cell medium to expand the MART-1aa26-35*A27L-specific T-cells.
Ifn-γ ELISPOT assay
CD8+ cells were purified from the MART-1aa26-35*A27L-activated T-cell population by positive immunomagnetic cell sorting (MACS-system, Miltenyi Biotec). Purified CD8+ cells were then incubated with the MART-1aa26-35*A27L peptide or irrelevant peptide-pulsed T2 cells (loaded by a 2-h incubation in serum-free RPMI 1640 media containing 10 µg/ml peptide) for 24 h in anti-IFN-γ antibody- (Mabtech) coated nitrocellulose-plates (Millipore) in an effector cell to target cell (E:T) ratio of 1:5. Subsequently, plate-bound IFN-γ was detected as previously described.Citation26
Non-specific activation of T-cells and IL-2 enzyme-linked immunosorbent assay (ELISA)
To analyse the non-specific activation of T-cells, CD3+ cells were isolated from MNCs and were activated with anti-CD3/CD28 microbeads (Dynabeads, Invitrogen Dynal) for 24 h at a cell:bead ratio of 1:4. Afterwards, the concentration of IL-2 in the supernatants of the T-cell activation cultures was determined with an IL-2 ELISA kit (Mabtech) according to the manufacturer’s instructions.
RT-PCR
Gene expression analyses were measured by TaqMan real-time reverse transcription polymerase chain reaction (RT-PCR) assays. Total RNA was extracted from CD3+ T-cells using RNeasy Kit (Qiagen, Germany). For cDNA reverse transcription and PCR amplification reaction TaqMan RNA-to-CT™ 1-Step Kit (Thermo Fisher scientific, Germany) has been used according to the manufacturer’s protocol. The following primer-probe sets from Life Technologies were used: IKZF1 (Hs00958474_m1), IKZF3 (Hs00232635_m1), 18S (Hs03003631_g1). Analysis was performed on a StepOnePlus™ RT-PCR System (Applied Biosystems) in a 96-well plate. Relative expression levels were calculated using the ΔΔCT method.
Statistical and survival analyses
Differences in the number of spots per well in the IFN-γ ELISPOT assay experiments between T2 cells loaded with MART-1aa26-35*A27L and T2 cells loaded with an irrelevant peptide were calculated by Student’s t-test using Statistica software for Windows (StatSoft, Tulsa OK, USA). ELISPOT assays were defined as positive if MART-1aa26-35*A27L peptide activation achieved at least 10 spots more than the control peptide and if the difference was significant (p < 0.05). Unless otherwise specified, the comparisons between different patient groups were measured with t-tests using the GraphPad (version 5.01) software, and the difference was considered statistically significant at (p < 0.05). Kaplan-Meier plots were generated using GraphPad software, and regardless of cause, OS was considered as the time from the first diagnosis of MMIII until death; patients alive at the last follow-up were censored. PFS was measured from the first diagnosis of asymptomatic MMI until the first observed progression to symptomatic MM; patients who did not progress until the last follow-up were censored. The log-rank (Mantel-Cox) test was used to compare the differences between different groups in the OS and PFS analyses, and P-value p < 0.05 was considered to be statistically significant.
Conflict of interest statement
Mohamed H.S. Awwad: travel support Bristol-Myers Squibb and Celgene.
Katharina Kriegsmann: research funding by BMS and Celgene.
Julian Plaumann: no conflicts.
Michael Benn: no conflicts.
Jens Hillengass: honoraria from Amgen, BMS, Celgene,
Marc Raab: research funding from Novartis, Amgen, Morphosys.
Uta Bertsch: no conflicts.
Markus Munder: Consultancy: Janssen-Cilag, BMS, Takeda, Amgen, Celgene.
Katja Weisel: advisory board und honoraria: AMGEN, BMS, Celgene, Janssen, Novartis, Takeda, Onyx; research funding: Janssen, Celgene.
Hans Jürgen Salwender: honoraria and travel support: Janssen Cilag, Celgene, BMS.
Mathias Hänel: honoraria Roche, Novartis.
Roland Fenk: Honoraria and travel grants: Celgene, Janssen, BMS; research funding: Celgene.
Jan Dürig: advisory Board Bristol-Myers Squibb, Celgene, Janssen; speakers bureau Celgene, Janssen; research funding Celgene.
Carsten Müller-Tidow: no conflicts.
Hartmut Goldschmidt: Research Support Celgene, Janssen, Chugai, Novartis and BMS; Advisory Boards Janssen, Celgene, Novartis, Amgen, Takeda, BMS; Honoraria: Celgene, Janssen, Novartis, Chugai, BMS.
Michael Hundemer: Research Support BMS, Celgene, Sanofi, Morphosys.
Supplemental Material
Download MS Word (363.4 KB)Supplemental Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kastritis E, Dimopoulos MA. 2007. Thalidomide in the treatment of multiple myeloma. Best Practice & Research. Clinical Haematology. 20(4):681–699. doi:10.1016/j.beha.2007.09.001.
- Dimopoulos M, Spencer A, Attal M, et al. 2007. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357(21):2123–2132. doi:10.1056/NEJMoa070594.
- Dumortier A, Jeannet R, Kirstetter P, et al. 2006. Notch activation is an early and critical event during T-Cell leukemogenesis in Ikaros-deficient mice. Mol Cell Biol 26(1):209–220. doi:10.1128/MCB.26.1.209-220.2006.
- Le Geimer Lay A-S, Oravecz A, Mastio J, et al. 2014. The tumor suppressor Ikaros shapes the repertoire of notch target genes in T cells. Sci Signal 7(317):ra28. doi:10.1126/scisignal.2004545.
- Song C, Pan X, Ge Z, et al. 2016. Epigenetic regulation of gene expression by Ikaros, HDAC1 and Casein Kinase II in leukemia. Leukemia 30(6):1436–1440. doi:10.1038/leu.2015.331.
- Caballero R, Setien F, Lopez-Serra L, et al. 2007. Combinatorial effects of splice variants modulate function of Aiolos. J Cell Sci 120(Pt 15):2619–2630. doi:10.1242/jcs.007344.
- Uckun FM, Ma H, Zhang J, et al. 2012. Serine phosphorylation by SYK is critical for nuclear localization and transcription factor function of Ikaros. Proc Natl Acad Sci U S A 109(44):18072–18077. doi:10.1073/pnas.1209828109.
- Hung K-H, Su S-T, Chen C-Y, et al. 2016. Aiolos collaborates with Blimp-1 to regulate the survival of multiple myeloma cells. Cell Death Differ 23(7):1175–1184. doi:10.1038/cdd.2015.167.
- Kelley CM, Ikeda T, Koipally J, et al. 1998. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol CB 8(9):508–515. doi:10.1016/S0960-9822(98)70202-7.
- Ma H, Qazi S, Ozer Z, Zhang J, Ishkhanian R, Uckun FM. 2013. Regulatory phosphorylation of Ikaros by Bruton’s tyrosine kinase. PloS One 8(8):e71302. doi:10.1371/journal.pone.0071302.
- Gómez-Del Arco P, Maki K, Georgopoulos K. 2004. Phosphorylation controls Ikaros’s ability to negatively regulate the G(1)-S transition. Mol Cell Biol 24(7):2797–2807. doi:10.1128/MCB.24.7.2797-2807.2004.
- Kronke J, Udeshi ND, Narla A, et al. 2014. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science (New York, N.Y.). 343(6168):301–305. doi:10.1126/science.1244851.
- Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. 2007. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood 109(7):2878–2886.
- Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. 2007. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol (Baltimore, Md.: 1950) 179(11):7305–7315. doi:10.4049/jimmunol.179.11.7305.
- Kronke J, Kuchenbauer F, Kull M, et al. 2017. IKZF1 expression is a prognostic marker in newly diagnosed standard-risk multiple myeloma treated with lenalidomide and intensive chemotherapy: a study of the German Myeloma Study Group (DSMM). Leukemia doi:10.1038/leu.2016.384.
- Sehgal K, Das R, Zhang L, et al. 2015. Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: impact of immune activation and cereblon targets. Blood 125(26):4042–4051. doi:10.1182/blood-2014-11-611426.
- Zhu YX, Braggio E, Shi C-X, et al. 2014. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 124(4):536–545. doi:10.1182/blood-2014-02-557819.
- Pourabdollah M, Bahmanyar M, Atenafu EG, Reece D, Hou J, Chang H. 2016. High IKZF1/3 protein expression is a favorable prognostic factor for survival of relapsed/refractory multiple myeloma patients treated with lenalidomide. J Hematol Oncol 9(1):123. doi:10.1186/s13045-016-0354-2.
- Bolomsky A, Hubl W, Spada S, et al. 2017. IKAROS expression in distinct bone marrow cell populations as a candidate biomarker for outcome with lenalidomide-dexamethasone therapy in multiple myeloma. Am J Hematol 92(3):269–278. doi:10.1002/ajh.24634.
- Gorgun G, Calabrese E, Soydan E, et al. 2010. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 116(17):3227–3237. doi:10.1182/blood-2010-04-279893.
- Neuber B, Herth I, Tolliver C, et al. 2011. Lenalidomide enhances antigen-specific activity and decreases CD45RA expression of T cells from patients with multiple myeloma. J Immunol (Baltimore, Md.: 1950) 187(2):1047–1056. doi:10.4049/jimmunol.1002460.
- Goldschmidt H A Phase III Trial on the Effect of Elotuzumab in \VRD Induction/Consolidation and Lenalidomide Maintenance in Patients With Newly Diagnosed Myeloma (GMMG-HD6): identifier: NCT02495922. https://clinicaltrials.gov/ct2/show/NCT02495922. Updated October 26, 2017. Accessed January 31, 2018.
- Merz M, Hielscher T, Seckinger A, et al. 2016. Baseline characteristics, chromosomal alterations, and treatment affecting prognosis of deletion 17p in newly diagnosed myeloma. Am J Hematol 91(11): E473-E477. doi:10.1002/ajh.24533.
- Granzow M, Hegenbart U, Hinderhofer K, et al. 2017. Novel recurrent chromosomal aberrations detected in clonal plasma cells of light chain amyloidosis patients show potential adverse prognostic effect: first results from a genome-wide copy number array analysis. Haematologica 102(7):1281–1290. doi:10.3324/haematol.2016.160721.
- Christensen O, Lupu A, Schmidt S, et al. 2009. Melan-A/MART1 analog peptide triggers anti-myeloma T-cells through crossreactivity with HM1.24. J Immunotherapy (Hagerstown, Md.: 1997) 32(6):613–621. doi:10.1097/CJI.0b013e3181a95198.
- Hundemer M, Schmidt S, Condomines M, et al. 2006. Identification of a new HLA-A2-restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma. Exp Hematol 34(4):486–496. doi:10.1016/j.exphem.2006.01.008.