ABSTRACT
It has been recently recognized that the tumor microenvironment (TME) is an essential factor that defines the efficiency of chemotherapy. The local TME, consisting of immune cells with diverse phenotypes and functions, can strongly modulate the response to chemotherapy. Tumor-associated macrophages (TAMs) that display pronounced heterogeneity and phenotypic plasticity are the major innate immune component in the microenvironment of solid tumors. In our review, we elucidate the complex role of TAMs in the progression of different types of solid tumors, summarize the current knowledge about the effects of different anticancer chemotherapeutic agents on monocytes/macrophages, and describe the mechanisms of chemotherapy resistance mediated by TAMs.
Introduction
Enhancing the efficiency of antitumor therapy is the most relevant challenge in clinical oncology. The main goal of the established cytostatic therapeutic schemas is to achieve the maximal cytoreduction of the primary tumor and metastatic foci by inducing apoptosis or necrosis or blocking uncontrolled cancer cell proliferation.Citation1 The outcome of cytostatic treatment depends on the biological (including genetic) characteristics of tumor cells and their sensitivity or resistance to therapeutic agents.Citation2 On average, only 40–60% of the cancer patients benefit from antitumor chemotherapy (CT).Citation3,Citation4 Drug resistance in surviving cancer cells, in fact, leads to an inability to achieve complete pathological regression.Citation5 However, even when complete pathological regression is achieved, tumors can relapse in 10–40% of the cases.Citation3,Citation6
The therapeutic sensitivity of tumors significantly depends on the complex interaction of cancer cells with different components of the tumor microenvironment (TME), particularly with immune cells.Citation6–Citation8 It was shown that effective cytostatic treatment is associated with a manifestation of the cytotoxic activity of immune effector cells that can be enhanced by radiation.1,Citation9 Cytostatic agents can stimulate the antigenic properties of cancer cells, facilitating their recognition by the immune system and can also enhance the systemic effects of chemotherapy, resulting in immunosuppressive cell depletion.Citation10,Citation11
The key cells of the immune system that define the intratumoral immune status and interaction of cancer cells with the immune component of the microenvironment are the tumor-associated macrophages (TAMs).Citation12 There are two main directions of phenotypic and functional macrophage polarization: classically activated pro-inflammatory M1 macrophages with antitumor properties and alternatively activated anti-inflammatory M2 macrophages with tumor-supporting functions. In the majority of solid tumors, TAMs have a pronounced M2 phenotype that strongly supports primary tumor growth and metastatic spread.Citation13 However, there are also experimental data suggesting that TAMs can combine the properties of M1 and M2 macrophages.Citation14 The effect of TAMs on tumor progression can depend on the tumor type, the type of tumor microenvironment and the localization of TAMs in specific intratumoral compartments.Citation15–Citation17 Reprogramming the M2 macrophage phenotype toward the pro-inflammatory M1 phenotype is recognized as a promising therapeutic approach for cancer treatment.Citation18 However, the complexity of the interaction of the components of the tumor microenvironment, in particular, TAMs with chemotherapeutic agents, has to be considered for the elaboration of novel efficient therapeutic schemas.
In our review, we focused on recent advances in understanding the role of the microenvironment, particularly, the role of TAMs in defining the efficiency of chemotherapeutic treatment on solid tumors.
Macrophages and tumor progression
TAMs originate from two major sources: (a) tissue-resident macrophages that are long-living and embryonically derived (yolk sac), and (b) macrophages derived from the circulating monocytes that originate out of bone marrow and are recruited to tumor tissue by growth factors and chemokines, such as M-CSF, CCL2, and CCL5.Citation18,Citation19 The tumor microenvironment affects the programming of both resident and infiltrating macrophages into tumor-specific macrophages. It is believed that resident macrophages are the first to be reprogrammed by growing tumors into pro-tumoral phenotypes.Citation18 TAMs originating from resident macrophages can mediate DNA damage, transformation and the survival of transformed cells and cancer-related inflammation. Monocytes/macrophages recruited to the tumor site further promote the proliferation and survival of tumor cells and angiogenesis.Citation20
A significant number of data indicate a supportive role for macrophages in cancer development, as shown in different experimental models and in clinical observations.Citation21 In solid tumors, TAMs can promote primary tumor growth, induce angiogenesis, lymphangiogenesis, stromal remodeling, metastasis, and suppression of immunity.Citation22,Citation23 TAMs express molecules that directly affect cancer cell proliferation, including epidermal growth factor (EGF), members of the fibroblast growth factor (FGF) family, and transforming growth factor beta (TGFβ).Citation14,Citation24 The ability of TAMs to promote tumor progression and accelerate vessel growth is mediated through the upregulation and release of several pro-angiogenic factors, such as vascular endothelial growth factor A (VEGF-A), tumor necrosis factor α (TNFα), FGF, thymidine phosphorylase (TP), urokinase plasminogen activator (uPA), adrenomedullin (ADM), and semaphorin 4D (Sema4D).Citation23,Citation24 TAMs produce several factors that are responsible for the induction of lymphangiogenesis, including VEGF-C, VEGF-D, VEGF-A, MMP2, MMP9, CXCL8 and many others.Citation14,Citation23,Citation24 TAMs support stromal remodeling, tumor cell invasion and metastasis by releasing various enzymes, including plasmin, uPA, matrix metalloproteinases (MMPs), cathepsin B, platelet-derived growth factor (PDGF) and TGF-β1.Citation22,Citation23 TAMs also secrete a number of cytokines and chemokines, such as CCL3, CCL4, CCL5, CCL22, TGFβ, and IL10, which recruit natural regulatory T cells (nTreg) to the tumor microenvironment and suppress CD4+ and CD8 + T cell effector functions.Citation12,Citation21
TAMs are able to support cancer stem cell functions.Citation13,Citation20 Cancer stem cells (CSCs) represent a tumor subset with an enhanced ability to initiate tumor progression, dissemination, and relapse.Citation20 Thus, in non-small-cell lung carcinoma, TAMs accompany enhanced stemness by increasing the expression of CD133+ cells (CSCs) and inflammation-associated genes, including Sox2 and NF-κB.Citation25 In glioma cells, M2-TAMs promote stemness and migration by secreting TGF-β1.Citation26
The tumor-supporting functions of TAMs have been demonstrated for many types of malignancies, such as breast cancer, lung adenocarcinoma, cervical cancer, ovarian cancer, prostate cancer, melanoma, renal cell carcinoma, and esophageal cancer.Citation27–Citation33 However, there is much experimental clinical evidence that indicates a dual role of TAMs in tumor progression and survival, and the antitumor activities of TAMs have been identified (). M. Bogels and coworkers showed that the pro- or antitumor activity of macrophages depends on the type of tumor.Citation22 For example, breast carcinoma cells induce the secretion of tolerogenic cytokines in human monocyte-derived macrophages, while intestinal carcinoma cells stimulate the secretion of inflammatory cytokines.Citation22
Table 1. Correlations between common macrophage markers and the progression of several cancers.
In , we summarized the latest results concerning the tumor-promoting and antitumor activities of TAMs in different types of cancer and the association of the main macrophage markers with tumor progression. Thus, CD68 was indicative of poor prognosis and reduced survival in many types of cancer, including non-small-cell lung cancer (NSCLC), ovarian cancer, stomach cancer, melanoma, and breast cancer. In NSCLC, the tumor-infiltrating CD68-expressing macrophage density negatively correlated with patient survival and positively correlated with tumor IL-8 expression, which may contribute to the increased tumor angiogenesis.Citation28 High levels of CD68-positive infiltrating TAMs in gastric cancer (GC) were associated with metastasis and poor prognosis and strongly correlated with EMT features (loss of E-cadherin and positivity of vimentin).Citation29 In melanoma tissue, a high number of CD68+ macrophages was associated with a worse prognosis and high melanoma-specific mortality.Citation30
Controversial reports were found for CD68 expression in patients with breast cancer (BC). Triple-negative breast cancer (TNBC) with a large number of infiltrating CD68+ TAMs had a high risk of distant metastasis and low rates of disease-free survival (DFS) and overall survival (OS).Citation31 In another study of breast tumors, high numbers of CD68 macrophages were significantly associated with worse breast cancer-specific survival and shorter DFS.Citation32 A study of 100 breast cancer samples demonstrated a significant association of CD68+ TAM infiltration with TNM stages and tumor size, and the high-infiltration of TAMs in tumors correlated with poor outcome and decreased OS.Citation33 On the other hand, the average score of CD68 expression was found to be lower in cases with lymph node (LN) metastases compared to negative LN, both without NAC and after NAC in BC patients.Citation16,Citation34
The opposite results, indicating an antitumor effect of macrophages, were demonstrated for colorectal cancer where TAMs had pro-inflammatory properties and expressed a number of cytokines such as IFN-γ, IL-1, and IL-6, which activated cytotoxic Th1 cells, mediating the antitumor immune response.Citation28,Citation54 In a study of 446 patients with colorectal cancer treated in the Department of Surgery, Umea University Hospital (Sweden), Forsell J and colleagues showed that high levels of CD68+ TAMs localized in the tumor/stroma line were correlated with better survival in colon cancer.Citation35 In another study of 208 patients with colorectal cancer who were treated in the Humanitas Research Hospital (Italy), a high amount of TAMs was associated with better DFS and OS, independent of nodal status and vascular invasion, in patients with colorectal cancer.Citation36
Several independent clinical studies demonstrated that the M2 phenotype of TAMs is associated with poor survival in patients with breast cancer, ovarian cancer, gastric cancer, renal cell carcinoma, hepatocellular carcinoma, multiple myeloma, and osteosarcoma.Citation37–Citation46,Citation55 CD206 and CD163 are the most frequently used biomarkers to histologically identify the M2 phenotypes of TAMs in tumor tissues.Citation15 Zhang et al. showed that a decrease in the number of CD206+ TAMs in gastric cancer was associated with a longer DFS, which can be considered a significant prognostic factor.Citation38 A positive correlation between CD206+ TAM macrophage infiltration and poor survival rates was found for ovarian cancer, renal cell carcinoma, and hepatocellular carcinoma.Citation39,Citation40 Similarly, an increased density of CD163+ was found in the advanced stages of ovarian cancer, multiple myeloma, gastric cancer, breast cancer, osteosarcoma and positively correlated with worse progression-free survival (PFS) and OS.Citation41–Citation46,Citation55 ()
Stabilin-1 is a multifunctional scavenger receptor that plays important roles in the clearance of “unwanted” self-substances.Citation47 The expression of stabilin-1 was found on alternatively activated macrophages.Citation56 A high number of stabilin-1+ TAMs was found in metastasizing primary human breast tumors and was shown to support tumor growth in a mammary adenocarcinoma mouse model.Citation47
Chitinase-like proteins (CLPs) are evolutionarily conserved lectins, and their elevated levels of gene expression or secretion are indicative of tumor progression, metastasis or response to therapy.Citation57 Thus, circulating levels of YKL-40 are increased in glioblastoma, breast, colorectal, lung, prostate, bladder, stomach, and endometrial cancers among others and predict a poor outcome or short DFS.Citation48–Citation51,Citation58 For example, YKL-40 may contribute to the proliferation of malignant cells, stimulate angiogenesis, and regulate extracellular tissue remodeling.Citation57 YKL-39 is considered a biological marker for the progression of osteoarthritis.Citation59 Recently, we identified that an elevated gene expression of YKL-39, a new pro-angiogenic and monocyte-recruiting factor in breast cancer tumors, after neoadjuvant chemotherapy (NAC) is predictive for the increased risk of distant metastasis and for a poor response to NAC.Citation52 In patients who received anthracycline-containing NAC, the absence of a clinical response was associated with the presence of M2-type TAMs identified by the expression of YKL-39 or CCL18.Citation53
These numerous studies demonstrate the important roles of both the amount and phenotypes of TAMs in tumor progression and metastasis and indicate the necessity to understand the mechanism of the interaction between TAMs and chemotherapeutic agents to predict the efficiency of chemotherapy and to design therapeutic schemas enhancing the antitumor activities of TAMs.
Cancer chemotherapy, chemoresistance, and immunomodulation
In patients with the operable forms of cancer, systemic chemotherapy is divided into preoperative (neoadjuvant) and postoperative (adjuvant) chemotherapy. The goal of adjuvant therapy is the long-term suppression of distant metastasis and the eradication of micrometastases after surgical treatment, which results in an increase in patient survival and a prolongation of disease-free (metastases-free) period.Citation60 Neoadjuvant chemotherapy (NAC) is used to reduce the volume of the primary tumor and the level of regional lymphadenopathy, enabling radical surgery.
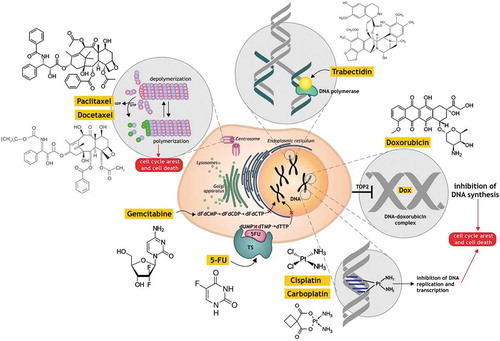
The mechanisms of action of the main chemotherapeutic agents are illustrated in and summarized in . Anthracyclines (for example, doxorubicin) intercalate between base pairs of nucleic acids and disrupt topoisomerase-II-mediated DNA repair, preventing the DNA double helix from being resealed and thereby inhibiting DNA replication.Citation79 Taxanes (paclitaxel and docetaxel) target tubulin and stabilize the microtubule polymer, protecting it from disassembly, which blocks mitosis and reverses to the G0 phase of the cell cycle without cell division.Citation80 Alkylating agents (cisplatin, oxaliplatin, carboplatin, which are platinum drugs, and cyclophosphamide) form DNA crosslinks both between and within DNA strands (known as interstrand and intrastrand cross-linkages, respectively). This process is irreversible and leads to cell apoptosis.Citation81 5-Fluorouracil (5-FU) acts as an inhibitor of thymidylate synthase (TS), which methylates deoxyuridine monophosphate (dUMP) to form thymidine monophosphate (dTMP), which in turn blocks the synthesis of the pyrimidine thymidine that is required for DNA replication.Citation82 Gemcitabine, after the attachment of the three phosphates, becomes pharmacologically active as gemcitabine triphosphate (dFdCTP) and is incorporated into new DNA strands.Citation83 Trabectedin binds to the minor groove of the DNA, causing the DNA helix to bend toward the major groove, which causes the inhibition of transcription and reparation.Citation84
Table 2. The effects of different anticancer chemotherapy agents on monocytes/macrophages.
Figure 1. The mechanisms of action of the main chemotherapeutic agents.
There are several mechanisms by which chemotherapeutic agents act in the cancer cell (a) intercalate into DNA and disrupt topoisomerase-II-mediated DNA repair (doxorubicin); (b) promote microtubule polymerization and stabilization (paclitaxel and docetaxel); (c) form DNA crosslinks (cisplatin, carboplatin); (d) inhibit thymidylate synthase (TS) (5-fluorouracil); (e) act as pyrimidine nucleoside antimetabolite (gemcitabine); (f) bind to the minor groove of the DNA (trabectedin). Commonly, all of them cause inhibition the DNA replication and transcription and cancer cell death. The detailed explanation is given in the paragraph “Cancer chemotherapy, chemoresistance and immunomodulation”.
Chemoresistance and chemotherapy-induced immunosuppression can result in the relapse of tumors and is critical for survival in cancer patients.Citation7,Citation85 In the past few decades, several studies have examined the molecular mechanisms that promote the chemoresistance of cancer cells, such as the induction of anti-apoptotic regulators, ABC transporters, aberrant transcription factor nuclear factor‐κB (NF-κB) activity, and the mechanisms of damaged DNA repair.Citation7,Citation86,Citation87 CT can also lead to the selective expansion of resistant cancer clones.Citation88
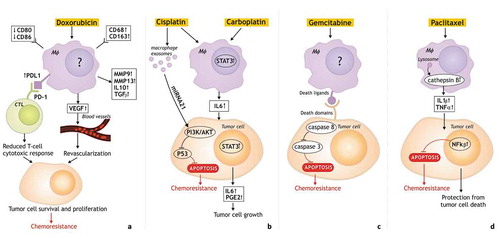
It was found that myeloid cells, particularly TAMs, generally accumulate in tumors after chemotherapy and contribute to tumor recurrence by initiating the physiological regenerative program that is a beneficial action of macrophages in wound healing but detrimental in the case of tumor relapse.Citation62,Citation85 Concomitant mechanisms include the macrophage-induced suppression of T cell immunity, the maintenance of tumor cell survival and the activation of tumor revascularization ().
Figure 2. The mechanisms of macrophage-mediated resistance to chemotherapy.
The pathways responsible for the tumor-promoting function of TAMs after chemotherapy and chemoresistance include the increased recruitment of immunosuppressive TAMs, the pro-tumor polarization, the reduced T-cell cytotoxic response, the activation of anti-apoptotic programs in malignant cells. a. TAMs which contribute to tumor resistance to doxorubicin have high expression of CD68, CD206, CD163, PD-L1, but low expression of CD80 and CD86. Moreover, TAMs release immunosuppressive cytokines (IL10 and TGFβ), factors which promote invasion (MMP9, MMP13), pro-angiogenic factor VEGF which cause revascularization. Increased expression of PDL1 in TAMs limits tumor response to chemotherapy by suppression the antitumor functions of cytotoxic T cells resulting in tumor cell survival and proliferation, and chemoresistance. b. TAM-mediated resistance to carboplatin is associated with STAT3 signaling and macrophage-produced IL6 promoting tumor cell growth. TAM exosomes are involved in cisplatin resistance via the activation of the PI3K/AKT signaling pathway in tumor cells. c. Chemoresistance to gemcitabine is mediated by decreasing the activation of caspase-3 and reducing the apoptosis in tumor cells. d. Cathepsin proteases (cathepsin B and S) secreted by TAMs mediate chemoprotection through NF-κβ activation, or indirectly through IL-6 expression and STAT3 activation. There are unknown mechanisms of chemotherapy influence on macrophages.
Alternatively, activated M2 macrophages can mediate chemoresistance by secreting growth factors and inhibiting cell death signaling pathways in tumor cells, protecting them from the cytotoxic effects of chemotherapy.Citation89 Thus, in patients with esophageal cancer who received neoadjuvant chemotherapy (two courses of 5-fluorouracil (5-FU), cisplatin, and adriamycin), the infiltration of CD68+ and CD163+ macrophages in tumor mass significantly correlates with tumor depth, lymphatic and venous invasion, and poor prognosis.Citation66 Treatment with cyclophosphamide (CTX), paclitaxel (PTX) and doxorubicin (DOX) for a mouse Lewis lung carcinoma model (LLC1s) and mouse models of breast cancer metastasis (MMTV-PyMT) resulted in a significant increase in the number of CD206+ TAMs, accumulating mostly in the vascularized chemokine CXCL12-rich regions of tumors after chemotherapy that caused tumor revascularization and relapse.Citation85 In breast cancer patients undergoing neoadjuvant chemotherapy and in the PyMT mouse model after paclitaxel (PTX) treatment, a dramatic accumulation of macrophages protecting tumors was found.Citation90 The application of chemotherapeutic agents can also be cytotoxic for monocytes and macrophages, as in the case of the DNA-damaging agent trabectedin (), which has strong antitumor activity.Citation89 In transplantable tumor models of fibrosarcoma, ovarian carcinoma, and Lewis lung carcinoma, treatment with trabectedin significantly delayed tumor growth and decreased the production of the major monocyte chemoattractant CCL2 by TAMs. Decreased levels of CCL2 resulted in macrophage depletion in tumor tissues, which was suggested as an essential mechanism of the antitumor activity of trabectedin.Citation78
Chemotherapy was also considered to create the conditions for the activation of cytotoxic immune responses against tumor cells. Thus, the application of antitumor drugs that damage DNA in tumor cells results in immunogenic cell death (ICD) due to the expression of neoantigens on tumor cells following cellular DNA disruption.Citation17 Carboplatin is a platinum compound with DNA-damaging agents.Citation91 An increased tumor pathological complete response (pCR) was shown in 53.2% of the patients with stage II-III triple-negative breast cancer (TNBC) treated with carboplatin against 36.9% without carboplatin.Citation92 In the BrighTNess trial, the pCR rate in TNBC increased from 31% without carboplatin to 58% with carboplatin.Citation92 In gastric cancer, a high amount of TAMs before treatment correlated with prolonged survival in patients who received 5-fluorouracil (FU)-based postoperative chemotherapy.Citation76 In patients with stage III colorectal cancer treated with 5-FU adjuvant therapy, the high macrophage density before the treatment significantly correlated with a better improved prognosis.Citation36 In pancreatic adenocarcinoma, high levels of CD68+ TAMs before treatment were associated with an established prognosis only in patients who received adjuvant gemcitabine-based chemotherapy but not in untreated patients. In vitro gemcitabine (GEM) re-educated macrophages to an antitumor phenotype by dramatically increasing the cellular reactive oxygen species (ROS) production that is responsible for the tumor cytotoxic effect.Citation77 The GEM-modified polarization of macrophages was characterized by an upregulation of the expression of the M1 markers HLA-DR, CD40 and the chemokine receptor CCR7, a downregulation of the expression of M2 markers CD163 and CD206, and an activation of the pro-inflammatory program in macrophages.Citation77
Thus, TAMs have a controversial role not only in tumor progression but also in cancer chemoresistance. Macrophages modify the effect of chemotherapeutic drugs, but the direction of these changes (enhancement or decrease) depends on both the type of CT agent and the type of cancer. However, the ways in which different types of TAMs respond to CT agents must be understood at the mechanistic level.
How does chemotherapy edit macrophages?
Chemotherapeutic agents can edit macrophages in tumor-protective or antitumor directions, where three major mechanisms must be considered: 1) changes in the macrophage phenotype; 2) induced recruitment of monocytes or macrophages to the tumor site; and 3) systemic depletion of monocytes/macrophages. The main effects of chemotherapeutic agents on monocytes/macrophages are summarized in .
1) Changes in macrophage phenotype during chemotherapy treatment
In vitro, paclitaxel was found to re-educate macrophages to induce genes encoding the mediators of inflammation such as TNF-α, IL-12, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), transcription factors, colony-stimulating factors, adrenomedullin, and cytokines that can activate other immune cells, including dendritic cells (DCs), natural killer cells (NKs) and tumor-specific cytotoxic T-lymphocytes (CTLs). Such activity of PTX-treated TAMs can result in the suppression of tumor growth and the enhancement of immune cell function against tumors.Citation61
In vitro, the short exposure of cisplatin (a platinum agent) on peritoneal macrophages isolated from BALB/c mice and coincubated with L929 cells (a mouse fibroblast cell line) resulted in the enhanced production of NO, TNF-a, IL-1B, IL-12 and IFNg, linked to the enhanced expression of Toll-like receptor (TLR)-2 and TLR-4 genes, and the activation of the mitogen-activated protein (MAP) kinases and NF-kB pathways.Citation64 The increased antigen-presenting ability of peritoneal macrophages isolated from BALB/c mice treated with cisplatin was regulated not only by the continuous presence of cisplatin in the culture medium but also by the macrophage release of soluble factors, such as IL-1, IL-6, and IL-8, and TNF-a.Citation93 Cisplatin-activated murine peritoneal macrophages in coculture with L929 cells were able to release the tumor cell-specific cytotoxic factors FasL and TNF and to facilitate the apoptosis of tumor cells.Citation94 The induction of tumor cell death by apoptosis was also confirmed by an enhanced activation of apoptosis-mediating molecules such as caspase-8, caspase-3, CAD, PARP, Bid, Bax, cytochrome c, as well as DNA fragmentation and the downregulation of Bcl-2.Citation94
At the same time, Dyikgraaf and colleagues showed that in an in vitro model, M2 macrophages were more sensitive to cisplatin and carboplatin compared to M1-macrophages and DCs. The impact of platinum chemotherapy on human cervical and ovarian cancer cell lines resulted in the increased production of prostaglandin E2 (PGE2) and IL-6 by tumor cells in a STAT-dependent manner, which skewed the differentiation of monocytes toward the M2-like macrophage phenotype and led to chemoresistance. The blockage of these molecular pathways increased the efficacy of cisplatin and carboplatin.Citation65
Cyclophosphamide (CTX) was found to enhance the production of the pro-inflammatory cytokines IL-6 and IL-12 and to decrease the production of the anti-inflammatory cytokines IL-10 and TGF-b in mouse peritoneal macrophages in vivo, potentiating immune responses by activating Th1 cells and antigen-specific macrophages.Citation67 DOX and CTX can activate macrophages to maintain the antitumor response in breast and leukemia models.Citation89 DOX causes the ICD of tumor cells and the recruitment and differentiation of myeloid cells into antigen-presenting cells, resulting in the activation of effective adaptive responses.Citation72,Citation73 Leukemic cells treated with CTX released cytokines CCL4, CXCL8, VEGF and TNF that enhanced the recruitment and phagocytic activity of monocytes/macrophages.Citation68 In the B16F10 mouse model of melanoma, combined chemotherapy (vincristine, cyclophosphamide and doxorubicin) and immunotherapy (monoclonal anti-CD40+ cytosine–phosphate–guanosine-containing oligodeoxynucleotide 1826 (CpG-ODN)) treatment resulted in the enrichment of an M1-polarized TAM subpopulation.Citation69 This TAM subpopulation was characterized by the upregulation of molecules associated with the M1 phenotype (CD40, CD80, CD86, major histocompatibility complex (MHC) class II, IFN-γ, tumor necrosis factor-α (TNF-α) and IL-12) and the downregulation of molecules associated with the M2 phenotype (IL-4Rα, B7-H1, IL-4 and IL-10).Citation69
Thus, chemotherapeutic agents can have several potentially beneficial effects via M1-programming TAMs. However, whether the pro-inflammatory activity of TAMs is helpful for antitumor responses or whether it can create conditions of low-grade inflammation remains an open question. Moreover, the same chemotherapeutic agent, depending on the type of tumor and therapy schemas, can also enhance the tumor-supporting M2 phenotype of TAMs. The interactions of the chemotherapeutic agents and TAMs must be carefully investigated for each type of tumor, and individual differences between patients must be considered. Deep insight into the mechanisms of TAM response to CT must be obtained in ex vivo experimental models.
2) Recruitment of monocytes or macrophages to the tumor site
A variety of studies demonstrate that anticancer therapies induce the recruitment of monocytes to the tumor sites where chemotherapy-damaged cells are localized, which monocytes consider as persistent nonhealing wounds to be repaired.Citation95 In this case, tumor-infiltrating macrophages initiate the regenerative program that supports the proliferation of cancer cells.Citation96 Thus, it was shown that chemotherapy, ionizing radiation, and the vascular disrupting agent combretastatin A4-P cause the increased production of the mononuclear phagocyte growth factor CSF-1 and the chemokines CCL2 and CXCL12 that can trigger monocyte recruitment and TAM accumulation in tumor sites.Citation85 In patients with advanced pancreatic cancer receiving gemcitabine, an overall increase in CD14+ monocytes, CD11c+ myeloid DCs and CD123+ plasmacytoid DCs was observed more frequently in comparison with untreated patients.Citation10
In mouse mammary tumors, chemotherapy increased the expression of CSF-1 by tumor cells, which then recruited large numbers of CSF1R-expressing macrophages.Citation62 In vivo, murine MDA-MB435 breast tumors treated with Abraxane (paclitaxel+albumin) showed a significantly higher infiltration of CD45+ CD169+ macrophages in comparison with the tumors from the untreated group. Flow cytometry analysis confirmed a significantly increased population of F4/80+ macrophages in MDA-MB-435 tumors but not in MDA-MB-435R (paclitaxel-resistant) tumors. The authors supposed that in this case, TAMs mediated the clearance of chemotherapy-induced apoptotic tumor cells.Citation97 A metronomic CTX regimen was shown to stimulate the recruitment of DCs, macrophages, and NK cells to the tumor site in mouse models and activate NK-dependent antitumor immunity in cancer patients.Citation70,Citation71 HER2/Neu-driven mammary carcinomas under the protein kinase inhibitor lapatinib and/or doxorubicin treatment were also highly infiltrated with immature macrophages in a STAT1-dependent manner. These cells had a phenotype of CD11b+F4/80+ Gr-1(Ly6C/Ly6G)+ and were possibly involved in antigen presentation and the induction of antitumor T cell immunity. The accumulation of immature macrophages and reduction of mature TAMs at the tumor site after lapatinib/doxorubicin treatment correlated with reduced tumor growth.Citation74
Nakasone and colleagues showed that in MMTV-PyMT (mouse model of breast cancer metastasis) tumors treated with DOX, the recruitment of monocytes was increased, and MMP9 produced by the recruited myeloid cells limited drug delivery to the tumors due to the decreased blood vessel permeability, suggesting that an increased vascular permeability is associated with a better response to DOX.Citation75 Moreover, the different populations of myeloid cells were differentially correlated with drug response. Thus, the CD206+ macrophages promoted the increased vascular leakage that resulted in a better DOX response, but the CCR2-dependent recruitment of monocytes was associated with tumor relapse. In contrast, CCR2 null mice responded better to treatment with doxorubicin or cisplatin.Citation75 Therefore, the impact of the chemotherapy-induced infiltration of monocytes/macrophages in the tumor site is still controversial and depends on the nature of chemotherapeutic agents and the context of the local tumor microenvironment, and it still has to be profoundly studied in human cancer.
3) Depletion of monocyte-macrophage lineage cells due to chemotherapy treatment
The antitumor activity of the chemotherapeutic agent docetaxel was shown in 4T1-Neu mammary tumor implants and involves the depletion of immunosuppressive (M2-like) TAMs and the activation of antitumoral (M1-like) monocytes/MDSCs, which can enhance the cytotoxic T cell responses in tumors.Citation63
In mouse tumor models, treatment with trabectedin led to a significant reduction in the number of monocytes (CD45+ CD11b+ CD115+) in the bloodstream, mature monocytes (CD11b+ CD115+) in the bone marrow and splenic F4/80+ macrophages via the TRAIL-R2-dependent pathway activating caspase-8-dependent apoptosis. The effect of trabectedin was selectively cytotoxic for cells of the monocyte/macrophage lineage but not for neutrophils and lymphocytes. Moreover, the production of CCL2, a major chemotactic factor that induces monocyte recruitment in tumors, was also decreased in treated mice. In patients with soft tissue sarcoma receiving trabectedin in a neoadjuvant regimen, a strong decrease in the density of TAMs and blood vessels was observed after treatment.Citation78
Macrophages contribute to tumor drug resistance and relapse after chemotherapy treatment
The tumor-protective function of macrophages was found in many in vivo and in vitro studies for some antitumor agents, including doxorubicin (adriamycin), platinum compounds, 5-fluorouracil (5-FU), gemcitabine, and paclitaxel.Citation65,Citation89 The major mechanisms of chemotherapy resistance are illustrated in . The pathways responsible for the tumor-promoting function of TAMs after chemotherapy include the increased recruitment of immunosuppressive TAMs, pro-tumor polarization, the activation of a tumor-promoting Th17 response, and the activation of anti-apoptotic programs in malignant cells and others.Citation89 M2 macrophages are known to have strong tumor-supporting functions as they contribute to the establishment of a local immunosuppressive microenvironment.Citation98 Macrophage-mediated resistance to paclitaxel, doxorubicin and etoposide was found in coculture studies combining mammary carcinoma cell lines and bone marrow-derived macrophages.Citation90
Thus, Baghdadi M and colleagues investigated the influence of the tumor supernatants of doxorubicin-resistant (DR) and doxorubicin-sensitive (DS) cell lines on monocyte polarization. They found that in monocytes stimulated with tumor supernatants from the doxorubicin-resistant cell line of lung adenocarcinoma A549-DR, the expression levels of CD68 and the М2 marker CD163 were significantly higher in comparison with the DOX-sensitive group A549-DS. Moreover, monocytes stimulated with chemoresistant tumor supernatants from A549-DR cells differentiated into M2 macrophages and acquired an immunosuppressive phenotype via an elevated expression of PD-L1 and a low expression of the MHC class I and II costimulatory molecules CD80 and CD86. Moreover, CD68+ CD163+ TAMs released various factors that contributed to tumor progression and chemoresistance, such as immunosuppressive cytokines (IL10 and TGFβ), pro-angiogenic factor VEGF, and factors that promote invasion (MMP9, MMP13).Citation7 ()
Jinushi et al. demonstrated that the in vivo resistance of pancreatic ductal adenocarcinoma cells (PDAC) to carboplatin was associated with STAT3 signaling and macrophage-produced IL6 promoting tumor cell growth.Citation99 () There is evidence that the exosomes of M2-macrophages are involved in the mechanisms of cisplatin resistance due to microRNA-21. Exosomes are considered to act as extracellular communicators between tumor cells and the tumor microenvironment.Citation100 As shown by Zheng et al. in in vitro models and an athymic nude mouse model, miRNA-21 from the macrophage culture suppresses apoptosis and enhances the activation of the PI3K/AKT signaling pathway in tumor cells, resulting in tumor progression.Citation100 () TAMs also increase the chemoresistance of PDAC (in vitro cell line and in vivo mouse model) to gemcitabine by reducing the level of apoptosis, particularly by decreasing the activation of caspase-3.Citation101 ()
Numerous studies have demonstrated that cathepsins play a pivotal role in tumor chemoresistance by diverse mechanisms. Cysteine proteases, or cathepsins, comprise a family of proteins that are localized in the endosomal/lysosomal compartment and are responsible for the proteolytic degradation of a wide variety of intracellular and extracellular substances.Citation102 Cathepsins can be secreted by malignant cells and cells of the tumor microenvironment, such as fibroblasts and macrophages.Citation103 In cancer, cathepsins, particularly cathepsin B and S, are involved in apoptosis, angiogenesis, cell proliferation, and invasion.Citation103 Cathepsin B is capable of degrading various components of the extracellular matrix, including type IV collagen, laminin, and fibronectin, facilitating the growth and invasion of tumor cells into surrounding tissue and vasculature.Citation104,Citation105 Cathepsins were found to be overexpressed in various human tumors (including breast, colorectal, gastric, urinary bladder carcinomas, glioblastomas, lung and prostate tumors, melanoma, chondrosarcoma, and many others), and their proteolytic activity correlated with poor prognosis and the invasive phenotype of colon and bladder cancers, esophageal adenocarcinomas, and breast cancer.Citation104,Citation106
Shree et al. demonstrated that cathepsin protease activity (cathepsin B and S) may influence the production of chemoprotective factors by macrophages in PTX-treated mice and that macrophages contribute to breast cancer resistance to therapy via the secretion of cathepsins.Citation90 Cathepsin B is important in the trafficking of TNF-α-containing vesicles to the surface of macrophages, which mediates chemoprotection through NF-κβ activation, or indirectly through IL-6 expression and STAT3 activation.Citation107 Macrophage-derived cathepsins B and S were able to protect the breast cancer cell line against PTX-induced cell death in vitro ().Citation90
DeNardo and colleagues supposed that the ability of TAMs to limit tumor response to chemotherapy can be mediated by the suppression of the antitumor functions of cytotoxic T cells.Citation108 It was found that breast tumors with high amounts of TAMs and low amounts of cytotoxic T cells respond weakly to neoadjuvant chemotherapy.Citation62 It was reported that in chemotherapy-treated mouse tumors, the M2 subpopulation of TAMs (CD206+ TIE2HiCXCR4Hi) accumulated around blood vessels, where they promoted tumor revascularization and relapse, in part, via VEGF-A release. A similar subpopulation of TAMs was clustered after chemotherapy in human breast carcinomas and bone metastases. Moreover, CXCL12, a ligand of CXCR4, was upregulated in the perivascular sites after chemotherapy and was responsible for the chemotaxis of CD206+ TAMs.Citation85
These findings demonstrated that macrophages contribute to drug resistance and relapse after chemotherapy treatment via different pathways based on the interaction of TAMs and cancer cells. However, the mechanisms of the direct action of chemotherapeutic drugs on TAMs as well as the mechanisms of TAM-mediated chemoresistance in tumors still require deep investigation.
Approaches to TAM targeting that can improve the antitumor effect of chemotherapy
Chemotherapy is primarily applied to selectively kill tumor cells or arrest their growth. However, chemotherapy-induced resistance frequently limits the cytotoxic effect of drugs in tumor sites. The immune system, especially TAMs, considerably contributes to chemoresistance; therefore, the depletion of M2-like TAMs or their reprogramming into the M1-like phenotype can enhance the efficacy of treatment and can be an efficient way to suppress tumor regression. Understanding the complex interaction between cancer cells and the immune system provides new therapeutic approaches to improve the antitumor effect in chemotherapy-treated patients.
Currently, an increasing number of studies have focused on complex therapeutic approaches in cancer treatment, including not only chemotherapy regimens but also immunotherapy, designed to activate immune responses to increase the efficacy of CTLs against cancers, and immune checkpoint blockade therapy, inhibiting immune suppressor molecules. For example, recently, the application of anti-PD-1, anti-PD-L1 and anti-CTLA-4 agents demonstrated significant benefits in the survival of patients with metastasis, and these agents have become advanced drugs in cancer treatment.
Macrophages express the ligands of the inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), which are normally upregulated in activated immune effector cells such as T cells, B cells, and NKT cells. The activation of PD-1 and CTLA-4 by their ligands (PD-L1, PD-L2 and B7-1 [D80], B7-1 [CD86], respectively) directly inhibits T cell and B cell responses by suppressing proliferation and cytokine production.Citation12
Experimental studies and clinical trials demonstrate the beneficial effects of the combination of chemotherapy and immunotherapy. Breedveld showed that in breast cancer, the overall response rate was significantly greater in the group of patients treated with trastuzumab (anti-HER-2 monoclonal antibodies) and chemotherapy.Citation109 Weir et al. demonstrated that the combined treatment with a class I restricted peptide-based cancer vaccine, metronomic cyclophosphamide (mCPA) and anti-PD-1 antibody, increases the clonality and activity of tumor-infiltrating antigen-specific T cells in a murine tumor model expressing HPV16 E7 (C3).Citation110 Soares et al. also proposed that the immunosuppressive pathways, including those of regulatory T cells (Tregs) and the immune check-point molecule CTLA-4 expression on T cells, can be inhibited by the addition of the vaccine or a low dose of cyclophosphamide to the PD-1 blockade.Citation111
B7-H4 is a member of the B7 superfamily that is expressed on TAMs and implicated in the suppression of T cells. Blocking B7-H4 or depleting B7-H4+ TAMs may represent a novel strategy to enhance T cell immunity in cancer.Citation12,Citation112 In human ovarian cancer, the inhibition of B7-H4 was found to contribute to tumor regression.Citation112
Several studies demonstrated the positive effect of the suppression of macrophage recruitment to the tumor site. Thus, the blockade of colony-stimulating factor-1 (CSF-1) was shown to limit macrophage infiltration and improve the response of breast cancer xenografts in mice to chemotherapy.Citation113,Citation114 Paclitaxel was found to improve the survival of mammary tumor-bearing mice in combination with the blockade of macrophage recruitment with CSF1R-signaling antagonists by limiting tumor development and reducing pulmonary metastasis.Citation62,Citation108 TAM depletion was found to enhance the efficacy of paclitaxel in MMTV-PyMT mouse mammary tumors and combination chemotherapy (cyclophosphamide, methotrexate, and 5-fluorouracil) in breast cancer xenografts in immunodeficient mice.Citation108 Genetic knockout of CSF-1, the use of neutralizing antibodies, low molecular weight inhibitors or antisense RNA for inhibiting CSF-1R signaling reduce the aggressiveness of tumor xenografts, which is associated with the lack of TAMs.Citation21,Citation115,Citation116 In mouse models of pancreatic tumors, targeting tumor-infiltrating macrophages by inhibiting either CSF1R or chemokine (C-C motif) receptor 2 (CCR2) improves the efficacy of gemcitabine therapy and reduces the number of metastases by activating antitumor T cell responses.Citation117
The combination of TAM depletion and chemotherapy was found to reduce tumor-vessel density by 50%. TAM depletion in tumor mass may normalize the vessels by skewing perivascular TAMs from pro-angiogenic cells to angiostatic cells that leads to increased blood flow and the delivery of chemotherapeutic agents to tumors, promoting tumor destruction.Citation62 Vascular endothelial growth factor A (VEGF-A) attracts macrophage progenitor cells, which then differentiate into TAMs (M2 macrophages) under the influence of IL-4.Citation118 The removal of these macrophages inhibits growth, angiogenesis, and tumor invasion. The reduction of lymphangiogenesis can be achieved by inhibiting the activity of macrophages via blocking the VEGF-C/VEGFR3 axis in chemotherapy-treated tumors.Citation119
Duhamel M and colleagues demonstrated a new therapeutic strategy combining paclitaxel and proprotein convertase 1/3 (PC1/3) inhibition to switch macrophages toward an antitumoral phenotype. PC1/3 knock-down (KD) macrophages activated by paclitaxel showed the inhibition of the anti-inflammatory pathway STAT3 and secreted more pro-inflammatory cytokines that can inhibit glioma growth in a cocultured experiment.Citation120 The approach of the combined treatment in MMTV-PyMT mice with paclitaxel plus an inhibitor of the c-kit receptor tyrosine kinases (PLX3397) showed a significant reduction in primary tumor burden, a reduction in the CD31+ vessel density within the mammary tumors, and an activation of the cytotoxic effector T lymphocytes compared to the treatment with a single agent.Citation120
Concluding remarks
It is evident that to overcome the chemoresistance of solid tumors, the effects of chemotherapeutic agents on TAMs must be considered. Many studies have demonstrated the involvement of TAMs in tumor progression in different cancers, including breast, prostate, colorectal, gastric, ovarian, melanoma, glioblastoma and others, and indicate a controversial role of TAMs in tumors. The fact that chemotherapeutic agents do not kill TAMs and can support the recruitment of the precursors of TAMs to the tumor site indicates that it is essential to identify not only how macrophages affect the sensitivity of cancer cells to the chemotherapeutic agent but also to focus on the long-term program induced in TAMs by chemotherapeutic agents.
It must be clearly understood for each type of cancer and each therapeutic agent or their combinations, whether the treatment leads to the activation of the antitumor activities of TAMs or programs TAMs to create beneficial conditions for tumor replacement and metastasis. Recent studies revealed that macrophages contribute to drug resistance and relapse after chemotherapy treatment via different pathways: promoting tumor revascularization, suppressing cytotoxic T cell immunity, and activating anti-apoptotic programs in cancer cells; however, the mechanisms of the direct action of chemotherapeutic drugs on TAMs remain unknown.
Understanding the mechanisms of the interaction of TAMs with chemotherapeutic agents in a tumor-specific context can lead to the prospective use of macrophages and other inflammatory and stromal components as targets for therapeutic effects to modify the tumor microenvironment in the direction of inhibiting tumor growth and reducing the risk of metastasis.Citation121
Such a strategy can lead to the possibility of using macrophages and other inflammatory and stromal components as targets for chemotherapeutic agents, where decisions about the specific treatment will be aimed at initiating the tumor-killing activity of macrophages and eliminating the macrophage types that can support tumor replacement. The functional “reorientation” of macrophages to the antitumor phenotype triggers a cascade of events leading to a disruption of the ecosystem by promoting tumor growth that results in the blocking of tumor cell proliferation, their metastatic potential and creating conditions for achieving a kind of parity between the tumor and the host organism, resulting in the inhibition of disease progression.
In summary, understanding the fundamentally important role of TAMs in determining the effectiveness of antitumor cytostatic therapy opens the prospect of developing new therapeutic approaches for the treatment of malignant neoplasms based on the balanced synergistic action of cytostatic agents and innovative immunomodulatory approaches.Citation6,Citation12
Abbreviations
5-FU 5-fluorourocile
BC breast cancer
COX cyclooxygenase-2
CSF1 colony stimulating factor
CT chemotherapy
CTL cytotoxic T-lymphocyte
CTX cyclophosphamide
DC dendritic cell
DFS disease-free survival
DOX doxorubicin
ECM extracellular matrix
ICD immunogenic cell death
INFγ interferon gamma
iNOS inducible nitric oxide synthase
NAC neoadjuvant chemotherapy
NK natural killer
OS overall survival
PTX paclitaxel
TAM tumor-associated macrophage
TME tumor microenvironment
TGF beta transforming growth factor beta
TLR toll-like receptor
TNBC triple-negative breast cancer
TNFα tumor necrosis factor alpha
VEGF vascular endothelial growth factor
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Funding
References
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi:10.1038/nri2216.
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi:10.1038/nrc3599.
- Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012 May;19(5):1508–1516. doi:10.1245/s10434-011-2108-2.
- Liu B, Ezeogu L, Zellmer L, Yu B, Xu N, Joshua Liao D. Protecting the normal in order to better kill the cancer. Cancer Med. 2015;4(9):1394–1403. doi:10.1002/cam4.488.
- Thomas F, Fisher D, Fort P, Marie JP, Daoust S, Roche B, Grunau C, Cosseau C, Mitta G, Baghdiguian S, et al. Applying ecological and evolutionary theory to cancer: a long and winding road. Evol Appl. 2013;6(1):1–10. doi:10.1111/eva.12021.
- Stakheyeva M, Riabov V, Mitrofanova I, Litviakov N, Choynzonov E, Cherdyntseva N, Kzhyshkowska J. Role of the immune component of tumor microenvironment in the efficiency of cancer treatment: perspectives for the personalized therapy. Curr Pharm Des. 2017;23(32):4807–4826. doi:10.2174/1381612823666170714161703.
- Baghdadi M, Wada H, Nakanishi S, Abe H, Han N, Putra WE, Endo D, Watari H, Sakuragi N, Hida Y, et al. Chemotherapy-induced IL-34 enhances immunosuppression by tumor-associated macrophages and mediates survival of chemoresistant lung cancer cells. J Clin Pathol. 2012;65:159–163. doi:10.1158/0008-5472.CAN-16-1170.
- Senthebane DA, Rowe A, Thomford NE, Shipanga H, Munro D, Mazeedi MAMA, Almazyadi HAM, Kallmeyer K, Dandara C, Pepper MS, et al. Role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. 2017;21;18(7):E1586. doi:10.3390/ijms18071586.
- Mozaffari F, Lindemalm C, Choudhury A, Granstam-Björneklett H, Helander I, Lekander M, Mikaelsson E, Nilsson B, Ojutkangas ML, Osterborg A, et al. NK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapy. Br J Cancer. 2007;97(1):105–111. doi:10.1038/sj.bjc.6603840.
- Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi:10.1038/cdd.2013.67.
- Vacchelli E, Aranda F, Eggermont A, Galon J, Sautès-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3(1):e27878. doi:10.4161/onci.27878.
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi:10.1016/j.immuni.2014.06.010.
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012 Mar;122(3):787–795. doi:10.1172/JCI59643.
- Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(2):195–205. doi:10.1111/j.1365-2249.2011.04515.x.
- Yang M, McKay D, Pollard JW, Lewis CE. Diverse functions of macrophages in different tumor microenvironments. Cancer Res. 2018 Oct 1;78(19):5492–5503. doi:10.1158/0008-5472.CAN-18-1367.
- Mitrofanova I, Zavyalova M, Telegina N, Buldakov M, Riabov V, Cherdyntseva N, Kzhyshkowska J. Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology. 2017 Jan;222(1):101–109. doi:10.1016/j.imbio.2016.08.001.
- Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42(3):161–170. doi:10.3109/07853890903405753.
- Krishnan V, Schaar B, Tallapragada S, Dorigo O. Tumor associated macrophages in gynecologic cancers. Gynecol Oncol. 2018;149(1):205–213. doi:10.1016/j.ygyno.2018.01.014.
- Gupta V, Yull F, Khabele D. Bipolar tumor-associated macrophages in ovarian cancer as targets for therapy. Cancers (Basel). 2018;10(10):1–13. doi:10.3390/cancers10100366.
- Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: A roadmap for multitargeting strategies. Oncogene. 2016;35(6):671–682. doi:10.1038/onc.2015.132.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi:10.1016/j.cell.2010.03.014.
- Bögels M, Braster R, Nijland PG, Gül N, van de Luijtgaarden W, Fijneman RJ, Meijer GA, Jimenez CR, Beelen RH, van Egmond M. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunol. 2012;1(6):798–809. doi:10.4161/onci.20427.
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–322. doi:10.1007/s10555-006-9001-7.
- Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5 MAR(March):1–13. doi:10.3389/fphys.2014.00075.
- Huang W, Chan M, Chen M, Tsai T. Modulation of macrophage polarization and lung cancer cell stemness by MUC1 and development of a related small-molecule inhibitor pterostilbene. Oncotarget. 2016;7(26):1;13. doi:10.18632/oncotarget.8101.
- Liu Z, Kuang W, Zhou Q, Zhang Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int J Mol Med. 2018;42(6):3395–3403. doi:10.3892/ijmm.2018.3923.
- Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi:10.1155/2012/948098.
- Chen JJW, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737.
- Zhang J, Yan Y, Yang Y, Wang L, Li M, Wang J, Liu X, Duan X, Wang J. High infiltration of tumor-associated macrophages influences poor prognosis in human gastric cancer patients, associates with the phenomenon of EMT. Med(United States). 2016;95(6):1–6. doi:10.1097/MD.0000000000002636.
- Makitie T, Summanen P, Tarkkanen A, Kivelä T. Tumor-infiltrating macrophages (CD68+ Cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421.
- Yuan ZY, Luo RZ, Peng RJ, Sen WS, Xue C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther. 2014;7:1475–1480. doi:10.2147/OTT.S61838.
- Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65(2):159–163. doi:10.1136/jclinpath-2011-200355.
- Yang J, Li X, Liu XP, Liu Y. The role of tumor-associated macrophages in breast carcinoma invasion and metastasis. Int J Clin Exp Pathol. 2015;8:6656–6664.
- Buldakov M, Zavyalova M, Krakhmal N, Telegina N, Vtorushin S, Mitrofanova I, Riabov V, Yin S, Song B, Cherdyntseva N, et al. CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer. Immunobiolog. 2017;222(1):31–38. doi:10.1016/j.imbio.2015.09.011.
- Forssell J, Öberg Å, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13(5):1472–1479. doi:10.1158/1078-0432.CCR-06-2073.
- Malesci A, Bianchi P, Celesti G, Basso G, Marchesi F, Grizzi F, Di Caro G, Cavalleri T, Rimassa L, Palmqvist R, et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology. 2017;6(12):1–11. doi:10.1080/2162402X.2017.1342918.
- Xu L, Zhu Y, Chen L, An H, Zhang W, Wang G, Lin Z, Xu J. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann Surg Oncol. 2014;21(9):3142–3150. doi:10.1245/s10434-014-3601-1.
- Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015 Oct;18(4):740–750. doi:10.1007/s10120-014-0422-7.
- Shu QH, Ge YS, Ma HX, Gao XQ, Pan JJ, Liu D, Xu GL, Ma JL, Jia WD. Prognostic value of polarized macrophages in patients with hepatocellular carcinoma after curative resection. J Cell Mol Med. 2016;20(6):1024–1035. doi:10.1111/jcmm.12787.
- Le Page C, Marineau A, Bonza PK, Rahimi K, Cyr L, Labouba I, Madore J, Delvoye N, Mes-Masson AM, Provencher DM, et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PLoS One. 2012;7:6. doi:10.1371/journal.pone.0038541.
- Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12(3):259–267. doi:10.7785/tcrt.2012.500312.
- Yafei Z, Jun G, Guolan G. Correlation between macrophage infiltration and prognosis of ovarian cancer-a preliminary study. Biomed Res. 2016;27:305–312.
- Chen X, Chen J, Zhang W, Sun R, Liu T, Zheng Y, Wu Y. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget. 2017;8(68):112685–112696. doi:10.18632/oncotarget.22340.
- Cheng Z, Zhang D, Gong B, Wang P, Liu F. CD163 as a novel target gene of STAT3 is a potential therapeutic target for gastric cancer. Oncotarget. 2010;8(50):87244–87262. doi:10.18632/oncotarget.20244.
- Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi:10.1186/1471-2407-12-306.
- Zhang WJ, Wang XH, Gao ST, Chen C, Xu XY, Sun Q, Zhou ZH, Wu GZ, Yu Q, Xu G, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res. 2018;222:93–101. doi:10.1016/j.jss.2017.09.035.
- Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I, Gratchev A, Llopis Verdiell M, Sticht C, Schmuttermaier C, et al. Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget. 2016;7:21. doi:10.18632/oncotarget.8857.
- Bi J, Lau SH, Lv ZL, Xie D, Li W, Lai YR, Lai YR, Zhong JM, Wu HQ, Su Q, et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum Pathol. 2009;40(12):1790–1797. doi:10.1016/j.humpath.2009.07.005.
- Thorn AP, Daugaard S, Christensen LH, Christensen IJ, Petersen MM. YKL-40 protein in osteosarcoma tumor tissue. Apmis. 2016;124(6):453–461. doi:10.1111/apm.2016.124.issue-6.
- Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46(3):333–340. doi:10.1016/j.lungcan.2004.05.010.
- Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95(2):267–274. doi:10.1002/cncr.10644.
- Liu T, Larionova I, Litviakov N, Riabov V, Zavyalova M, Tsyganov M, Buldakov M, Song B, Moganti K, Kazantseva P, et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. Oncoimmunology. 2018 Mar 13;7(6):e1436922. doi:10.1080/2162402X.2018.1436922.
- Litviakov N, Tsyganov M, Larionova I, Ibragimova M, Deryusheva I, Kazantseva P, Slonimskaya E, Frolova I, Choinzonov E, Cherdyntseva N, et al. Expression of M2 macrophage markers YKL-39 and CCL18 in breast cancer is associated with the effect of neoadjuvant chemotherapy. Cancer Chemother Pharmacol. 2018 Jul;82(1):99–109. doi:10.1007/s00280-018-3594-8.
- Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJ, Wong WC, Yang H, Schwarz H, Lim KH, et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012 Jan;42(1):89–100. doi:10.1002/eji.201141825.
- Aljabery F, Olsson H, Gimm O, Jahnson S, Shabo I. M2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancer. Urol Oncol Semin Orig Investig. 2018;36(4):159.e19-159.e26. doi:10.1016/j.urolonc.2017.11.020.
- David C, Nance JP, Hubbard J, Hsu M, Binder D, Wilson EH. Stabilin-1 expression in tumor associated macrophages. Brain Res. 2012;24(1481):71–78. doi:10.1016/j.brainres.2012.08.048.
- Larionova IV, Sevastyanova TN, Rakina AA, Cherdyntseva NV, Kzhyshkowska JG. Chitinase-like proteins as promising markers in cancer patients. Sib J Oncol. 2018;17(4):99–105. doi:10.21294/1814-4861-2018-17-4-99-105.
- Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem. 2016 Mar;397(3):231–247. doi:10.1515/hsz-2015-0269.
- Knorr T1, Obermayr F, Bartnik E, Zien A, Aigner T. YKL-39 (chitinase 3-like protein 2), but not YKL-40 (chitinase 3-like protein 1), is up regulated in osteoarthritic chondrocytes. Ann Rheum Dis. 2003 Oct;62(10):995–998. doi:10.1136/ard.62.10.995.
- Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, Tonin PN, Provencher DM, Mes-Masson A-M. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110(2):244–254. doi:10.1002/cncr.22789.
- Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38(4):283–290. doi:10.1016/j.ejps.2009.08.009.
- De Palma M, Lewis CE. Macrophages limit chemotherapy. Cancer Discov. 2011;1(1):54–67. doi:10.1038/472303a.
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi:10.1158/1078-0432.CCR-10-0733.
- Chauhan P, Sodhi A, Shrivastava A. Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology. 2009;214(3):197–209. doi:10.1016/j.imbio.2008.07.012.
- Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LTC, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, Sh VDB. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73(8):2480–2492. doi:10.1158/0008-5472.CAN-12-3542.
- Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111(6):752–759. doi:10.1002/jso.23881.
- Bryniarski K, Szczepanik M, Ptak M, Zemelka M, Ptak W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharmacol Rep. 2009;61(3):550–557. doi:10.1016/S1734-1140(09)70098-2.
- Pallasch CP, Leskov I, Braun CJ, Vorholt D, Drake A, Soto-Feliciano YM, Bent EH, Schwamb J, Iliopoulou B, Kutsch N, et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell. 2014;156(3):590–602. doi:10.1016/j.cell.2013.12.041.
- Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, Rakhmilevich AL. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132(2):226–239. doi:10.1111/j.1365-2567.2010.03357.
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4 +CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi:10.1007/s00262-006-0225-8.
- Liu P, Jaffar J, Hellstrom I, Hellstrom KE. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J Immunother. 2010;33(1):53–59. doi:10.1097/CJI.0b013e3181b56af4.
- Kroemer G, Galluzzi L, Zitvogel L. Immunological effects of chemotherapy in spontaneous breast cancers. Oncoimmunology. 2013;2(12):41–44. doi:10.4161/onci.27158.
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741. doi:10.1016/j.immuni.2013.03.003.
- Laoui D, Van Overmeire E, Van Ginderachter JA. Unsuspected allies: chemotherapy teams up with immunity to fight cancer. Eur J Immunol. 2013;43(10):2538–2542. doi:10.1002/eji.201344042.
- Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Ewald AJ, Fein M, Rasch MG, Tan YX, et al. Imaging Tumor-Stroma Interactions during Chemotherapy Reveals Contributions of the Microenvironment to Resistance. Cancer Cell. 2012;21(4):488–503. doi:10.1016/j.ccr.2012.02.017.
- Wang B, Xu D, Yu X, Ding T, Rao H, Zhan Y, Zheng L, Li L. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol. 2011;18(9):2585–2593. doi:10.1245/s10434-011-1609-3.
- Di Caro G, Cortese N, Castino GF, Grizzi F, Gavazzi F, Ridolfi C, Capretti G, Mineri R, Todoric J, Zerbi A, et al. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2015;65(10):1710–1720. doi:10.1136/gutjnl-2015-309193.
- Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi:10.1016/j.ccr.2013.01.008.
- Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):10430. doi:10.1097/FPC.0b013e32833ffb56.
- Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi:10.1091/mbc.E14-04-0916.
- Basu A, Krishnamurthy S. Cellular responses to cisplatin-induced DNA damage. J Nucleic Acids. 2010;2010. doi:10.4061/2010/201367.
- Miura K, Kinouchi M, Ishida K, Fujibuchi W, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, et al. 5-FU metabolism in cancer and orally-administrable 5-FU drugs. Cancers (Basel). 2010;2(3):1717–1730. doi:10.3390/cancers2031717.
- Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Futur Oncol. 2005;1(1):7–17. doi:10.1517/14796694.1.1.7.
- D’Incalci M, Badri N, Galmarini CM, Allavena P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br J Cancer. 2014;111(4):646–650. doi:10.1038/bjc.2014.149.
- Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75(17):3479–3491. doi:10.1158/0008-5472.CAN-14-3587.
- Harrison DJ. Molecular mechanisms of drug resistance in tumours. J Pathol. 1995;175(1):7–12. doi:10.1002/path.1711750103.
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002 Jan 1;2:48. doi:10.1038/nrc706.
- Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi:10.1038/nature10762.
- Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212(4):435–445. doi:10.1084/jem.20150295.
- Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25(23):2465–2479. doi:10.1101/gad.180331.111.
- Castrellon AB, Pidhorecky I, Valero V, Raez LE. The role of carboplatin in the neoadjuvant chemotherapy treatment of triple negative breast cancer. Oncol Rev. 2017;11(1):7–12. doi:10.4081/oncol.2017.324.
- Su Y-W, Hung C-Y, Lam H-B, Chang Y-C, Yang P-S. A single institution experience of incorporation of cisplatin into adjuvant chemotherapy for patients with triple-negative breast cancer of unknown BRCA mutation status. Clin Med Insights Oncol. 2018;12. doi:10.1177/1179554918794672.
- Singh RAK, Sodhi A. Antigen presentation by cisplatin-activated macrophages: role of soluble factor(s) and second messengers. Immunol Cell Biol. 1998;76(6):513–519. doi:10.1046/j.1440-1711.1998.00769.
- Chauhan P, Sodhi A, Tarang S. Cisplatin-treated murine peritoneal macrophages induce apoptosis in L929 cells: role of Fas-Fas ligand and tumor necrosis factor-tumor necrosis factor receptor 1. Anticancer Drugs. 2007;18(2):187–196. doi:10.1097/CAD.0b013e3280104b11.
- Owen W, Thurs K. Basal cell carcinoma presenting as a nonhealing wound case report. Adv Skin Wound Care. 2009;August:353–355. doi:10.1097/01.ASW.0000358640.76210.49.
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016 Mar 15;44(3):450–462. 2016;15(5):477–91. doi:10.1016/j.immuni.2016.02.015.
- Cao Q, Yan X, Chen K, Huang Q, Melancon MP, Lopez G, Cheng Z, Li C. Macrophages as a potential tumor-microenvironment target for noninvasive imaging of early response to anticancer therapy. Biomaterials. 2018;152:63–76. doi:10.1016/j.biomaterials.2017.10.036.
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: A magic bullet?. Science. 2013 Jan 18;339(6117):286–291. doi:10.1126/science.1232227.
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci. 2011;108(30):12425–12430. doi:10.1073/pnas.1106645108.
- Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):1–13. doi:10.1186/s13046-017-0528-y.
- Weizman N, Krelin Y, Shabtay-Orbach A, Amit M, Binenbaum Y, Wong RJ, Gil Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33(29):3812–3819. doi:10.1038/onc.2013.357.
- Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120(10):3421–3431. doi:10.1172/JCI42918.
- Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004;3(12):1516–1519. doi:10.4161/cc.3.12.1289.
- Podgorski I, Sloane BF. Cathepsin B and its role(s) in cancer progression. Biochem Soc Symp. 2003;70:263–276. doi:10.1042/bss0700263.
- Vasiljeva O, Papazoglou A, Krüger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66(10):5242–5250. doi:10.1158/0008-5472.CAN-05-4463.
- Sevenich L, Schurigt U, Sachse K, Gajda M, Werner F, Muller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J, et al. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc Natl Acad Sci. 2010;107(6):2497–2502. doi:10.1073/pnas.0907240107.
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472. doi:10.1016/j.ccell.2015.02.015.
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi:10.1158/2159-8274.CD-10-0028.
- Breedveld FC. Therapeutic monoclonal antibodies. Lancet. 2000;355:735–740.
- Weir GM, Hrytsenko O, Quinton T, Berinstein NL, Stanford MM, Mansour M. Anti-PD-1 increases the clonality and activity of tumor infiltrating antigen specific T cells induced by a potent immune therapy consisting of vaccine and metronomic cyclophosphamide. J Immunother Cancer. 2016;4(1):1–13. doi:10.1186/s40425-016-0169-2.
- Soares KC, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Wamwea A, Bigelow E, Lutz E, Liu L, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2014;00(00):1–11. doi:10.1097/CJI.0000000000000062.
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi:10.1084/jem.20050930.
- Aharinejad S, Schäfer R, Paulus P, Sioud M, Hofmann M, Zins K, Stanley ER, Abraham D. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res. 2004;64(15):5378–5384. doi:10.1158/0008-5472.CAN-04-0961.
- Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66(8):4349–4356. doi:10.1158/0008-5472.CAN-05-3523.
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. doi:10.1158/0008-5472.CAN-06-1823.
- Abraham D, Zins K, Sioud M, Lucas T, Schäfer R, Stanley ER, Aharinejad S. Stromal cell-derived CSF-1 blockade prolongs xenograft survival of CSF-1-negative neuroblastoma. Int J Cancer. 2010;126(6):1339–1352. doi:10.1002/ijc.24859.
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. doi:10.1158/0008-5472.CAN-12-2731.
- Linde N, Lederle W, Depner S, Van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227(1):17–28. doi:10.1002/path.3989.
- Alishekevitz D, Gingis-Velitski S, Kaidar-Person O, Gutter-Kapon L, Scherer SD, Raviv Z, Merquiol E, Ben-Nun Y, Miller V, Rachman-Tzemah C, et al. Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep. 2016;17(5):1344–1356. doi:10.1016/j.celrep.2016.09.083.
- Duhamel M, Rose M, Rodet F, Murgoci A-N, Zografidou L, Régnier-Vigouroux A, Abeele FV, Kobeissy F, Nataf S, Pays L, et al. Paclitaxel treatment and PC1/3 knockdown in macrophages is a promising anti-glioma strategy as revealed by proteomics and cytotoxicity studies. Mol Cell Proteomics. 2018;mcp.RA117.000443. doi:10.1074/mcp. RA117.000443.
- Heindryckx F, Gerwins P. Targeting the tumor stroma in hepatocellular carcinoma. World J Hepatol. 2015;7(2):165–176. doi:10.4254/wjh.v7.i2.165.
