ABSTRACT
Gastrointestinal stromal tumor (GIST) is a devastating disease, especially in the setting of metastasis. The natural progression of GIST has been significantly altered by the development of small molecule tyrosine kinase inhibitors (TKIs), including imatinib, sunitinib, and regorafenib, all of which are FDA approved. However, TKIs are not always well-tolerated, and the refractory disease continues to be a problem. For these reasons, alternative treatments are needed. In this report, we discuss a patient with metastatic wild-type (WT) GIST refractory to multiple TKIs, but with a durable clinical response to the anti-programmed cell death protein 1 (PD-1) antibody, nivolumab. This report suggests that continued research evaluating checkpoint inhibitors in GIST is warranted.
Introduction
Gastrointestinal stromal tumor (GIST) is one of the most prevalent soft tissue sarcoma subtypes. Each year, between 4,000 and 6,000 new cases are diagnosed in the United States.Citation1,Citation2 GIST most often occurs in middle-aged and elderly persons, with ~60% located in the stomach, 30% in the small intestine, and 10% along the rest of the gastrointestinal (GI) tract.Citation3 The most important prognostic factors are tumor size, mitotic rate, and location of the primary lesion.Citation4 Most GISTs are sporadic with mutations in c-Kit or PDGFRA; however, these may be absent in the setting of an NF-1 mutation or SDH deficiency.Citation5
Currently, the first-line treatment for patients with primary, localized high-risk GIST is surgical resection followed by at least 3 years of adjuvant imatinib. Despite improvements in GIST management, treatment is rarely curative for patients with metastatic disease, and imatinib resistance inevitably emerges. Sunitinib, a multi-targeted TKI, has been approved for the treatment of patients with GIST after progression on imatinib therapy,Citation6 while regorafenib is FDA approved as a third-line therapy for metastatic GIST based on the phase III GRID trial.Citation7
New studies continue to search for improved alternatives. A single center study of 60 consecutive patients with advanced/inoperable metastatic GIST after failure on at least imatinib and sunitinib, treated with sorafenib showed a 1-year PFS rate of 23%, and a median PFS of 7.7 months suggesting potential benefit in the refractory setting.Citation8 Pazopanib was studied in similar patients as a third-line option vs best supportive care alone and showed a significant improvement of PFS (3.4 vs 2.3 months).Citation9 Dasatinb was studied in patients with imatinib-resistant GIST, and objective tumor response was observed in 25% of patients.Citation10 Further, two new TKIs, ripretnib, and avapritinib, are currently in development and may be highly active (NCT03673501, NCT02508532).
PD-1 inhibitors, such as pembrolizumab and nivolumab, may be viable options for patients with metastatic GIST that evolve TKI resistance/intolerance. Nivolumab is currently approved by the FDA in treating melanoma, squamous non-small cell lung cancer, and renal cell carcinoma.Citation11-Citation13 However, little has been written about the clinical utility of anti-PD-1 for GIST patients. While the advent of tyrosine kinase inhibitors has improved long-term survival, they have not proven curative for metastatic GIST. Here we report our experience using nivolumab in a patient with refractory, metastatic GIST.
Results
The patient is a 40-year-old woman who presented in June 2000 with anorexia and unintentional weight loss. CT abdomen showed multiple masses in her stomach. The tumors were surgically resected, and pathology was consistent with WT GIST. The patient was scheduled for endoscopic surveillance every 6 months – 1 year. After 5 years the patient abandoned monitoring, but re-presented in April 2007 with fatigue and diffuse pain. Endoscopy was abnormal, and disease had recurred. The patient underwent partial gastrectomy whereby 2/2 lymph nodes were found to have focal extension consistent with metastatic GIST. Following surgery, the patient started imatinib in June 2007, but was unable to tolerate the side effects (fatigue, diarrhea, painful rash, and mouth sores) and was consequently switched to sunitinib in October 2007. The patient progressed in January 2009, and was switched back to imatinib. The patient continued imatinib in-spite of fatigue, diarrhea and rash, until cancer progression in February 2013, at which time treatment was changed to regorafenib. In March 2014, regorafenib was stopped due to disease progression. The patient was enrolled in a Phase I clinical trial of the phosphoinositide 3-kinase inhibitor, BKM-120, used in conjunction with imatinib (NCT01468688). The BKM-120 was stopped after the patient developed persistently elevated creatinine, and sorafenib was initiated in October 2015. In December 2015, the patient developed hand-foot syndrome which limited her activities to an extent where she expressed reluctance to try another TKI.
With limited systemic options and progressive disease, the decision was made to pursue compassionate use nivolumab. Of note, nivolumab with concomitant TKI was recommended to the patient given demonstrated synergyCitation14 without increasing the likelihood adverse effects,Citation15 however, the patient refused the TKI because of prior experiences mentioned above. After 1 cycle of nivolumab, the patient noted some joint pain, especially in her wrist where several years prior she had a surgical excision of a desmoid tumor. However, this pain lasted less than 2 weeks and was not severe enough to impair her routine daily activities. Further, after cycle 15, the patient developed bilateral lower-extremity edema, requiring management with furosemide for less than 1 month before spontaneously resolving. While the patient also experienced intermittent fatigue and pruritis, overall she had a much improved quality of life compared to previous treatment regimens. The patient was able to be active with her wife and her daughter, whereas with previous agents she was frequently bed bound related to pain, severe fatigue, and adverse side effects.
After cycle 64 of nivolumab, CT chest/abdomen/pelvis (3/14/2018) showed an overall decrease in size/number/conspicuity of hepatic metastases, with stable pulmonary nodules and no new signs of metastases (). Interestingly, after cycle 59, the patient was found to have an acute appendicitis treated by a surgical appendectomy. Pathologic specimen analysis was negative for GIST involvement. Unfortunately, after 33.5 months (71 cycles), the patient was found to have progressive disease on surveillance CT.
Image 1. Contrast-enhanced CT images of the upper abdomen prior to initiating nivolumab (a, d) demonstrate low attenuation metastases within hepatic segments VI and II (arrows). Follow-up CT 2 years later (b, e) demonstrates that the metastasis in segment VI has decreased while the metastasis in segment II has completely resolved (arrows). Similarly, follow-up CT on 3/14/2018 (c, f) after cycle 64 of nivolumab demonstrates that the segment VI metastasis has again decreased and the segment II metastasis is still resolved (arrows)
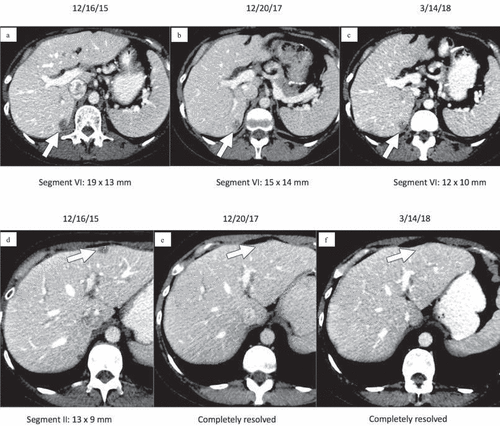
Figure 1. Tumor tissue and tonsil (control) stained for CD3, CD4, CD163, PD-L1. Few CD3+, or CD4+ cells were detected in tumor. FOXP3 was detected in tumor, but not with CD3+ or CD4+ cells. Majority of myeloid tumor cells expressed PD-L1
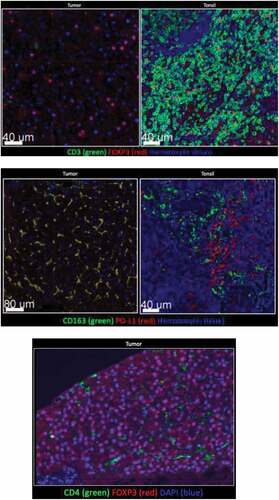
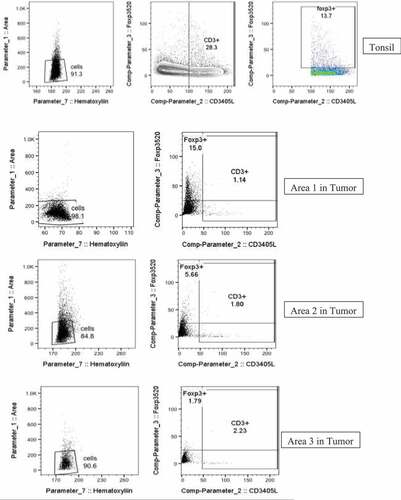
Tissue collected before nivolumab initiation was stained for CD3, CD163, FOXP3, PD1, and PD-L1 (). Interestingly, all macrophages expressed PD-L1, while most CD3 + T cells were Foxp3+ regulatory T cells – collectively reflecting an immunosuppressive environment. There were also a high number of PD-L1+ CD163 – spherical cells that resembled monocytes, which would likely differentiate into immunosuppressive macrophages.
Discussion
Uncovering the dynamic intricacies of the tumor immune microenvironment in GIST, especially in relation to treatment, is critical for improving clinical outcomes using check-point inhibitors. While this patient had nearly 3 years of durable disease regression with minimal toxicity, this case is certainly exceptional. Of note, other investigators have demonstrated stable disease for 33% (3/9) in patients with metastatic or unresectable GIST treated with checkpoint inhibitor-based trials.Citation16
Interestingly, this tumor had a low density of CD3 + T cells, which is unique for this patient given that higher levels of CD3+ infiltrates may portend a superior PFS.Citation17 Furthermore, while the co-localization of CD163+ and PD-L1 was expected, the expression of FOXP3 largely in the absence of CD3+ and CD4+ was surprising. While other studies have shown that some cancer cell types can express FOXP3,Citation18-Citation20 this has not yet been demonstrated previously in sarcoma to our knowledge.
For many patients, additional targets may be concomitantly needed with anti-PD1 therapy. One team analyzed 129 GIST tissue microarrays and found that nearly 90% of tumors were IDO-positive, while 69% were positive for PD-L1 – suggesting that IDO is a critical target.Citation21 Because imatinib has activity against IDO in the GIST microenvironment,Citation22 the combination of imatinib and anti-PD1 has been investigated in murine models with promising synergy,Citation14 but this has been less evident in a phase I clinical trial.Citation16 However, PD1 is probably critical because when KIT inhibition was combined with the CTLA-4 inhibitor ipilimumab, clinical outcomes did not appear improved.Citation23 PD-L1 expression in GIST can be heterogenous, particularly in higher-risk tumors, further strengthening the argument for combination immunotherapy, and a trial is now ongoing at UCLA (NCT02880020) comparing single-agent nivolumab (anti-PD-1) alone or in combination with ipilimumab (anti-CTLA-4).
Here, we describe a patient with metastatic WT GIST for which the role of immunotherapy is not well defined. There are no published case reports of refractory WT GIST with a clinical response to nivolumab. The clinical benefit of nivolumab in metastatic disease is noteworthy, and our findings suggest that nivolumab is a potentially effective and well-tolerated agent in this setting. To date, no study has been published to assess the response rate of nivolumab in patients with advanced WT GIST, however, an ongoing trial (NCT02880020) will provide prospective data and could alter our current treatment paradigm.
Materials & methods
The diagnosis of GIST was confirmed by an experienced soft tissue pathologist. Pathology was evaluated for gene mutation analysis, and results were negative for KIT and PDGFRA gene mutations, consistent with wild-type GIST. The patient was treated with nivolumab dose escalation beginning at 170 mg (max 240 mg) as an intravenous infusion over 1 h, once every 2 weeks for a total of 71 cycles. Re-staging CT scans were generally performed after every four cycles assuming no signs or symptoms suggestive of progressive disease.
The tissue was obtained by a core biopsy of a metastatic lesion from segment two of the liver. Multiplex IF staining was performed on formalin-fixed paraffin-embedded sections after deparaffinization and antigen retrieval (AR). The section was interrogated reveal immune cell subsets including T cells (CD3+), regulator T cells (FoxP3+), Macrophages (CD163+), and their expression of PD-1 and PDL-1. High‐resolution composite images were acquired with Leica SP8 confocal scanning microscope and spectral spill over was calculated and corrected for using single color stains for each fluorophore. Images were analyzed using Imaris (Bitplane). Image Cytometry analysis were performed as previously published.Citation24 Briefly, imaging datasets were segmented based on their nuclear staining signal and intensities of all channels as well parameters such as area, sphericity, positional co-ordinates, etc., were calculated on the iso-surfaces generated on nuclear signals. Data were then exported as single comma-separated values format which were imported into FlowJo for further analysis. Scatter Plots were generated using FlowJo ().
Abbreviations
| GIST | = | gastrointestinal stromal tumor |
| TKIs | = | tyrosine kinase inhibitors |
| PD-1 | = | programmed cell death protein 1 |
| GI | = | gastrointestinal |
| CT | = | computed tomography |
| PFS | = | progression free survival |
| PDGFRA | = | platelet-derived growth factor receptor alpha |
| IDO | = | indoleamine-pyrrole 2,3-dioxygenase |
| CTLA-4 | = | cytotoxic T-lymphocyte associated protein-4 |
| WT | = | wild-type |
Authors’ contributions
BAS wrote and edited the manuscript. KK performed immunohistochemistry with oversight from RHP. RBO evaluated CT images. TSK and RLJ cared for the patient. SMP cared for the patient and edited the manuscript. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Consent for publication
Written informed consent was obtained from the patient for publication of their individual details and accompanying images in this manuscript. The consent is held by the authors and is available for review by the Editor-in-Chief.
Ethics approval and consent to participate
The patient was treated on a compassionate use basis with the appropriate consent. No other ethical approvals were needed. Consent for the administration of this agent was obtained from this patient.
Acknowledgments
We acknowledge the support of Bristol-Myers Squibb Co. in providing the Nivolumab used to treat this patient.
References
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–6. doi:10.1200/JCO.2004.05.140.
- Plesec TP. Gastrointestinal mesenchymal neoplasms other than gastrointestinal stromal tumors: focusing on their molecular aspects. Patholog Res Int. 2011;2011:952569.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42(2):399–415. doi:10.1016/j.gtc.2013.01.001.
- Rajendra R, Pollack SM, Jones RL. Management of gastrointestinal stromal tumors. Future Oncol. 2013;9(2):193–206. doi:10.2217/fon.12.178.
- Postow MA, Robson ME. Inherited gastrointestinal stromal tumor syndromes: mutations, clinical features, and therapeutic implications. Clin Sarcoma Res. 2012;2:11. doi:10.1186/2045-3329-2-16.
- George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, DePrimo SE, Harmon CS, Law CNJ, Morgan JA, Ray-Coquard I, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968. doi:10.1016/j.ejca.2009.02.011.
- Demetri GD, Reichardt P, Kang YK, Blay J-Y, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicenter, randomized, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi:10.1016/S0140-6736(12)61857-1.
- Rutkowski P, Jagielska B, Andrzejuk J, Bylina E, Lugowska I, Switaj T, Kosela-Paterczyk H, Kozak K, Falkowski S, Klimczak A. The analysis of the long-term outcomes of sorafenib therapy in routine practice in imatinib and sunitinib resistant gastrointestinal stromal tumors (GIST). Contemp Oncol. 2017;21:285–289.
- Mir O, Cropet C, Toulmonde M, Cesne AL, Molimard M, Bompas E, Cassier P, Ray-Coquard I, Rios M, Adenis A, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomized, multicenter, open-label phase 2 trial. Lancet Oncol. 2016;17(5):632–641. doi:10.1016/S1470-2045(16)00075-9.
- Schuetze SM, Bolejack V, Thomas DG, von Mehren M, Patel S, Samuels B, Choy E, D’Amato G, Staddon AP, Ganjoo KN, et al. Association of dasatinib with progression-free survival among patients with advanced gastrointestinal stromal tumors resistant to imatinib. JAMA Oncol. 2018;4(6):814–820. THIS IS REFERENCE 50. doi:10.1001/jamaoncol.2018.0601.
- Hazarika M, Chuk MK, Theoret MR, Mushti S, He K, Weis SL, Putman AH, Helms WS, Cao X, Li H, et al. U.S. FDA approval summary: nivolumab for treatment of unresectable or metastatic melanoma following progression on ipilimumab. Clin Cancer Res. 2017;23:3484–3488. doi:10.1158/1078-0432.CCR-16-0712.
- Kazandjian D, Khozin S, Blumenthal G, Zhang L, Tang S, Libeg M, Kluetz P, Sridhara R, Keegan P, Pazdur R. Benefit-risk summary of nivolumab for patients with metastatic squamous cell lung cancer after platinum based chemotherapy: a report from the US Food and Drug Administration. JAMA Oncol. 2016;1:118–122. doi:10.1001/jamaoncol.2015.3934.
- Xu JX, Maher VE, Zhang L, Tang S, Sridhara R, Ibrahim A, Kim G, Pazdur R. FDA approval summary: nivolumab in advanced renal cell carcinoma after anti-angiogenic therapy and exploratory predictive biomarker analysis. Oncologist. 2017;3:311–317. doi:10.1634/theoncologist.2016-0476.
- Seifert AM, Zeng S, Zhang JQ, Kim TS, Cohen NA, Beckman MJ, Medina BD, Maltbaek JH, Loo JK, Crawley MH, et al. PD-1/PD-L1 blockade enhances T cell activity and antitumor efficacy of imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2017;2:454–465. doi:10.1158/1078-0432.CCR-16-1163.
- Reilley MJ, Bailey A, Subbiah V, Janku F, Naing A, Falchook G, Karp D, Piha-Paul S, Tsimberidou A, Fu S, et al. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J Immunother Cancer. 2017;5:35. doi:10.1186/s40425-017-0238-1.
- Groisberg R, Hong DS, Behrang A, Hess K, Janku F, Piha-Paul S, Naing A, Fu S, Benjamin R, Patel S, et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer. 2017;5:100. doi:10.1186/s40425-017-0301-y.
- Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, Vimond N, Concha A, Garrido F, Isambert N, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73(12):3499–34510. doi:10.1158/0008-5472.CAN-13-0371.
- Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, Gedye C, Moss D, Ng SP, MacGregor D, et al. The regulatory T cell—associated transcription factor FOXP3 is expressed by tumor cells. Cancer Res. 2008;68(8):3001–3009. doi:10.1158/0008-5472.CAN-07-5664.
- Karanikas V, Spletetas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI, Germenis AE. Foxp3 expression in human cancer cells. J Transl Med. 2008;6(19). doi:10.1186/1479-5876-6-19.
- Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C, Ungefroren H, Saeger H-D, et al. Foxp3 expression I pancreatic carcinoma as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67(17):8344–8350. doi:10.1158/0008-5472.CAN-06-3304.
- Blakely AM, Matoso A, Patil PA, Taliano R, Machan JT, Miner TJ, Lombardo KA, Resnick MB, Wang L-J. Role of immune microenvironment in gastrointestinal stromal tumours. Histopathology. 2018;72(3):405–413. doi:10.1111/his.2018.72.issue-3.
- Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–1100. doi:10.1038/nm.2438.
- D’Angelo SP, Shoushtari AN, Keohan ML, Dickson MA, Gounder MM, Chi P, Loo JK, Gaffney L, Schneider L, Patel Z, et al. Combined KIT and CTLA-4 blockade in patients with refractory GIST and other advanced sarcomas: a phase Ib study of dasatinib plus ipilimumab. Clin Cancer Res. 2017;23(12):2972–2980. doi:10.1158/1078-0432.CCR-16-2349.
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain R. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;24(37):364–376. doi:10.1016/j.immuni.2012.07.011.