ABSTRACT
Hepatocellular Carcinoma (HCC) is one of the leading causes of cancer-related mortality worldwide. Current systemic therapies result only in modest benefits and new therapeutic options are critically needed. Some patients show promising clinical responses to immune checkpoint inhibitors, however, additional immunotherapeutic approaches, such as adoptive cell therapies (ACT), need to be developed. This review summarizes recent ACT studies and discusses the promise and obstacles of this approach. We further discuss ways of improving the efficacy of ACT in HCC including the use of combination therapies and locoregional delivery methods.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer type and the third leading cause of cancer-related death worldwide. In the United States, the age-adjusted incidence of liver cancer has steadily increased over the last several decades and this year more than 42,000 new cases are expected in the United States alone.Citation1 This recent increase has been largely attributed to the spread of hepatitis C infection in this country, however increasing rates of metabolic syndrome and nonalcoholic fatty liver disease (NAFLD) are also suspected to be major contributory factors.Citation2
In recent years, important advances have been made in the detection and management of HCC. The treatment of HCC is dictated by the size of the tumor, the underlying performance status of the patient (Child-Pugh), and the eligibility for transplant according to the Milan criteria, or more recently, the UCSF or Barcelona Clinic Liver Cancer (BCLC) criteria.Citation3 Patients with good underlying liver function (Child-Pugh A-B) and early stage disease defined as single tumors (up to 5cm) or small, multinodular tumors (<3 up to 2cm) can be cured through liver transplant and/or tumor resection/ablation. In the setting of intermediate stage disease, patients typically present with large and/or polynodular tumors that are less amenable to the curative treatments. In these patients, the intra-arterial approaches such as Transarterial Embolization (TAE), Transarterial Radioembolization (TARE) and Transarterial Chemoembolization (TACE) represent first line treatments according to most guidelines, although some patient subpopulations may benefit initially from hepatic resection or radiation therapy.Citation4 In patients with advanced disease and poor underlying liver function (Child-Pugh B-C), treatment options are limited and prognosis is poor with 5-year survival rates as low as 2%.Citation1 Currently, systemic therapy with sorafenib represents the mainstay of treatment for these patients, but it only extends median survival by 2–3 months obviating the need for additional therapeutic options.Citation5,Citation6 Building on the successes of sorafenib, newer multi-kinase inhibitors including lenvatinib, regorafenib, cabozantinib, and ramucirumab have recently been tested as first line and second line agents. While these medications have led to modest survival benefits, they are not the therapeutic breakthrough many hoped for.Citation7 To this end, novel strategies are currently being investigated to improve survival in patients with this devastating cancer. In recent years, immunotherapy including checkpoint inhibition and adoptive cell transfer (ACT) have emerged as treatment options for a variety of different solid tumors, however, no standard immunotherapeutic approach has been defined for HCC.Citation8 This review will cover some future immunotherapeutic options for patients with HCC with an emphasis on ACT and methodologies for improving its efficacy in solid tumors.
The immune microenvironment of HCC
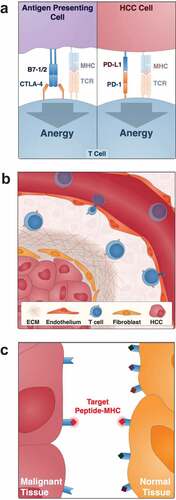
It has become increasingly recognized that tumors are not just a collection of malignant cells, but instead a complex mixture of enzymes, growth factors, signaling factors, and immune cells which together modulate tumor development and progression.Citation8 The microenvironment of HCC is thought to be highly immunosuppressive resulting in the production of a generally weak and inefficient anti-tumor immune response. As with many solid tumors, this is in part due to the high metabolic status of HCC. Rapid cellular growth and division creates a hypoxic, acidic, and nutrient-poor microenvironment which restricts T cell proliferation and cytokine production.Citation9 More specific to HCC, by virtue of the fact that the liver is continuously exposed to antigens from the gut microbiota, the organ itself has developed a tolerance to immune activation. This immuno-tolerance is imparted by a high concentration of immunosuppressive T-regulatory cells (Tregs) relative to effector T cells.Citation10 The role of a Treg is to dampen the local immune system directly and indirectly by increasing the content of inhibitory cytokines like TGF-B, IL-4, and IL-10 as well as other inhibitory signals like CTLA-4 and PD-1 (), which attenuate the proliferation of activated T-cells.Citation11 Furthermore, since the majority of HCCs develop in chronically inflamed and fibrotic livers secondary to viral hepatitis, alcohol abuse, and metabolic disease, these malignancies evade the immune system simply by undermining homing of immune cells into the tumor tissue (). The few immune effector cells that still arrive in the immediate tumor environment will face a dramatic downregulation of human leukocyte antigen (HLA) molecules presenting peptide fragments of tumor antigens () and the co-stimulatory molecules CD80 and CD86.Citation12 However, as our understanding of the tumor microenvironment has evolved, approaches to modulate the local immune system to counter the immunosuppressive factors in HCC have emerged.
Figure 1. Obstacles to the use of ACT in HCC
Spontaneous anti-tumor immune responses in HCC
The immune contexture of HCC includes a wide variety of cells that are necessary for innate and adaptive immune system activation. This includes amongst many others, the liver sinusoidal endothelial cells (LSECs) which function as APCs, CD8+ cytotoxic T lymphocytes (CTLs), and CD4+ T helper cells. Despite the aforementioned challenges, spontaneous and durable immune responses do occur in HCC and have been associated with improved survival and recurrence outcomes after surgical resection.Citation13,Citation14 Such immune responses are predominantly CD8+ T cell-driven and are directed against well-established tumor associated antigens (TAA) such as alpha-fetoprotein (AFP) and glypican-3 (GPC3).Citation15 These data suggest that all of the essential elements for local T cell activation are present in principle in HCC and that the immune system can hopefully be leveraged to attack the tumor.
Immunotherapies targeting HCC
Recent research efforts in cancer treatment have been geared toward mobilizing the patient’s endogenous immune system to attack cancer cells. Immune checkpoint inhibitors (CKI), for example, are an antibody-based immunotherapy that effectively unleashes the immune system from a suppressed state by blocking cancer-mediated repressors of the immune system such as CTLA-4 and PD-1.Citation16 So far checkpoint inhibition has produced mixed results in advanced HCC disease.Citation17 In one of the first published trials (checkpoint 040), 47 patients with sorafenib-resistant HCC including 33 with extrahepatic metastasis and 6 with vascular invasion were treated with the anti-PD1 monoclonal antibody nivolumab. The response rate and disease control rates (DCR) were favorable with 19% of patients demonstrating disease regression and 67% of patients showing stable disease.Citation18 These data led to the accelerated FDA approval of nivolumab for HCC patients who failed sorafenib and served as the impetus for numerous subsequent trials exploring the expanded clinical use of nivolumab and other checkpoint inhibitors both as first-line monotherapy and as combination therapies in HCC.Citation19,Citation20 Unfortunately, however, new unpublished data from these extension studies including the checkpoint 459 trial, which looked at nivolumab against sorafenib as a first line treatment, and the Keynote-240 trial, which investigated pembrolizumab against placebo as a second line treatment, both showed no statistically significant survival benefit leaving the door open for other immunotherapeutic approaches.Citation21,Citation22
Adoptive cell transfer (ACT), the focus of this review, encompasses cell-based immunotherapies that target cytotoxic T-cells to tumor cells using tumor-specific antigens.Citation23 Included in this class of immunotherapies are the tumor-infiltrating lymphocytes (TILs), T cell receptor (TCR)-engineered T cells, and chimeric antigen receptor (CAR) T cells (). Of these subtypes, TILs were the earliest development whereby a sub-population of endogenously produced tumor-specific T cells from the patient’s blood or, preferentially, tumor tissue were enriched. Following in vitro expansion, the anti-tumor T-cells were then transfused into the host to target and destroy tumor cells. Although the results from this approach have been promising in a few specific cancers including one study on HCC,Citation24 the application of TILs is limited by the natural T cell repertoire of the host and technical difficulties in isolating and expanding these cells on an individual basis.Citation25 In light of these limitations, a second iteration of ACT came with the development of TCR-engineered T cells. Here, using genome engineering techniques, TCRs targeting specific tumor antigens are transduced into patient- or donor-derived T cells before expansion and transfusion. While this development has demonstrated considerable promise in pre-clinical studies () targeting GPC3Citation26 and AFP,Citation27,Citation28 these cells are dependent on the antigens being presented in an HLA context, the expression of which, as discussed above, is downregulated in many cancers.Citation29 This downregulation may explain the unfortunate failure of HBV-TCR T-cell therapy as demonstrated in one patient with an extrahepatic HCC metastasis.Citation30
Table 1. Clinical trials investigating systemic TCR/CAR T-cell delivery in HCC
Figure 2. Production and optimization of TCR-engineered T cells, cytokine-induced killer cells, and CAR T cells
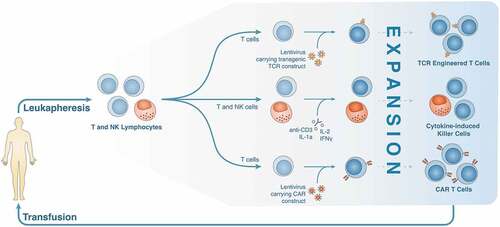
To circumvent the HLA requirement, a third iteration of ACT came with the development of chimeric antigen receptor (CAR) T cells. In this technique, chimeric T cell receptors composed of an extracellular single chain variable fragment (scFv) antigen-specific target binding domain, a costimulatory transmembrane domain, and a CD3ζ-derived intracellular signaling and activation domain are artificially expressed in T cells. This approach in principle enables the same limitless targeting potential as TCR-engineered T cells without the dependence on HLA/TCR interactions.Citation31 CAR T cell therapy has proven effective in a variety of hematologic cancers, most significantly in the treatment of CD-19 expressing B cell acute lymphoblastic leukemia and B cell lymphomas.Citation32 The use of CAR T cell therapy in solid tumors including HCC, however, has been stalled by several obstacles that will be outlined below.Citation33
Obstacles of ACT in HCC
The widespread use of ACT in solid tumor cancer treatment has been hampered by four major issues which together affect their in vivo efficacy and tolerability. This includes: (1) the lack of well-defined solid-tumor associated antigens (TAA) and, in the case of TCR-transduced T cells, the loss of HLA molecules on the tumor cells, (2) the limited ability of exogenous T cells to traffic to the organ of interest and penetrate the fibrotic tumor stroma, (3) the intrinsically immunosuppressive tumor microenvironment and (4) off-target effects and toxicity (–). Here, we review the evidence behind these limitations and highlight methodologies for overcoming these challenges to work toward the goal of expanding ACT to HCC.
Targeting the tumor: finding better antigens
A major barrier concerning the use of ACT in solid tumor immunotherapy is the identification of tumor-associated antigens (TAA). A preferable TAA is one that is universally highly expressed in tumor cells and negligibly expressed in normal tissues.Citation23 Unlike hematologic cancers, solid tumors often lack the expression of specific surface molecules necessary for CAR T cells. However, members of a unique class of intracellularly expressed proteins, cancer-testis (CT) antigens or cancer-germline (CG) antigens, have recently emerged as preferable immunotherapeutic targets in a number of solid tumor cancers, including HCC. More than 200 genes at present make up the CT antigen gene family. CT gene expression is normally restricted to male germ cells and early embryonic development, however, many CT genes are aberrantly expressed in human cancers including HCC.Citation34 In recent years, CT antigen gene function has been extensively studied with the hypothesis that embryonic or gametogenic transcriptional programs, which includes the CT genes, are reactivated in cancers and serve as a driving force for tumorigenesis. Through this work, we have learned that a number of CT antigens including MAGE-A, MAGE-C2, SSX, and PRAME withhold potent oncogenic properties by conferring resistance to apoptosis, invasive/metastatic potential, and uncontrolled growth.Citation34–Citation36
In addition to being cancer-specific, CT antigens are immunogenic meaning they are capable of evoking a robust spontaneous and vaccine-induced immune response. Several recent trials using CT antigens as immuno-targets have provided encouraging results. To this end, Odunsi et al. showed that treating advanced stage melanoma and ovarian carcinoma with an NY-ESO-1 vaccine induces a CD8+ T cell response which correlates with progression free survival.Citation37 Likewise, using an NY-ESO-1 directed TCR-based therapy, multiple independent groups have succeeded in inducing durable anti-tumor responses and tumor regression in patients with metastatic melanoma and synovial sarcoma.Citation38,Citation39 Most recently, Stevanovic et al. induced complete cancer regression in a patient with metastatic HPV-positive cervical carcinoma using TILs immunodominant for the CT antigen KK-LC-1.Citation40
Although the expression and specificity of many different CT antigens have been confirmed in HCC, the targets with highest priority currently are NY-ESO-1 and MAGE-A1.Citation41 NY-ESO-1 and MAGE-A1 are expressed in approximately 45% and 70% of HCC tumors, respectively, and have been associated with HCC tumor grade, metastasis, and recurrence post resection.Citation42,Citation43 As with GPC3 and AFP, mentioned above, spontaneous immune responses against NY-ESO-1 and MAGE-A1 have also been described in a subset of HCC patients with advanced disease. Importantly, in these patients, the presence of naturally occurring CTA-specific CD8+ cytotoxic T cells is associated with improved survival outcomes.Citation14 In line with these observations, multiple clinical trials are now underway targeting CTAs in HCC using TILs, TCR engineered T cells, and even CAR T cells ().
Improving T cell trafficking and tissue penetration: local delivery of cellular immunotherapies
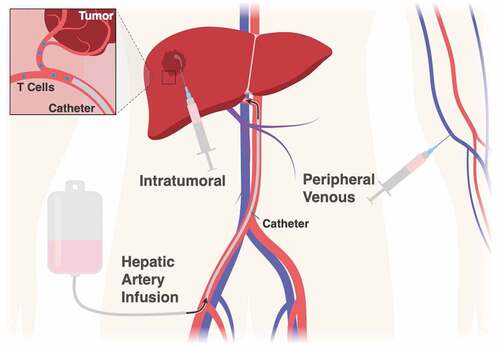
Another factor regulating the efficacy of ACT transfer in HCC and other solid tumor malignancies is the relative inability of systemically infused T cells to migrate to and infiltrate the tumor stroma.Citation23 In part, the poor trafficking of adoptive T cells may be linked to a chemokine and chemokine-receptor mismatch between the tumor and the engineered cells. Innovative strategies, including the use of CAR-T cells co-expressing CCR2, the receptor for a functional tumor trafficking chemokine, have been developed but have led to only modest increases in anti-tumor activity indicating multiple additional barriers to successful trafficking.Citation44 One consideration is that many HCC tumors develop in the setting of cirrhosis () which could make it difficult for systemically delivered T-cells to physically infiltrate the tumor and generate an efficacious response.Citation45 The extent to which this is true needs to be investigated as does the safety of this cytotoxic anti-tumor treatment in such a compromised organ.
With on-going advancements in imaging technology and intra-vascular techniques, a promising new strategy involves delivering ACT immunotherapy loco-regionally, either directly into the organ of interest or intratumorally if the surrounding fibrosis proves too tough for the cells to penetrate on their own (). Not only would such an approach mitigate the trafficking issue, it could also decrease the well-known off-target and maybe even off-tissue toxicities associated with systemic infusion of manipulated T cells. Using a mouse model of human pleural malignancy, Adusumilli et al. demonstrated that intrapleural delivery of anti-mesothelin CAR-T cells outperformed intravenously delivered CAR T cells, which were dosed 30-fold higher, with respect to the magnitude and durability of tumor regression.Citation46 More recently, a pre-clinical mouse study of HER2+ breast cancer metastatic to the brain showed that intra-cranial delivery of anti-HER2 CAR T cells facilitated complete tumor regression whereas systemic delivery, even with higher doses of CAR-T cells, resulted in only partial tumor regression.Citation47 The first human trial investigating loco-regional delivery of immunotherapy to the liver was performed in metastatic colorectal cancer patients. In this study, anti-CEA CAR T cells were delivered directly to the liver through hepatic artery infusion (HAI).Citation48 In one patient, the CAR T cells were undetectable in the peripheral blood three days after delivery but they persisted in the liver for up to three months.Citation49 Clinically in these patients, localized and sustained CAR-T cell targeting coincided with increased tumor necrosis and fewer off-target effects compared to prior experiments using systemic infusion.Citation50 Based on these studies, multiple clinical trials are now underway looking at the efficacy of local T-cell delivery in primary and metastatic liver cancers ().
Table 2. Clinical trials investigating liver directed ACT
Combined ACT approaches to circumvent local immunosuppression
In theory, even if tumor-specific T cells can be effectively delivered to the liver, the immunosuppressive microenvironment intrinsic to HCC must simultaneously be overcome. One strategy for doing so could be to strategically combine ACT with other systemic or locoregional treatments such as the tyrosine kinase inhibitors (e.g. sunitinib, sorafenib) or CKI (e.g. nivolumab, pembrolizumab, tremelimumab). Interestingly, in addition to its tumoricidal effects, sunitinib- but not sorafenib- has been shown to produce a strong immunomodulatory effect and suppress HCC progression when combined with immunotherapy.Citation51 A CKI combination would likely also enhance the local immune response thus improving T cell effector function, survival, and proliferation within a tumor. It is important to point out however that these combinations must be tailored to specific populations of HCC patient as there are marked differences in the endogenous immune response mounted in patients with HCC cancers caused by nonalcoholic steatohepatitis (NASH) compared to those caused by chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. For example, the CD8+ T cells in NASH-related HCC predominantly express CTLA4 and thus would most likely benefit from combinations with ipilimumab whereas those in HBV-related cancers which express PD-1 would perhaps benefit more from nivolumab.Citation52 Another consideration is the mutation profile of the HCC which likely affects its immunogenicity. Tumors carrying the CTNNB1 mutation for example are poorly infiltrated by immune cells and tend to be resistant to CKI whereas tumors containing a TP53 mutation, which is associated with a high tumor mutational burden, are more likely to respond.Citation53,Citation54 How the molecular features of a cancer affect the efficacy of ACT, if at all, remains to be studied. In addition to selecting specific checkpoint inhibitors based on the molecular and immune profile of the tumor, it is also conceivable that one could administer checkpoint treatment intratumorally to avoid the side-effects of systemic treatment. Along these lines, multiple clinical trials are currently open investigating the efficacy and toxicity of intratumoral ipilimumab in head and neck cancer (NCT02812524), glioblastoma (NCT03233152), colorectal cancer (NCT03982121), and melanoma (NCT02857569). Alternative approaches to combine ACT with checkpoint inhibition involve using CAR-T cells containing a genetically engineered mutant PD-L1 receptor, potentially preventing tumor-induced downregulation of effector T cell function. So far, these studies have demonstrated comparable efficacy while avoiding the common toxicities associated with checkpoint inhibition such as fatigue, rash, and diarrhea.Citation55,Citation56
Another strategy for enhancing the efficacy of CAR T-cells in vivo is to combine their delivery with tumor ablation or ionization radiation (IR) administered through external beam techniques (e.g. stereotactic body radiation therapy, SBRT) or with brachytherapy (i.e. Y90 radioembolization). Both ablation and IR affect the tumor microenvironment and the immune system in numerous ways. In addition to directly damaging tumor cell DNA, these treatments likely help provoke a productive immune response which may boost CAR-T cell function. In HCC, radiofrequency ablation (RFA) has been shown to increase the number of tumor antigen specific T-cells leading to improvements in recurrence-free survival.Citation57,Citation58 Likewise, in a model of pancreatic cancer it was recently demonstrated that IR increases the susceptibility of antigen-negative tumor cells to CAR T cell therapy reducing the risk of tumor relapse.Citation59 One plausible explanation for this effect is that IR promotes antigen availability and presentation in otherwise antigen-negative tumor cells. A second and non-mutually exclusive explanation is that IR simply promotes a pro-inflammatory tumor microenvironment through tumor-intrinsic production of Type 1 Interferon (IFN) leading to the activation of M1 macrophages that drive tumor rejection.Citation60,Citation61 This mechanism is thought to account for the rare anti-tumor response that occurs at distant disease sites after radiation therapy known as the abscopal effect.Citation62
As IR can exacerbate underlying liver disease, alternative strategies for increasing antigen availability within the HCC tumor microenvironment should also be explored including local ablation and epigenetic inhibitors. One recently published study examined the use of epigenetic therapy consisting of small molecule inhibitors against DNMT1 and EZH2 in combination with CKI. Here, the authors were able to show that epigenetic therapy induces tumor cell expression of neoantigens like NY-ESO1 and stimulates better T cell trafficking and tumor cell apoptosis which together enhanced tumor regression compared to immunotherapy alone.Citation63 Perhaps the use of these inhibitors can be combined with ACT to improve trafficking and prevent antigen escape.
Conclusions and perspectives
The incidence of HCC continues to rise with limited treatment options currently available for many patients. Recent breakthroughs in cancer immunotherapy have led to renewed hope and enthusiasm for improved HCC treatments. Clinical responses have been achieved in a subset of patients with the use of immune checkpoint inhibitors. Clinical studies are already exploring the use of ACT such as CAR T cells and TCR-transduced T Cells with some promising preliminary findings. In addition, combination immunotherapies are emerging as further strategies to further improve outcomes, particularly in the setting of an immunosuppressive microenvironment. Further studies to evaluate timing and locoregional delivery approaches of immunotherapies are necessary to identify the ideal approaches to improve treatment response and simultaneously reduce systemic toxicities.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PGH, DA and GCF reviewed the literature and wrote the manuscript. MLO wrote the manuscript and prepared the figures. TL and TH wrote the manuscript. SW wrote the manuscript and prepared the tables.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–8.
- Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, McGlynn KA. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–1765.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Sun J-Y, Yin T, Zhang X-Y, Lu X-J. Therapeutic advances for patients with intermediate hepatocellular carcinoma. J Cell Physiol. 2019;234:12116–12121.
- Copur MS. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498–authorreply2498–9.
- Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang T-S, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
- Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616.
- Menon S, Shin S, Dy G. Advances in cancer immunotherapy in solid tumors. Cancers (Basel). 2016;8:106.
- D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 2018;9:282.
- Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–218.
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700.
- Kim HY, Park J-W. Current immunotherapeutic strategies in hepatocellular carcinoma: recent advances and future directions. Therap Adv Gastroenterol. 2017;10:805–814.
- Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414.
- Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426.
- Xu Y, Li H, Gao RL, Adeyemo O, Itkin M, Kaplan DE. Expansion of interferon-gamma-producing multifunctional CD4+ T-cells and dysfunctional CD8+ T-cells by glypican-3 peptide library in hepatocellular carcinoma patients. Clin Immunol. 2011;139:302–313.
- Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. LIC. 2017;6:1–12.
- Heinrich B, Czauderna C, Marquardt JU. Immunotherapy of Hepatocellular Carcinoma. ORT. 2018;41:292–297.
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim T-Y, Choo S-P, Trojan J, Welling TH, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502.
- Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24:S3–S10.
- Harding JJ. Immune checkpoint blockade in advanced hepatocellular carcinoma: an update and critical review of ongoing clinical trials. Future Oncol. 2018;35:4001.
- Bristol-Myers Squibb Announces Results from CheckMate. 459 study evaluating opdivo (nivolumab) as a first-line treatment for patients with unresectable hepatocellular carcinoma. 2019 June 24. [accessed 2019 Sep 20]. https://news.bms.com/press-release/bmy/bristol-myers-squibb-announces-results-checkmate-459-study-evaluating-opdivo-nivol
- Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim H-Y, Breder VV, Edeline J, Chao Y, Ogasawara S, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;37:4004.
- Schubert M-L, Hoffmann J-M, Dreger P, Müller-Tidow C, Schmitt M. Chimeric antigen receptor transduced T cells: tuning up for the next generation. Int J Cancer. 2018;142:1738–1747.
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. The Lancet. 2000;356:802–807.
- Ma W, Wu L, Zhou F, Hong Z, Liu Z, Yuan Y. T cell–associated immunotherapy for hepatocellular carcinoma. CPB. 2017;41:609–622.
- Dargel C, Bassani-Sternberg M, Hasreiter J, Zani F, Bockmann J-H, Thiele F, Bohne F, Wisskirchen K, Wilde S, Sprinzl MF, et al. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells In vitro and in mice. Gastroenterology. 2015;149:1042–1052.
- Zhu W, Peng Y, Wang L, Hong Y, Jiang X, Li Q, Liu H, Huang L, Wu J, Celis E, et al. Identification of α-fetoprotein-specific T cell receptors for hepatocellular carcinoma immunotherapy. Hepatology. 2018;68:574–589.
- Sun L, Guo H, Jiang R, Lu L, Liu T, He X. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumor Biol. 2015;37:799–806.
- Li H, Zhao Y. Increasing the safety and efficacy of chimeric antigen receptor T cell therapy. Protein Cell. 2017;8:573–589.
- Bertoletti A, Brunetto M, Maini MK, Bonino F, Qasim W, Stauss H. T cell receptor-therapy in HBV-related hepatocellularcarcinoma. Oncoimmunology. 2015;4:e1008354.
- Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. 2016;37:220–230.
- Yeku O, Li X, Brentjens RJ. Adoptive T-cell therapy for solid tumors. Am Soc Clin Oncol Educ Book. 2017;37:193–204.
- Yong CSM, Dardalhon V, Devaud C, Taylor N, Darcy PK, Kershaw MH. CAR T‐cell therapy of solid tumors. Immunol Cell Biol. 2017;95:356–363.
- Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6:15772–15787.
- Yang F, Zhou X, Miao X, Zhang T, Hang X, Tie R, Liu N, Tian F, Wang F, Yuan J. MAGEC2, an epithelial-mesenchymal transition inducer, is associated with breast cancer metastasis. Breast Cancer Res Treat. 2014;145:23–32.
- Song X, Hao J, Wang J, Guo C, Wang Y, He Q, Tang H, Qin X, Li Y, Zhang Y, et al. The cancer/testis antigen MAGEC2 promotes amoeboid invasion of tumor cells by enhancing STAT3 signaling. Oncogene. 2017;36(11):1476.
- Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA. 2012;109:5797–5802.
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924.
- Robbins PF, Kassim SH, Tran TLN, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027.
- Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200–205.
- Sideras K, Bots SJ, Biermann K, Sprengers D, Polak WG, IJzermans JNM, de Man RA, Pan Q, Sleijfer S, Bruno MJ, et al. Tumour antigen expression in hepatocellular carcinoma in a low-endemic western area. Br J Cancer. 2015;112:1911–1920.
- Li K, Y L, Wang J, Liu L. Chimeric antigen receptor–engineered T cells for liver cancers, progress and obstacles. Tumor Biol. 2017;39:1010428317692229.
- Seifi-Alan M, Shamsi R, Ghafouri-Fard S. Application of cancer-testis antigens in immunotherapy of hepatocellular carcinoma. Immunotherapy. 2018;10:411–421.
- Moon EK, Carpenito C, Sun J, Wang L-CS, Kapoor V, Predina JD, Powell DJ, Riley J, June CH, Albelda SM. Expression of a functional CCR2 receptor enhances tumor localization and eradication by human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17(14):4719–4730. clincanres.0351.2011
- Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740.
- Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151.
- Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, Chang W-C, Ostberg JR, Neman J, Jandial R, et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin Cancer Res. 2018;24:95–105.
- Katz SC, Burga RA, McCormack E, Wang LJ, Mooring JW, Point G, Khare PD, Thorn M, Ma Q, Stainken BF, et al. Phase I Hepatic Immunotherapy for Metastases study of intra-arterial chimeric antigen receptor modified T cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21(14):3149–3159. clincanres.1421.2014.
- Sridhar P, Petrocca F. Regional delivery of chimeric antigen receptor (CAR) T-cells for cancer therapy. Cancers (Basel). 2017;9:92.
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626.
- Liu D, Qi X, Manjunath Y, Kimchi ET, Ma L, Kaifi JT, Staveley-O’Carroll KF, Sunitinib LG. sorafenib modulating antitumor immunity in hepatocellular cancer. J Immunol Res Ther. 2018;3:115–123.
- Inada Y, Mizukoshi E, Seike T, Tamai T, Iida N, Kitahara M, Yamashita T, Arai K, Terashima T, Fushimi K, et al. Characteristics of immune response to tumor-associated antigens and immune cell profile in patients with hepatocellular carcinoma. Hepatology. 2019;69:653–665.
- Xu W, Liu K, Chen M, Sun J-Y, McCaughan GW, Lu X-J, Ji J. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019.
- Wang L, Yan K, Zhou J, Zhang N, Wang M, Song J, Zhao J, Zhang Y, Cai S, Zhao Y, et al. Relationship of liver cancer with LRP1B or TP53 mutation and tumor mutation burden and survival. J Clin Oncol. 2019;37:1573.
- Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, Du G, Luo H, Wu X, Wang Y, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118.
- Pan Z, Di S, Shi B, Jiang H, Shi Z, Liu Y, Wang Y, Luo H, Yu M, Wu X, et al. Increased antitumor activities of glypican-3-specific chimeric antigen receptor-modified T cells by coexpression of a soluble PD1-CH3 fusion protein. Cancer Immunol Immunother. 2018;67:1621–1634.
- Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Yamashita T, Fushimi K, Kaneko S. Enhancement of tumor‐associated antigen‐specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–1457.
- Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551.
- DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, Sadelain M. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26:2542–2552.
- Keane FK, Hong TS, Zhu AX. Evolving systemic therapy in hepatocellular carcinoma: current management and opportunities for integration with radiotherapy. Semin Radiat Oncol. 2018;28:332–341.
- Textor A, Listopad JJ, Wührmann LL, Perez C, Kruschinski A, Chmielewski M, Abken H, Blankenstein T, Charo J. Efficacy of CAR T-cell therapy in large tumors relies upon stromal targeting by IFNγ. Cancer Res. 2014;74:6796–6805.
- Chino F, Pollis KE, Choi S, Salama JK, Palta M. Stereotactic body radiation therapy-induced abscopal effect on hepatocellular carcinoma after treatment for lung cancer: a case report. Hepatology. 2018;68:1653–1655.
- Hong YK, Li Y, Pandit H, Li S, Pulliam Z, Zheng Q, Yu Y, Martin RCG. Epigenetic modulation enhances immunotherapy for hepatocellular carcinoma. Cell Immunol. 2019;336:66–72.