ABSTRACT
Objective: Nivolumab has been used for treating non-small cell lung cancer (NSCLC) worldwide. Whether neutrophil-lymphocyte ratio (NLR) can predict the prognosis of NSCLC treated with Nivolumab is still under debate. This meta-analysis was to assess the significance of NLR as a predictive factor in NSCLC patients receiving Nivolumab.
Methods: Databases including PubMed, Embase, and the Cochrane library were searched to identify eligible studies evaluating the role of NLR in predicting prognosis of NSCLC treated with Nivolumab until March 2018 without language restrictions. The meta-analysis was performed using hazard ratio (HR) of progression free survival (PFS) and overall survival (OS) in NSCLC patients with various NLR.
Results: A total of 14 retrospective studies consisting of 1225 NSCLC patients were included. The combined results showed that relatively higher baseline NLR was associated with poor PFS (HR = 1.44; 95% confidence interval (CI):1.18–1.77; p < 0.05) and OS (HR = 1.75; 95% CI: 1.33–2.30; p < 0.05) after treatment of Nivolumab. Subgroup analysis suggested that NLR ≥ 5 was more reliable for PFS (HR = 1.73; 95%CI: 1.14, 2.62; p < 0.05) and OS (HR = 1.76; 95%CI: 1.47, 2.10; p < 0.05). In addition, post-treatment NLR also had predictive roles for PFS (HR = 3.17; 95%CI: 1.48, 6.82; p < 0.05) and OS (HR = 2.26; 95%CI: 1.05, 4.86; p < 0.05).
Conclusion: Our findings suggest that NLR can be used as a prognostic biomarker for NSCLC treating with Nivolumab, and the recommended cutoff value of NLR is 5.
Introduction
Non-small cell lung cancer (NSCLC) remains one of the most lethal malignant diseases worldwide.Citation1 The traditional therapy is mainly consisted of chemotherapy, radiotherapy, surgical resection and best supportive care.Citation1 In recent years, the therapeutic strategies for non-small cell lung cancer (NSCLC) have been enriched with small molecular targeted therapy and immunotherapy.Citation2 For targeted therapy, several positive and practical indicators of their efficacy have been identified by accumulating clinical and laboratory evidence, such as epidermal growth factor receptor (EGFR) mutation.Citation3 After failure of previous treatments, immunotherapy still shows favorable clinical benefits.Citation3 Moreover, there are evidences supporting first-line and/or second-line application of this novel treatment for NSCLC.Citation4 However, not of all the treated patients gain this advantage.Citation4 How to predict the efficacy of immunotherapy in a feasible and reliable way is important during the process of selecting regimen from multiple options for oncologists and patients.
Immune checkpoint inhibitors such as Nivolumab have gained much attention in clinical practice because of their superiority in controlling cancer.Citation5,Citation6 A series of factors like PD-L1 expression, tumor infiltrating lymphocytes (TILs), and tumor mutational burden(TMB) have been tested whether they are suitable to serve as predictor of immunotherapy effectiveness.Citation7–Citation9 PD-L1 expression is associated with survival with Nivolumab in NSCLC patients, especially non-squamous histology subset. However, responses to Nivolumab are also observed in patients without discernible expression of PD-L1, challenging the reliance of this factor. TILs is also reported to be associated with survival during immunotherapy. But the detection of this biomarker is not so convenient and cheap. Although TMB is also a remarkable biomarker for immunotherapy, it exhibits similar shortcomings as PD-L1 and TILs. Therefore, the searching for feasible, cheap and optimal predictive marker is still under exploring.
Recently, several studiesCitation10-Citation12 have reported that inflammatory status is important for survival in NSCLC patients, and the most promising one is neutrophil-lymphocyte ratio (NLR). NLR is a marker of systemic inflammation and suggested to be associated with clinical benefits in various cancer patients. Inflammation plays important roles in tumorigenesis, and it facilitates the progression of tumors by modulating microenvironment. Blood-based parameters, such as NLR, have been reported to predict outcomes in cancer patients. A number of meta-analysesCitation13–Citation15 have suggested that NLR is a good predictive biomarker in predicting efficacy of chemotherapy and molecular targeted therapy in various cancer types. Since 2017, retrospective studiesCitation5,Citation16–Citation19 evaluating the role of NLR in predicting outcomes of NSCLC patients receiving immune checkpoint inhibitors have been released. These studies showed that pre-treatment NLR might correlate with progression free survival (PFS) and overall survival (OS) in NSCLC patients receiving Nivolumab. More recently, two meta-analysesCitation20,Citation21 have addressed the question of pre-treatment NLR being the predictive biomarker in cancer patients treated with immunotherapy. However, these meta-analyses focused on various cancers with very limited data on NSCLC. Another question is about the optimal cutoff value for NLR to serve as a prognostic biomarker. Various cutoff values of NLR were reported in different studies. Most of them reported that NLR< 5 was associated with better survival in cancer patients treated with Nivolumab, and more powerful evidence is needed to prove it. In addition, whether post-treatment NLR has the same role as that of the pre-treatment NLR, it is also not well determined.
Therefore, we systematically searched databases to identify retrospective studies on the topic of relationships between NLR and outcomes of NSCLC patients treated with Nivolumab, extracted data of OS, PFS and ORR, and analyzed them by RevMan 5.3. The combined results supported the application of NLR to predict outcomes of NSCLC regardless of testing time of NLR.
Methods
Search strategy
The electronic databases including Pubmed, Emabse, and the Cochrane library were systematically searched to identify studies evaluating relationship between NLR and clinical outcomes in NSCLC patients receiving Nivolumab until March 2018. There was no limitation of language and study type. The keywords used during the search were as following: immunotherapy, immune checkpoint inhibitor, immune checkpoint blockade, Nivolumab, PD-1 inhibitor, PD-L1 inhibitor, lung cancer, non-small cell lung cancer, NSCLC, NLR, and neutrophil-lymphocyte ratio. The abstracts of meetings were included if they provided sufficient information of primary and secondary endpoints. For related data that was not available in the original full-text, the corresponding author was contacted for more detailed information.
Study selection
The NLR was defined as absolute neutrophil count (ANC) divided by absolute lymphocyte count (ALC). Clinical trials about assessing the predictive role of NLR in immunotherapy of NSCLC were included and analyzed. Inclusion criteria were as follows: (1) clinical trials of immune checkpoint inhibitor Nivolumab in treating NSCLC and the relationship between NLR and outcomes; (2) reported HRs in terms of OS, PFS, and ORR. Or the interested HRs could be calculated through survival curves; (3) no language limitation. Reviews, animal studies and comments were excluded. Two reviewers conducted the literature search process, independently.
Data extraction
The definitions of OS, PFS, ORR, DCR and NLR are the same as previously reported.Citation1,Citation22 Cutoff values of NLR were reported and extracted from each included study. The primary endpoints were HRs of NLR< cutoff value (low NLR) versus NLR≥ cutoff value (high NLR) for PFS, and the secondary endpoints were HRs of low NLR versus high NLR for OS, ORR, and DCR. The baseline characteristics such as title, author, publication year, age, number of participants, number of males and females, treatment, and pathological types were extracted. Two reviewers performed data extraction, independently. When there were inconsistencies about extracted data, a third reviewer was invited to solve it.
Quality assessment
To assess the overall quality of included studies, we used the NOS (Newcastle Ottawa Scale) that was recommended by the Cochrane Non-Randomized Studies Methods Working Group.Citation23 According to the protocol of NOS, three main perspectives including selection, comparability, and outcome were evaluated. There are four, one and three criteria items in concerns of selection, comparability and outcome, independently. The definition of a high quality study was at least six NOS criteria stars were achieved with low risk of selection, performance and reporting bias. The process of quality assessment was performed by Dedong Cao and Huilin Xu, independently. If there was a disagreement about the result of quality evaluation, a third reviewer (Ximing Xu) was involved to solve the concern.
Statistical analysis
Meta-analysis was performed using the Cochrane software RevMan 5.3. Briefly, the pooled analysis was conducted by using the original data obtained from the eligible studies. In order to assess the role of NLR in predicting progression free survival (PFS) and overall survival (OS) of non-small cell lung cancer patients receiving Nivolumab, hazard ratios (HRs) for PFS, OS, and overall response rate (ORR) were used. If there was only survival curve without direct information about HR, the method provided by the referred protocolCitation24 was applied to calculate an indirect HR of interest. The subgroup analyses were also performed in terms of study design, disease stage, cutoff value, NLR testing time, and region. The chi-squared (χ2, or Chi2) test and I2 value were used to assess the heterogeneity of the included studies, and different I2 indicated variable degree of heterogeneity according to the Cochrane Handbook 5.1.Citation25 According to the cited reference, a random effects model was used if the P value for heterogeneity was less than 0.1, and/or the I2 value was larger than 50%. Otherwise, the fixed effects model was used.Citation25 Egger’s test and Begg’s test were used to assess publication bias. For all combined analysis, a P that less than 0.05 is considered as there is a statistical significance.
Results
Search results
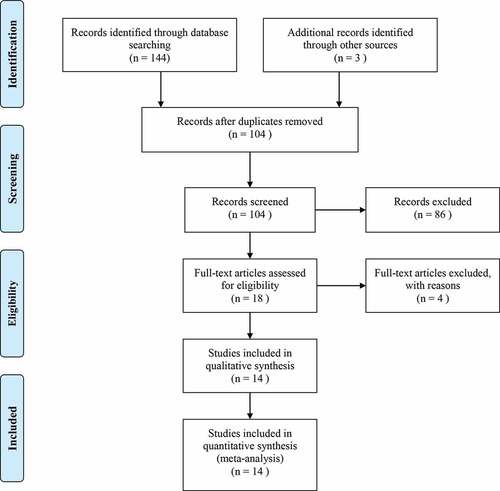
After systematically initial search, a total of 144 studies were collected. After removing duplication and reviewing titles and abstracts, 126 of them were discarded and the left 18 studies were included for more detailed selection. Finally, 130 of the initial studies were excluded and the left 14 retrospective studiesCitation16–Citation19,Citation26–Citation35 were considered as eligible for the combined analysis after reviewing the full text. We also planned to assess the role of NLR in predicting anti-PD-1/PD-L1 agents, including Pembrolizumab, Atezolizumab, and Durvalumab. However, there were not enough clinical studies on these agents.
The detailed information about study selection process is presented in .
There were 1225 NSCLC patients within these 14 studies published from 2017 to 2018. The median age of this population ranged from 47 to 71 years. The main immunotherapy agent was Nivolumab that used in all studies. The reported primary endpoints were PFS, OS and ORR. The cutoff value of NLR ranged from 2.8 to 6.5, and the value of 5 was applied in seven studies. The baseline characteristics of included studies are listed in .
Table 1. Baseline characteristics of included studies
Overall quality of included studies
As they were retrospective studies, we used NOS method to evaluate the quality of included studies. As illustrated by , five studies were considered as low quality because of less than 6 criteria star. The main disadvantages lowering the overall quality were selection and reporting outcomes. Six of them were meeting abstract and it was difficult to determine the quality of these studies just by limited information.
Table 2. Quality assessment of included studies by NOS
Combined results
PFS and OS
We extracted data of PFS and OS from individual studiesCitation16–Citation19,Citation26–Citation35. As shown in Supplemental Table 1, the median OS ranged from 6.5 to 17 months, while the median PFS was between 2.1 and 5.5 months. The median PFS of patients with low NLR ranged from 1.7 to 8.6 months, while it was 1.8 to 2.5 months in patients with high NLR. The median OS of low NLR were from 3.4 to 13.2, and it was 2.8 to 5.5 in high NLR (Supplemental Table 2).
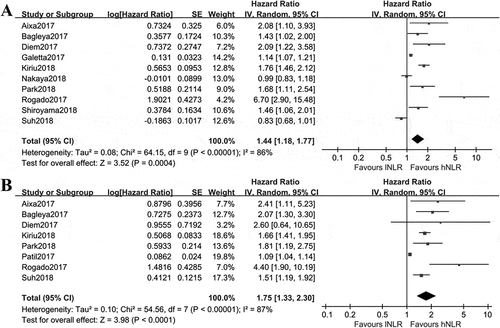
By combining HRs of high NLR versus low NLR with regard to PFS from 10 studies,Citation16–Citation19,Citation26,Citation28,Citation32,Citation33,Citation35,Citation36 the pooled result using random effect model showed that the estimated effect was 1.44(95%CI: 1.18, 1.77), indicating NSCLC patients with low pre-treatment NLR had a 1.44 times of getting better PFS(). As shown in , the pooled HRs of OS from eight studiesCitation16,Citation18,Citation19,Citation28,Citation30,Citation33,Citation35,Citation36 using random effect model was 1.75(95%CI: 1.33, 2.30), favoring NSCLC patients with low pre-treatment NLR.
ORR and DCR
Seven studiesCitation17–Citation19,Citation26,Citation29,Citation30,Citation33 reported number of patients achieved ORR and DCR after treatment of Nivolumab (Supplemental Table 3). In a total of 716 patients, 141 were considered as ORR and 236 as DCR, with percentages of 19.69% and 32.96%, respectively.
Subgroup analysis
The subgroup analysis was introduced to assess the impact of different cutoff value, disease stage, testing time of NLR and region. The overall results are listed in .
Table 3. Results of subgroup analysis in terms of stage, cutoff value, timing, and region
Disease stage
Most of the studies included patients with advanced NSCLC, and four studiesCitation27,Citation29,Citation30,Citation37 did not mention the disease stage. With extractable data, only one studyCitation30 with unclear disease stage was included when assessing NLR for OS, and all were advanced stage in assessment of NLR for PFS. The results showed that low NLR favored better OS (Supplemental Figure 1A) and PFS () regardless of disease stage.
Cutoff value of NLR
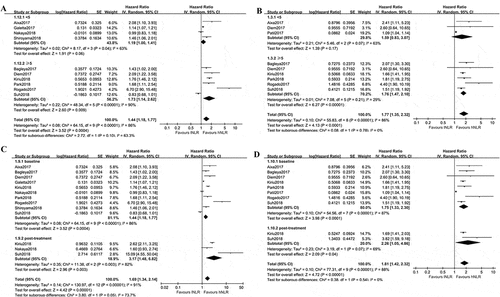
As there were several studiesCitation16,Citation18,Citation19,Citation28,Citation33,Citation35 set the cutoff value of pre-treatment NLR being 5, we used the value of 5 to find out the influence of different cutoff value on PFS and OS. The studiesCitation16-Citation19,Citation26,Citation28,Citation32,Citation33,Citation35,Citation36 included reported the HRs of high NLR versus low NLR on PFS in the treatment of advanced NSCLC. As shown in , the estimated effect of NLR < 5 was 1.19 (95%CI: 1.00, 1.41; p = 0.06), and it was 1.73(95%CI: 1.14, 2.62; p = 0.009) for NLR ≥ 5. A similar result was found in term of OS. The combined effect was 1.59(95%CI: 0.83, 3.07; p > 0.05) for NLR < 5 and 1.76(95%CI: 1.47, 2.10; p < 0.00001) for NLR ≥ 5 (). These findings suggested cutoff value of NLR ≥ 5 may be more reliable in predicting PFS and OS in NSCLC treated with Nivolumab.
Figure 3. Subgroup analyses of PFS and OS in Nivolumab treated NSCLC patients with different NLR. A, low baseline NLR versus high baseline NLR for PFS combined from different cutoff value; B, low baseline NLR versus high baseline NLR for OS combined from different cutoff value; C, low NLR versus high NLR for PFS, subsets were baseline NLR and post-treatment NLR; D, low NLR versus high NLR for OS, subsets were baseline NLR and post-treatment NLR. Baseline NLR was defined as NLR before Nivolumab treatment, post-treatment NLR was defined as NLR after Nivolumab treatment
Testing time of NLR
Next, we evaluated whether post-treatment NLR still hold the promise of predicting prognosis during immunotherapy in NSCLC. As shown in , the pooled effect of post-treatment NLR for predicting PFS was 3.17(95%CI: 1.48, 6.82; p = 0.003), with a statistically significance. Similarly, the estimated effect of post-treatment NLR for OS was 2.26(95%CI: 1.05, 4.86; p = 0.04, ).
Region of study
This subgroup analysis determined that patients with low baseline NLR could have beneficial PFS (Supplemental Figure 1B) and OS (Supplemental Figure 1C) from immunotherapy without limitation of regions.
Publication bias assessment and sensitivity analysis
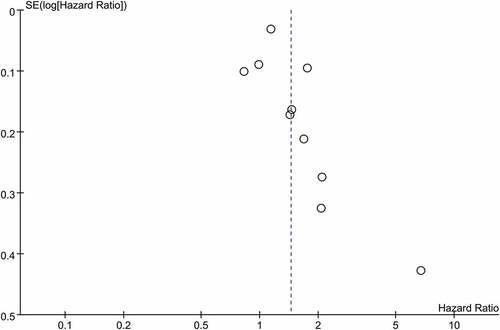
To assess the publication bias, we introduced funnel plot, Begg’s test and Egger’s test. As shown by , the funnel plot using data of PFS was not in a form of symmetry, indicating potential high risk of publication bias might exist. Then this was confirmed by the results of Egger’s (P = 0.031 < 0.05) and Begg’s test (P = 0.033 < 0.05), suggesting there might be unpublished or gray literature which should be included in the current analysis. The sensitivity analysis was conducted using data of PFS. As shown in Supplemental Figure 2, the study of Galetta was considered as the main source of heterogeneity for PFS analysis. The combined result of PFS was not significantly affected by excluding this study.
Discussion
Immunotherapy has been one of the most promising anti-cancer strategies as demonstrated by accumulating beneficial evidences.Citation38 Meanwhile, Nivolumab and Pembrolizumab are the recently approved immune checkpoint blockades for treating advanced non-small cell lung cancer after failure of traditional chemotherapy or not.Citation3 Several clinical studiesCitation17,Citation18,Citation20,Citation39 assessed relationship between NLR and immunotherapy, and some of themCitation17,Citation18,Citation20 argued that NLR could serve as a predictive factor while other found negative results.Citation39 Could NLR be a candidate for predicating prognosis of Nivolumab in NSCLC? How to feasibly and reliably determine NSCLC population who can gain a better benefit from Nivolumab? These are still in debates.
In this study, we tried to address this concern by combing NLR related data of individual studies through meta-analysis. The findings revealed that NLR before immune checkpoint inhibitor treatment might be a feasible prognostic biomarker for NSCLC. Patients with high baseline NLR was found to be associated with poor OS and PFS, whereas low baseline NLR was proved to be related with good OS and PFS. In subgroup analysis, the impact of disease stage, NLR cutoff value, NLR testing time and region on prognostic role of NLR were evaluated. The synthesized results showed that NLR remained to be effective regardless of different stage, testing time of NLR, and region, but not cutoff value. Pre-treatment NLR cutoff value ≥ 5 hold the potential to indicate worse PFS or OS. With data from 716 NSCLC, the combined ORR of Nivolumab was 19.69%, and the DCR was 32.96%.
Recently, several meta-analysesCitation13,Citation14 with regard to the prognostic role of NLR in cancer targeted therapy or chemotherapy were published. These studies have shown the value of NLR as a biomarker in predicting outcomes of patients with advanced lung cancer. The study of Yu et al. collected data from 18 studies involving 7219 lung cancer patients who received traditional therapies, and the results suggested that a NLR cut-off value ≥ 4 significantly predicted poor OS (HR = 1.56) and PFS (HR = 1.54), while it was suggested to be 5 in our study. More recently, two released meta-analysesCitation20,Citation21 assessed prognostic utility of baseline neutrophil-tolymphocyte ratio in cancer patients receiving immune checkpoint inhibitors. Jiang et alCitation21 included 24 retrospective studies with 4647 cancer patients who treated with anti-VEGF/VEGFR therapy or immunotherapy. Their results found that high pretreatment blood NLR was correlated with significant shorter OS (HR = 1.98) and PFS (HR = 1.78). However, they only included one study related to immunotherapy in treating NSCLC patients. Another meta-analysis performed by Sacdalan et al. also showed a favorable result confirming the prognostic role of pre-treatment NLR in cancer patients. They included three retrospective studies that assessed the relationship between NLR and immunotherapy in NSCLC patients. Compared to the limited number of included NSCLC studies on topic of NLR and immunotherapy, we included 14 retrospective studies. There might be gray literature as indicated by the significant risk of publication bias. Our results also found that not only increased pre-treatment NLR but also high post-treatment NLR were significantly associated with poor OS and PFS.
There are other predictive biomarkers with potential roles for immunotherapy, including tumor mutational burden(TMB), programmed cell death receptor 1(PD-1), gene expression profiles (GEP) and mismatch repair status. Compared to these factors, NLR has been used as a prognostic biomarker in several cancers, such as lung cancer and colorectal cancer. It is convenient to calculate NLR without additional harm and economy cost. However, the application of immunotherapy should not be determined by sole predictive factor. The optimal combination of these biomarkers should be recommended.
There were some limitations within our meta-analysis. Firstly, all the included studies were retrospective studies with limited number of participants, weakening the strength of our findings. Secondly, the baseline characteristics of included studies varied, introducing a high risk of heterogeneity across studies and affecting the reliability of our results. The characteristics of patients were different across studies, which may also contribute to heterogeneity. Third, six of them were meeting abstracts providing few available data, and this could be improved by updating with the latest data. Nevertheless, this was the latest evidence with plenty of studies to prove the prognostic role of NLR in NSCLC treated with immune checkpoint inhibitors.
Conclusion
Our study found baseline NLR that larger than cutoff value was associated with poor PFS and OS, suggesting NLR could be a feasible biomarker to predict NSCLC patients who could benefit better from Nivolumab. However, due to the limited strength of included studies, it should be cautious to use this evidence and large sample, multiple center, randomized controlled clinical trials are urgently needed to validate our findings.
Disclosure of Potential Conflict of Interest
The authors declared that there were no conflicts of interests.
Supplemental Material
Download Zip (3.2 MB)Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Dedong C, Huilin X, Anbing H, Ximing X, Wei G. The effect of ShenQi FuZheng injection in combination with chemotherapy versus chemotherapy alone on the improvement of efficacy and immune function in patients with advanced non-small cell lung cancer: A meta-analysis. PloS One. 2016;11:e0152270. doi:10.1371/journal.pone.0152270.
- Shroff GS, De Groot PM, Papadimitrakopoulou VA, Truong MT, Carter BW. Targeted therapy and immunotherapy in the treatment of non-small cell lung cancer. Radiol Clin North Am. 2018;56:485–495. doi:10.1016/j.rcl.2018.01.012.
- Remon J, Vilarino N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): approaches on special subgroups and unresolved burning questions. Cancer Treat Rev. 2018;64:21–29. doi:10.1016/j.ctrv.2018.02.002.
- Ernani V, Ganti AK. Immunotherapy in treatment naive advanced non-small cell lung cancer. J Thorac Dis. 2018;10:SS412–SS421. doi:10.21037/jtd.
- Bilen MA, Dutcher GMA, Liu Y, Ravindranathan D, Kissick HT, Carthon BC, Kucuk O, Harris WB, Master VA. Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with Nivolumab. Clin Genitourin Cancer. 2018;16:e563–e575. doi:10.1016/j.clgc.2017.12.015.
- Nomura S, Yokoi T, Kurata T. Platelet-related indices in patients with lung cancer with nivolumab. Platelets. 2018;29:207–208. doi:10.1080/09537104.2017.1356454.
- Akamine T, Takada K, Toyokawa G, Kinoshita F, Matsubara T, Kozuma Y, Haratake N, Takamori S, Hirai F, Tagawa T, et al. Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: A comprehensive analysis of systemic inflammatory markers. Surg Oncol. 2018;27:88–94. doi:10.1016/j.suronc.2018.01.002.
- Ren M, Dai B, Kong YY, Lv JJ, Cai X. PD-L1 expression in tumor infiltrating lymphocytes is a poor prognostic factor for primary acral melanoma patients. Histopathology. 2018. doi:10.1111/his.13527.
- Ren L, Matsuda T, Deng B, Kiyotani K, Kato T, Park JH, Seiwert TY, Vokes EE, Agrawal N, Nakamura Y. Similarity and difference in tumor-infiltrating lymphocytes in original tumor tissues and those of in vitro expanded populations in head and neck cancer. Oncotarget. 2018;9:3805–3814. doi:10.18632/oncotarget.23454.
- Lalani AA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, Duquette A, Bossé D, Bellmunt J, Van Allen EM, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6:5. doi:10.1186/s40425-018-0315-0.
- Tang Y, Li G, Wu S, Tang L, Zhang N, Liu J, Zhang S, Yao L. Programmed death ligand 1 expression in esophageal cancer following definitive chemoradiotherapy: prognostic significance and association with inflammatory biomarkers. Oncol Lett. 2018;15:4988–4996. doi:10.3892/ol.2018.7984.
- Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, Hata H, Tanaka R, Yamaguchi K, Nonomura Y, et al. Baseline neutrophil to lymphocyte ratio combined with serum LDH level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol. 2018;179:213–215. doi:10.1111/bjd.2018.179.issue-1.
- Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: A meta-analysis of 7,219 patients. Mol Clin Oncol. 2017;7:498–506. doi:10.3892/mco.2017.1342.
- Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5:12493. doi:10.1038/srep12493.
- Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med. 2015;8:3098–3106.
- Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim D-W, Heo DS, Lee JS. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67:459–470. doi:10.1007/s00262-017-2092-x.
- Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, Satsutani N, Ogata M, Miyara T, Nomura S. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 201823:634–640.
- Kiriu T, Yamamoto M, Nagano T, Hazama D, Sekiya R, Katsurada M, Tamura D, Tachihara M, Kobayashi K, Nishimura Y, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PloS One. 2018;13:e0193018. doi:10.1371/journal.pone.0193018.
- Park W, Kwon D, Saravia D, Desai A, Vargas F, El Dinali M, Warsch J, Elias R, Chae YK, Kim DW, et al. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with Nivolumab. Clin Lung Cancer. 2018;19:280–288.e4. doi:10.1016/j.cllc.2017.12.007.
- Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi:10.2147/OTT.S153290.
- Jiang T, Qiao M, Zhao C, Li X, Gao G, Su C, Ren S, Zhou C. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother. 2018;67:713–727. doi:10.1007/s00262-018-2126-z.
- Bartlett E, Ferraro RA, Stacruz JM, Postow MA, Coit D, Ariyan CE. Neutrophil to lymphocyte ratio (NLR) is associated with survival but varies with disease burden in melanoma patients treated with PD-1 inhibitor monotherapy. Ann Surg Oncol. 2018;25:S41.
- Cao DD, Xu HL, Xu XM, Ge W. The impact of primary tumor location on efficacy of cetuximab in metastatic colorectal cancer patients with different Kras status: a systematic review and meta-analysis. Oncotarget. 2017;8:53631–53641. doi:10.18632/oncotarget.19022.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1. 0. The Cochrane Collaboration. Confidence Intervals, 2011.
- Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, Nakahama K, Taniguchi Y, Isa S-I, Inoue T, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non–small cell lung cancer. Cancer Med. 2018;7:13–20. doi:10.1002/cam4.1234.
- Russo A, Scimone A, Picciotto M, Toscano G, Raiti F, Sava S, Battaglia A, Adamo V. Association between baseline absolute neutrophil count (ANC), derived neutrophil-to-lymphocyte ratio (dNLR), and platelet-to-lymphocyte ratio (PLR) and response to nivolumab (Nivo) in non-small cell lung cancer (NSCLC): A preliminary analysis add to collection. J Clin Oncol. 2017:15:e14617. doi:10.1200/JCO.2017.35.
- Rogado J, Fenor De La Maza MD, Pacheco-Barcia V, Serra JM, Toquero P, Vera B, Ballesteros A, Mondéjar R, Donnay O, Obispo B, et al. Are inflammatory markers predictive of nivolumab efficacy in advanced non-small-cell lung cancer (NSCLC)? J Thorac Oncol. 2017;12:SS2108–SS2109. doi:10.1016/j.jtho.2017.09.1205.
- Preeshagul IR, Sullivan KM, Paul D, Seetharamu N. The utilization of pretreatment neutrophil to lymphocyte ratio as a predictive marker for response to nivolumab therapy in non small cell lung cancer. J Clin Oncol. 2017;35:e20634. doi:10.1200/JCO.2017.35.
- Patil PD, Khunger M, Rakshit S, Stevenson J, Pennell NA, Elson P, Velcheti V. Pre-treatment hematological markers as a predictive biomarker for survival in patients with non-small cell lung cancer treated with nivolumab. J Clin Oncol. 2017;15:11547. doi:10.1200/JCO.2017.35.
- Gervais C, Boudou-Rouquette P, Jouinot A, Huillard O, Alexandre J, Arrondeau J, Giraud F, Chapron J, Alifano M, Revel MP, et al. Predictive and prognostic value of systemic inflammatory response biomarkers in patients receiving nivolumab for metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. 2017;15:3055. doi:10.1200/JCO.2017.35.
- Galetta D, Logroscino AF, Misino A, Montagna ES, Petrillo P, Ricci D, Catino A. P3.02c-085 neutrophil/lymphocyte ratio in advanced non-small cell lung cancer: correlation with prognosis and response to Anti-PD1 therapy. J Thorac Oncol. 2017;12:SS1329–SS1330. doi:10.1016/j.jtho.2016.11.1881.
- Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024.
- Bennati C, D’Arcangelo M, Minuti G, Vecchiarelli S, Landi L, Mazza V, Gili A, Puccetti M, Cappuzzo F. Programmed cell death ligand 1 and neutrophil to lymphocyte ratio to predict response to nivolumab in non-small cell lung cancer. J Thorac Oncol. 2017;12:SS2390–SS2391. doi:10.1016/j.jtho.2017.09.1939.
- Bagley S, Kothari S, Aggarwal C, Bauml J, Alley E, Evans T, Kosteva J, Ciunci C, Thompson J, Stonehouse-Lee S, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) predicts outcomes with nivolumab in non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12:S1318. doi:10.1016/j.jtho.2016.09.002.
- Soyano AE, Dholaria BR, Marin-Acevedo JA, Diehl NN, Hodge D, Lou Y. Baseline peripheral blood biomarkers associated with clinical outcome of advanced lung cancer in patients treated with anti-PD-1 antibody. J Clin Oncol. 2017;35:e20599–e.
- Bennati C, Mazza V, D’Arcangelo M, Minuti G, Vecchiarelli S, Attilia L, Gili A, Montanari M, Landi L, Cappuzzo F, Integrating programmed cell death ligand 1 (PD-L1) and neutrophil to lymphocyte ratio (NLR) as predictive panel of response to nivolumab in non-small cell lung cancer (NSCLC). Ann Oncol. 2017;28:vi56. doi:10.1093/annonc/mdx075.
- Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell. 2018;33:581–598. doi:10.1016/j.ccell.2018.03.005.
- Canova S, Bidoli P, Lissoni P, Abbate MI, Capici S, Casiraghi S, Cortinovis D. Predictive role of absolute lymphocyte count (ALC) and neutrophil/lymphocyte ratio (NLR) in patients with metastatic non small cell lung cancer (NSCLC) treated with nivolumab: results of a retrospective monocentric study. Ann Oncol. 2016;27. doi:10.1093/annonc/mdw141.