ABSTRACT
Background: Nivolumab and pembrolizumab, two PD1 inhibitors, trigger immune-related adverse events in approximately 50% of patients. Our objective was to determine whether these immune-related adverse events are associated with patient outcomes.
Patients and Methods: Retrospective cohort study, realized at the Institut Universitaire du Cancer de Toulouse, of all the patients treated with nivolumab or pembrolizumab off clinical trials. We included patients (i) diagnosed with unresectable stage III or stage IV melanoma or with recurrent stage IIIB or stage IV non-small cell lung cancer (ii) on nivolumab 3mg/kg or pembrolizumab 2mg/kg every 2 or 3 weeks respectively.
Results: Of the 311 patients included (of 641 eligible subjects), 120 (38.6%) had melanoma and 191 (61.4%) had non-small cell lung cancer; 241 (77.5%) were treated with nivolumab with a median follow-up of 24 months (20–29). We observed 166 immune-related adverse events in 116 (37.3%) patients, categorized as “early” (onset before 12 weeks in melanoma and before 8 weeks in lung cancer) in 63 (54.3%) patients. Early and late adverse events were significantly associated with an increase in overall survival: adjusted hazard ratio 0.58 [0.41–0.84] (p = .003) and 0.28 [0.16–0.50] (p < .001) respectively. The overall response rate was significantly increased in patients with an immune-related adverse event (53.9% vs 12.9%, p < .001)
Conclusions: This study validates the association between immune-related adverse events and anti-PD1 efficacy in real-life, especially if these events are delayed. Our results, along with further studies on the place of immunosuppressive drugs in the therapeutic strategy, could improve the management of these adverse events.
Introduction
The development of immune checkpoint inhibitors is one of the most recent major advances in oncology. These agents are intended to unleash the power of the immune system in order to destroy tumor cells. At present, immunotherapy in routine practice consists mainly of anti-PD1 (programmed cell death-1) and anti-PD-L1 (programmed cell death ligand-1) antibodies. Nivolumab and pembrolizumab are the first two anti-PD1 to have been studied and demonstrate a substantial survival benefit compared to prior standard melanomaCitation1,Citation2 and NSCLCCitation3–Citation5 (non-small cell lung cancer) treatments.
Although, immune-related adverse events (irAEs) occur in approximately 50% of patients treated with anti-PD1 monotherapy. These irAEs may concern any organ or system, with variable clinical severity and implications, leading to anti-PD1 discontinuation in approximately 10% of patients. Steroids or, more rarely, immunosuppressive drugs (IS) may be required to control irAEs. The management of irAEs has been the subject of recent ASCO guidelinesCitation6 and remains largely based on expert consensus.
The occurrence of irAEs has been found to be associated with favorable outcomes in melanoma, NSCLC and metastatic renal cell carcinoma.Citation7–Citation28 This supports the hypothesis of a mechanistic link between antitumor response and auto-immune reactivity. Although this relationship has been well documented with nivolumab and in clinical trials, it has been less studied in real life and with other anti-PD1 besides nivolumab. In addition, current data on the impact of specific types of irAEs on outcomes are not entirely consistent.
The aim of this cohort was to report on the efficacy and safety of nivolumab or pembrolizumab in patients with advanced melanoma or NSCLC with different clinical profiles, in real-life clinical practice.
Methods
Patients
We conducted a retrospective cohort study in all consecutive patients treated with nivolumab or pembrolizumab off clinical trials at the Toulouse University Cancer Institute (Institut Universitaire du Cancer de Toulouse {IUCT}, France), a tertiary referral center for cancer. All the patients who were included were 1) diagnosed with unresectable stage III or stage IV melanoma, or with recurrent stage IIIB or stage IV NSCLC, 2) over 18 years of age, 3) treated with anti-PD1 monotherapy, and 4) had received the first dose of anti-PD1 between September 19, 2014 and December 31, 2016. The patients were identified through the IUCT chemotherapy production unit register.
The following clinical, biological and radiological data were collected at baseline: a) age, gender, smoking status, ECOG-PS (Eastern Cooperative Oncology Group – Performance Status), medication; b) cancer type and histological subtype, mutational status, TNM staging according to the AJCC Cancer Staging Manual, 7th edition,Citation29,Citation30 metastatic sites, time since cancer diagnosis and the number of prior treatment lines.
Patients were treated with nivolumab 3mg/kg or pembrolizumab 2mg/kg every 2 or 3 weeks respectively until confirmation of disease progression or unacceptable toxicity. Tumor assessment was performed according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)Citation.31 In cases where pseudoprogression was suspected, tumor assessment was postponed until a subsequent assessment. IrAEs were recorded and reviewed by the principal investigator (RD) up to one month after the last administration. To be taken into account in this study, the causal relationship between the irAE and the anti-PD-1 had to be “certain” or “probable” according to the World Health Organization Uppsala Monitoring Center scaleCitation.32 The following data were reviewed: grading (according to Common Terminology Criteria for Adverse Events, version 5.0), medications administered to treat irAEs and the irAE outcomes.
Outcomes
The overall response rate (ORR) was defined as the proportion of patients in whom the best objective response was a complete response (CR) or a partial response (PR). Progression-free survival was defined as the time that elapsed between the date of the first injection of anti-PD1 treatment and disease progression or death (progression-free survival [PFS]). Overall survival was defined as the time that elapsed between the first treatment injection and death (overall survival [OS]). The cutoff date for early and late irAEs was set at 12 weeks for melanoma patients and 8 weeks for NSCLC patients. “Digestive” irAEs included immune-related diarrhea, colitis and hepatitis.
Statistical analyses
After corrections for aberrant or inconsistent data, the database was locked. We first described the patient characteristics using the appropriate descriptive statistics according to the type of variables. Descriptive statistics included the median (Inter-Quartile Range (IQR)) for continuous variables, and the number of observations with the frequency (%) for categorical variables. The ORR of the groups was compared using the χ2-test (or Fisher’s exact test for small data sets). For survival endpoints (OS and PFS), Kaplan–Meier survival curves were drawn and described using the median (IQR) and 1-year survival. Univariate analyses with a log-rank test were conducted to evaluate the relationship between survival and age, sex, tumor type, histological subtype, mutational status, cerebral metastases, time since cancer diagnosis, the number of prior treatment lines, the anti-PD1 type, time on anti-PD1, steroids at baseline, and irAEs. In the univariate analysis, differences in survival functions were tested using the log-rank test. In the multivariate analysis, HR and 95% confidence intervals (CI) were assessed with Cox model. Variables initially introduced in the multivariate survival analyses were all variables (potential confounding factors) associated with OS or PFS in the univariate analyses with a P-value < 0.20. A backward analysis was then applied until only variables significantly and independently associated with OS or PFS (P-value <0.05) remained. The proportional hazard assumption was tested for each covariate of the Cox model using “log-log” plot curves and was always affirmed. When the linearity hypothesis was not respected, continuous variables were transformed into ordered data using the median. Interactions between independent covariates were tested in the final models. All reported P-values were two-sided and the significance threshold was set at <0.05. Statistical analyses were performed using STATA 14.1 software (StataCorp LP, College Station, TX 77845 USA, www.stata.com).
Ethics
The data were collected as part of routine clinical care in compliance with good clinical practices. The study was approved by the institutional board ethics committee of Toulouse University Hospital and was conducted in accordance with the Declaration of Helsinki.
Results
Patient characteristics
We identified 641 patients treated with nivolumab or pembrolizumab at our institution on January 16, 2018. Three hundred and eleven of these patients met the selection criteria for study inclusion (Supplementary Figure 1). One hundred and ninety-four patients were men (62.4%) and the median age was 64 years (iqr: 56–70 years). Past medical history was remarkable for an auto-immune condition in 5 patients (1.6%). One hundred and twenty patients (38.6%) had melanoma and 191 (61.4%) had NSCLC. Two hundred and forty-one patients (77.5%) were treated with nivolumab and 70 (22.5%) with pembrolizumab. The median follow-up was 24 months (iqr 20–29).
Of the 120 (38.6%) melanoma patients, BRAFV600 mutation was identified in 38 (31.9%) tumors (). The anti-PD1 treatment was started within a median period of 2 years (iqr 1–5) after the diagnosis of cancer and was nivolumab in 50 cases (41.7%). The median duration of anti-PD1 treatment was 4 months (iqr 2–10).
Table 1. Characteristics of melanoma patients
Of the 191 (61.4%) NSCLC patients, all treated with nivolumab, 19 (10.2%) were never smokers (). The histological subtype was squamous in 44 cases (23.0%). An EGFR mutation was present in 9 (5.0%) tumors. The anti-PD1 treatment was started within a median period of 1 year (0.6–2) after the diagnosis of cancer. The median anti-PD1 treatment duration was 2 months (1–8).
Table 2. Characteristics of NSCLC patients
Efficacy
In patients with melanoma, 1-year OS and PFS were 49% [95%, CI 40–58] and 26% [18–34], respectively (Supplementary Table 1). Median OS and PFS were 11.6 (iqr 3.4–29.6) and 3.9 (2.2–12.6) months, respectively. The ORR was 33.9%. In patients with NSCLC, the 1-year OS and PFS were 44% [37–51] and 21% [16–27], respectively. Median OS and PFS were 9.1 (3.6–28.9) and 2.8 (1.6–10.4) months respectively. The ORR was 25.7%.
Safety analysis
During follow-up, 166 irAEs were reported in 116 (37.3%) patients ( and ). Multiple irAEs could occur in the same patient: two irAEs in 38 cases (12.2%), three irAEs in 3 cases (1.0%) and four in 2 cases (0.6%). Grade 3–4 irAEs occurred in 22 patients (7.1%) and 3 patients died as a result of myocarditis (n = 2) or pneumonitis (n = 1). The most frequent irAEs were cutaneous: 56 patients (18%), including rash: 28 (9.0%) and pruritus: 20 (6.4%), followed by diarrhea/colitis: 38 (12.2%), and dysthyroidism: 18 (5.8%). IrAEs occurred after a median of 2.3 months (iqr 1.0–5.1). Sixty-four patients (20.5%) experienced at least one early irAE, while 52 patients (16.7%) experienced only late irAE(s). Steroids were used in 43 cases (13.8%) and in conjunction with IS in 2 cases: infliximab (colitis, n = 1) and methotrexate (bullous pemphigoid, n = 1). Anti-PD1 was discontinued due to toxicity in 36 cases (11.6%).
Table 3. Summary of irAEs in melanoma patients
Table 4. Summary of irAEs in NSCLC patients
Digestive irAEs, which consisted of 38 cases of diarrhea/colitis and 5 cases of hepatitis, occurred in 41 patients (13.2% of the study population and 35.3% of the patients who experienced an irAE). These irAEs were given a grade of 3–4 in 9 patients (22.0%), required steroids/IS in 15 patients (36.6%) and the discontinuation of anti-PD1 in 12 patients (29.3%). Of the 5 patients with a preexisting auto-immune condition, each of 4 patients (80%) experienced one irAE, with a grade of 5 in one instance. In melanoma patients, rash, vitiligo and diarrhea/colitis occurred more frequently than in patients with NSCLC (14.2% vs 5.8%, 5.8% vs 0.5%, and 15.8% vs 9.9% respectively). Moreover, grade 3–5 irAEs were more frequent (10.8% vs 4.7%), steroids/IS were used more often (17.5% vs 11.5%) and anti-PD1 discontinuation was more common (13.3% vs 10.5%).
Relationship between irAEs and survival
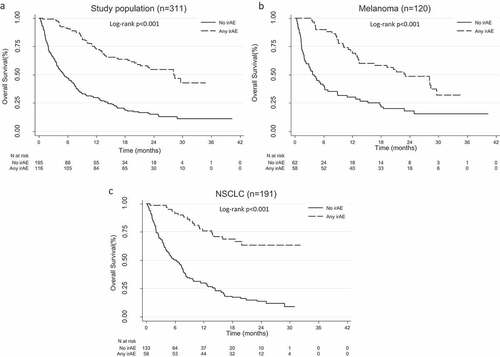
In the univariate analysis, any irAE, digestive irAEs, cutaneous irAEs, endocrine irAEs and irAEs grouped by early/late onset were significantly associated with an increase in OS: median 28.2 months (iqr 11.2 to not reached) vs 5.3 (2.0–14.4) (p < .001), 28.2 (9.1 to not reached) vs 8.7 (3.0–25.1) (p = .001), 29.6 (20.0 to not reached) vs 8.8 (3.3–28.1) (p < .001), not reached (28.2 to not reached) vs 8.8 (3.3–28.1) (p <.001), 16.5 (8.8–28.4) vs not reached (28.2 to not reached) (p < .001); and PFS: 11.5 months (5.8–25.8) vs 1.8 (1.2–3.7) (p < .001), 10.3 (2.8–24.8) vs 3.0 (1.6–9.1) (p = .001), 11.2 (8.8 to not reached) vs 2.9 (1.6–10.4) (p = .001), 12.3 (7.0 to not reached) vs 3.1 (1.6–10.4) (p < .001) and 8.0 (2.8–16.5) vs 18.8 (10.1 to not reached) (p < .001) (, Supplementary Table 2). In contrast, anti-PD1 discontinuation was not significantly associated with OS or PFS.
In the multivariate analysis, early and late irAEs were significantly associated with better OS: HR 0.58 [0.41–0.84] (p = .003) and 0.28 [0.16–0.50] (p < .001), and PFS: 0.36 [0.26–0.50] (p < .001) and 0.24 [0.16–0.37] (p < .001), respectively (). Anti-PD1 discontinuation was significantly related to better PFS in melanoma patients: HR 0.34 [0.14–0.80] (p = .013), but not in NSCLC patients (p = .383). Steroids >10mg/d at baseline were significantly related to worse OS: 1.80 [1.26–2.57] (p = .001) and PFS: 1.90 [1.34–2.68] (p < .001). Moreover, the time since cancer diagnosis and the number of prior treatment lines were significantly associated with survival.
Table 5. Multivariate analysis of overall and progression-free survival
Relationship between irAEs and tumor response
There was a significant increase in overall response rate in patients with an irAE regardless of the type (53.9% vs 12.9%, p < .001) (). Compared to patients who did not experience a specific type of irAE, the ORR was significantly higher in patients with cutaneous irAEs (66.1% vs 20.3%, p < .001), an immune-related rash (57.1% vs 26.0%, p < .005), endocrine irAEs (50.0% vs 27.3%, p = .024) and digestive irAEs (43.9% vs 26.6%, p = .023) (). In the irAE group, the ORR was significantly increased in patients with late irAEs compared to early irAEs (71.2% vs 39.7%, p < .001) but was not significantly different in patients treated with steroids/IS compared to untreated patients (47.6% vs 56.9%, p = .336).
Table 6. Association of overall response rate with irAEs
Discussion
Our results confirm that the occurrence of irAEs is a favorable prognostic indicator, with nivolumab or pembrolizumab. Furthermore, we identified a relationship between ORR and various types of irAEs, in particular digestive irAEs. Lastly, we found that the relationship between irAEs and treatment efficacy is stronger if the irAEs occurs late in the course of anti-PD1 treatment.
To the best of our knowledge, this is the first French retrospective series to describe the impact of nivolumab and pembrolizumab-induced irAEs on prognosis and survival in patients with melanoma and NSCLC. This study was conducted in a real-life setting by oncologists with an extensive clinical activity outside of clinical trials, in a cohort of consecutive patients with end-stage metastatic cancer. One hundred and five patients (33.8%) would not have met the eligibility criteria of the first clinical trialsCitation1–Citation4 because of active cerebral metastases or an ECOG-PS of 2 or 3. Moreover, 73 patients (23.5%) received nivolumab or pembrolizumab by means of an early-access program (the nominative temporary authorization). No NSLC patient received treatment with pembrolizumab because it was not yet approved at the time the study was conducted.
The relationship between any irAE and patient outcomes should be interpreted by taking into consideration individual contributions of specific types of irAEs. Cutaneous irAEs, rash and vitiligo in isolation, have been consistently associated with favorable outcomes,Citation8,Citation9,Citation13,Citation16,Citation17,Citation20,Citation23,Citation24,Citation26,Citation27 except in one study in which the frequency was fairly lowCitation.25 In our study, OS, PFS and ORR were consistently found to be superior in patients who experienced cutaneous irAEs or rash. Endocrine irAEs, all analyzed together or by taking into account single immune-related thyroid dysfunctions, have also been reported to be a favorable prognostic indicator,Citation9,Citation11,Citation20,Citation22,Citation24,Citation26 with only one exceptionCitation.25 In our study, endocrine irAEs were significantly associated with OS, PFS and ORR, even though only 7.1% of our patients experienced such irAEs, compared to approximately 10–15% in the literature.
The relationship between gastrointestinal and/or hepatic irAEs and treatment efficacy is unclear. Gastrointestinal irAEs have been most frequently described as favorableCitation20,Citation24,Citation26 or neutralCitation22,Citation28 prognostic factors. Nevertheless, Ksienski and al.Citation25 reported a negative univariate relationship between colitis and OS in 271 NSCLC patients treated with anti-PD1. However, this effect was not observed in the multivariate analysis. In our study, digestive irAEs were significantly associated with an increase in OS and PFS in the univariate, but not in the multivariate analysis. We believe this can be explained by different diarrhea/colitis profiles. In fact, 12.2% of the patients experienced diarrhea/colitis in our cohort, compared to 6.6% in the study by Ksienski and al., which probably reflects a higher incidence of grade 1–2 episodes in our series. In terms of hepatitis, the relationship with survival was found to be not significant in 4 studiesCitation20,Citation22,Citation25,Citation28 and significantly negative in oneCitation.26 In fact, in the work by Verzoni et al.Citation26 on 389 patients with renal cell carcinoma, the 1-year OS was 42% in the 7 patients who had hepatitis, versus 63% in the overall population. Conversely, the 1-year OS in our 5 hepatitis patients was 80% compared to 43% in the whole sample. Due to the small numbers and the cancer types that are different in the two studies, definite conclusions cannot be drawn on this point.
In this work, we describe a relationship between the onset of irAE and survival, with a 2- to 3-fold change in HR in favor of late vs early irAEs, and between the onset of irAE and ORR. On the contrary, Verzoni et al. found that the 1-year OS was comparable in the early onset (< 6 weeks) and late onset (> 6 weeks) irAE groups: 78.7% vs 85.2% (p = .34). These discrepancies may be due to different cutoff points between early and late irAEs, i.e. 12 weeks for melanoma patients and 8 weeks for NSCLC patients in our study. The cutoff points were chosen to match the median time to irAE (2.8 months for melanoma patients and 1.9 months for NSCLC patients, respectively). The rationale for a relationship between irAEs and outcomes dependent on the timing of the irAE onset remains elusive.
Steroids are often used in the management of anti-PD1 irAEs. Theoretically, steroids have immunosuppressive properties and could decrease the efficacy of anti-PD1. Current data is reassuring since the reported anti-PD1 outcomes are similar in patients treated with steroids for irAEs and patients experiencing irAEs who do not require the use of steroids.Citation18,Citation33 In this study, we show that steroids used to control irAEs have a negative impact on PFS, but not on OS. These results, obtained from real-life data, unlike prior reports,Citation18,Citation33 deserve further investigation to determine whether steroids could shorten antitumor response duration.
The retrospective nature of this study exposes us to the risk of information biases. However, we implemented a process of reviewing medical records that enabled standardization of the definition of irAEs, causal relationship assessment and grading. Furthermore, we believe that selection biases were prevented as a result of the exhaustive method of patient inclusion by consecutive patients from a cohort recorded in the pharmacy register. Various confusion factors were accounted for in the multivariate analysis, except for PD-L1 expression due to insufficient available data. In addition, the statistical analysis clustered melanoma and NSCLC patients for increased reliability. On one hand, this choice was justified by the similarity of patient outcomes in the two tumor groups, notably the 1-year OS: 48% in the melanoma group vs 43% in the NSCLC group. Our cohort was an extensive study on real-life utilization of nivolumab or pembrolizumab in patients with advanced cancers. Therefore, overall survival in the melanoma group tended to be lower than that reported in phase 3 studies, ranging between 68 and 73%.Citation1,Citation2 On the other hand, cancer type was not retained as significant during the variable selection phase in the multivariate modeling. Lastly, our data do not allow extrapolation of the prognostic impact of irAEs induced by checkpoint inhibitor combinations or by pembrolizumab in NSCLC patients.
In conclusion, in a real-life setting, this study validates the relationship between irAEs and anti-PD1 efficacy in melanoma and NSCLC patients who are either on nivolumab or pembrolizumab, and highlights the importance of the delay in the occurrence of irAE in this relationship. Nevertheless, proper management of cancer patients experiencing irAE remains a challenge in daily clinical practice. Further studies aimed at investigating optimal steroid dosing and timing, the place of immunosuppressive drugs in the therapeutic strategy and the benefits and risks of anti-PD1 rechallenge after discontinuation for toxicity will help to improve patient outcomes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download ()Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–8. doi:10.1056/NEJMoa1412082.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi:10.1056/NEJMoa1503093.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627.
- Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7.
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385.
- Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, Ishida T, Hirabayashi M, Hara S, Ishitoko M, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer. 2018;119:14–20. doi:10.1016/j.lungcan.2018.02.017.
- Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, Maruyama H, Fujisawa Y, Matsuya T, Fujimoto M, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. August 2016. doi:10.1111/1346-8138.13520.
- Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi:10.1001/jamaoncol.2017.2925.
- Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, Tsurumi K, Suzuki K, Shimizu H, Sugisaka J, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. The Oncologist. 2018 June;23:1358–1365. doi:10.1634/theoncologist.2017-0384.
- Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589. doi:10.1093/annonc/mdw640.
- Hasan Ali O, Diem S, Markert E, Jochum W, Kerl K, French LE, Speiser DE, Früh M, Flatz L. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology. 2016;5(11):e1231292. doi:10.1080/2162402X.2016.1231292.
- Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–1212. doi:10.1001/jamadermatol.2015.1916.
- Judd J, Zibelman M, Handorf E, O’Neill J, Ramamurthy C, Bentota S, Doyle J, Uzzo RG, Bauman J, Borghaei H, et al. Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. The Oncologist. 2017;22(10):1232–1237. doi:10.1634/theoncologist.2017-0133.
- Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, Enk A, Lorenz H-M, Hassel JC. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother. 2018;67(2):175–182. doi:10.1007/s00262-017-2069-9.
- Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–894. doi:10.1158/1078-0432.CCR-15-1136.
- Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi:10.1001/jamadermatol.2015.2707.
- Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi:10.1200/JCO.2015.66.1389.
- Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi:10.1016/j.lungcan.2017.11.019.
- Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485. doi:10.1007/s00432-018-2805-3.
- Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, Arranz R, Lorenzo A, Gullón P, Donnay O, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–27. doi:10.1016/j.ejca.2018.10.014.
- Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L, Blanchon M, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. October 2018. doi:10.1016/j.cllc.2018.10.002.
- Ishihara H, Takagi T, Kondo T, Homma C, Tachibana H, Fukuda H, Yoshida K, Iizuka J, Kobayashi H, Okumi M, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. 2019 March;37:355.e21–355.e29. doi:10.1016/j.urolonc.2019.03.003.
- Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, Bersanelli M, Bordi P, Santini D, Giusti R, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clinical Lung Cancer. 2019 February;20:237–247.e1. doi:10.1016/j.cllc.2019.02.006.
- Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z, Fenton D, Geller G, Glick D, Lesperance M, et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clinical Lung Cancer. 2019;20(1):e97–e106. doi:10.1016/j.cllc.2018.09.005.
- Verzoni E, Cartenì G, Cortesi E, Giannarelli D, De Giglio A, Sabbatini R, Buti S, Rossetti S, Cognetti F, Rastelli F, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer. 2019;7(1):99. doi:10.1186/s40425-019-0579-z.
- Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, Del Vecchio M. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019;145(2):511–521. doi:10.1007/s00432-018-2819-x.
- Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798–1805. doi:10.1016/j.jtho.2017.08.022.
- Balch CM, Gershenwald JE, Soong S-J, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi:10.1200/JCO.2009.23.4799.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi:10.1097/JTO.0b013e31812f3c1a.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026.
- Olsson S. The role of the WHO programme on international drug monitoring in coordinating worldwide drug safety efforts. Drug Saf. 1998;19(1):1–10. doi:10.2165/00002018-199819010-00001.
- von Pawel J, Syrigos K, Mazieres J, Cortinovis D, Dziadziuszko R, Gandara DR, Conkling P, Goldschmidt J, Thomas CA, Bordoni R, et al. 1314PAssociation between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol. 2017;28(suppl_5). doi:10.1093/annonc/mdx380.017.