ABSTRACT
The leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1) is an inhibitory receptor expressed on the majority of peripheral blood mononuclear cells and is important for the regulation of immune responses. The binding of LAIR-1 to its ligands results in the loss of immune function in the tumor microenvironment (TME) and a reduction in T cell function and immune responses of antigen-presenting cells. Using bioinformatics analysis, we showed that LAIR-1 is broadly upregulated in multiple types of cancer. By designing a LAIR-2-Fc recombinant protein to block the binding of LAIR-1 to its ligand collagen, we observed augmented cytotoxic T cell infiltration and function resulting in antitumor immune responses that eliminated cancer cells. Besides, LAIR-2-Fc fusion protein potentiated the antitumor effect of PD-1/L1 checkpoint blockade therapy. Collectively, our results support the targeting of LAIR-1 for potential immunotherapeutic applications.
Introduction
Inhibitory receptors containing ITIMs (immunoreceptor tyrosine-based inhibitory motifs) play an important role in the regulation of the immune system.Citation1 These receptors contain ITIM motifs in their intracellular domains and are classified as inhibitory receptors because these motifs can recruit phosphatases such as SHP-1, SHP-2, and SHIP to negatively regulate cell activation.Citation2 The leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1) is a collagen-binding ITIM-bearing inhibitory receptor important for the regulation of immune responses and is expressed on the majority of immune cell subsets, including T cells, NK cells, monocytes, macrophages, and dendritic cells.Citation3–Citation7 LAIR-1 can inhibit the cytotoxic activity of effector T cells upon CD3 cross-linkingCitation2,Citation8–Citation10 or antigen stimulation.Citation11 Furthermore, the inhibitory capacity of LAIR-1 in T cell clones correlates to surface expression density.Citation12
Collagens are functional ligands for LAIR-1 and directly inhibit immune cell activation in vitro in cell lines and primary cells.Citation13,Citation14 Under normal conditions, collagen forms a scaffold to provide strength and structure to tissues. However, many tumor types overexpress collagens, and high level of collagen expression correlates with enhanced metastatic capacity and unfavorable clinical outcome.Citation15,Citation16 Under the harsh conditions of the TME, by expressing extracellular matrix (ECM) collagens and transmembrane collagens, tumor cells or tumor stroma may use this LAIR-1-collagen interaction to downregulate antitumor responses by various effector cells.Citation17,Citation18 Furthermore, LAIR-1 expression was shown to be upregulated on immune cells in tumor patients, suggesting that tumor cells hijack this immune regulatory mechanism to evade anti-cancer immune response.Citation19
Given the broad expression profile of hLAIR-1 on immune cells and the high abundance of collagen molecules in the TME, strategies targeting the collagen-hLAIR-1 interaction are needed. LAIR-2 is a soluble homolog, sharing 84% sequence homology with hLAIR-1, which may function as a natural competitor for LAIR-1.Citation20 In this study, we designed a novel molecule to block LAIR-1-mediated immune suppression. By fusing human LAIR-2 protein with hIgG-Fc fragment, we successfully blocked the interaction between LAIR-1 and its binding partners, resulting in increased T cell function and restored antitumor immune activity both in vitro and in vivo. By analyzing mRNA databases, we also observed elevated expression of LAIR-1 in several cancers, including Brain, Kidney and Ovarian cancers. Besides, LAIR-1 expression levels negatively correlate with survival rates for different solid tumor types. To investigate whether LAIR-1 inhibition synergizes with other immune therapies, we combined LAIR-2-Fc and the checkpoint inhibitor in mouse tumor models and observed a synergistic effect of the combination therapy. Our results suggest that blocking LAIR-1-mediated immune suppression may provide a novel treatment and additional opportunities in combination with conventional therapies across a broad spectrum of cancers.
Materials and methods
Mice
All animal experiments were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Henan University of Science and Technology. SOD/SCID, C57BL/6 J and BALB/c female mice (6–8 weeks old) were purchased from SLRC Laboratory Animal Co., Ltd. (Shanghai, China) and housed in a pathogen-free animal facility at the experimental animal center of Henan University of Science and Technology. The mice were fed standard chow diet and given access to distilled water ad libitum. Fresh cages were provided weekly. Sodium pentobarbital (60 mg/kg) was used for anesthesia, and the mice were euthanized using 100 mg/kg sodium pentobarbital.
Subjects and cells
The human cell lines A431, BxPC-3, and U937 were obtained from the American Tissue Type Collection (ATCC) and cultured in DMEM medium (Life Technologies) or RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (Life Technologies). The mouse cell lines EL4 and A20 (ATCC) were stably transfected with the human COL1A1 gene (Addgene), and COL1A1-expressing clones were selected using cytofluorimetry. EL4-COL1A1 and A20-COL1A1 cells were maintained in RPMI-1640 medium (Gibco) supplemented with 2 mM glutamine and 10% (vol/vol) fetal calf serum (Life Technologies) at 37°C and 5% (vol/vol) CO2. Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of healthy volunteers by Ficoll‐Paque density gradient centrifugation. Cells were resuspended in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated FCS, penicillin, and streptomycin. For donation of peripheral blood, all donors gave written informed consent and approval was obtained from the Ethics Committee of the Second Affiliated Hospital of Henan University of Science and Technology.
Bioinformatics analysis
To determine the clinical significance of LAIR-1, we examined the mRNA levels of LAIR-1 in extensive cancers and its relation to survival outcomes and immune marker genes using Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn)Citation21 Tumor Immune Estimation Resource (TIMER, https://cistrome.shinyapps.io/timer/)Citation22 and UALCAN (http://ualcan.path.uab.edu/index.html)Citation23 databases.
Generation of LAIR-2-Fc
The recombinant human LAIR-2-Fc protein construct was generated by genetically linking the human LAIR-2 to both ends of the Fc portion of human IgG1. The Fc domain was mutated with N297A to prevent binding to Fc receptors as described previously,Citation24 and were further mutated with T250Q/M428L mutations to increase the binding ability to neonatal FcR (FcRn).Citation25 Anti-CD20 IgG1 (Rituximab; accession number: DB00073) introduced with the same N297A and T250Q/M428L mutations in its Fc portion served as the control. The recombinant protein was expressed transiently in suspended human embryonic kidney (HEK293F) cells. Supernatants were harvested 7 days post-transfection and purified by affinity chromatography using Protein A-SepharoseTM (GE Healthcare). The purity of each recombinant antibody was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Pharmacokinetic (PK) parameters were examined in NOD/SCID mice by injecting LAIR-2-Fc protein (n = 4) at 10 mg/kg, followed by determining the serum antibody concentrations at various time points using human IgG ELISA Kits (Bethyl Laboratories). PK parameters were calculated from the final dataset using PK solver software.
Cell-binding analysis
To examine binding ability of LAIR-2-Fc fusion protein to collagens, we incubated 5 × 105 A431 or BxPC-3 cells with LAIR-2-Fc at different concentrations for 1 h at 4°C, followed by two washes with PBS. The cells were then incubated with an anti-human Fc-FITC mAb (Invitrogen) for 30 min on ice in the dark before two further washes with PBS. The cell-binding activity of purified LAIR-2-Fc fusion protein was determined by flow cytometry. Dissociation constants (KD) were calculated using GraphPad Prism 7.0 (Graph Pad Software).
For fusion protein blocking studies, ninety-six-well plates were coated with Collagen I/Collagen III (2 μg/mL; Sigma-Aldrich) in 100 μL of PBS, supplemented with 2 mM acetic acid. After washings, wells were blocked with 1% (wt/vol) BSA. Subsequently, collagens were incubated with different concentrations of LAIR-2-Fc fusion protein or IgG control. 5 × 105 cells per well of CSFE-labeled U937 cells (1 mM CFSE; Life Technologies) expressing hLAIR-1 were allowed to interact for 3 h and were then washed and analyzed by flow cytometry.
LAIR-2-Fc blocking experiments in vitro
T cells were isolated from human PBMCs by depletion of non-T cells using the Pan T Cell Isolation Kit (Miltenyi Biotec). Purified T cells were further stimulated and expanded using the human T Cell Activation/Expansion Kit (Miltenyi Biotec). Anti-Biotin MACSiBead Particles were loaded with CD2, CD3, and CD28 antibodies. T cells were expanded using 1 loaded Anti-Biotin MACSiBead Particle per 2 T cells. Expansion is achieved by adding interleukin 2 (IL-2) and fresh medium every 3–4 days. At day 14, additional loaded Anti-Biotin MACSiBead Particles were added and cells were further expanded for 2 weeks. Stimulated T cells were then cultured in the presence of 10 µg/mL collagens (Sigma) or collagens plus LAIR-2-Fc at several concentrations overnight at 37°C. IFN-γ and TNF-α were measured using Human IFN-γ and TNF-α ELISA kits (Abcam).
In vivo tumor killing studies
The anti-tumor properties of LAIR-2-Fc in immune-deficient mice were determined using xenograft models in NOD/SCID mice reconstituted with human T lymphocytes. Human T cells were purified from human PBMCs by depletion of non-T cells using the Pan T Cell Isolation Kit (Miltenyi Biotec). A431 cells or BxPC-3 cells (8.5 x 106/mouse) were subcutaneously (s.c.) inoculated into female NOD/SCID mice. Animals were randomly assigned into treatment groups (6–8 per group), with the mean tumor volume for each group being 100–150 mm3. Tumor volumes were determined according to the formula: tumor volume (mm3) = longer diameter × (shorter diameter)2 × 0.5 mm3. NOD/SCID mice were intravenously injected on day 7 with PBS, anti-CD20 IgG control or LAIR-2-Fc protein (5 mg/kg). Human T cells (1 × 107/mouse) from healthy donors were intravenously injected on day 7 and day 14. All animals in the experimental groups transplanted with tumor cells and human T cells received an i.v. bolus of LAIR-2-Fc protein on days 7, 10, 13, 16,19 and 21. Tumor size was monitored twice a week.
To investigate the anti-tumor effect of the combination therapy, A431 cells or BxPC-3 cells (8.5 × 106/mouse) were injected s.c. into NOD/SCID mice. After the mean tumor volume for each group reached 100–150 mm3, LAIR-2-Fc protein was injected intravenously (i.v.), alone or in combination with the anti-human PD-1 antibody Pembrolizumab (KEYTRUDA®, Merck) at a dosage of 10 mg/kg twice a week. Human T cells (1 × 107/mouse) were intravenously injected on day 7 and day 14.
To study the anti-tumor activities in immune competent mice, 6–8-week-old female C57BL/6 J mice were subcutaneously injected with 5 × 105 EL4-COL1A1 cells mixed in solubilized basement membrane matrix. Then mice were randomly assigned to treatment groups (6–8 mice per group), with the mean tumor volume for each group being 100–150 mm3. Mice were treated with anti-CD20 IgG control or LAIR-2-Fc fusion protein at a dose of 1 mg/kg or 5 mg/kg on days 0, 4, and 8. Murine B lymphoma cells A20 were stably transfected with human COL1A1 and were subcutaneously injected into BALB/c mice (5 x 105/mouse). Mice were then treated with LAIR-2-Fc fusion protein or IgG control similarly. Tumor size was monitored twice a week.
Analysis of tumor-infiltrating T cells
Tumors were isolated and dissociated using a Mouse Tumor Dissociation Kit with the gentle MACS Octo Dissociator (Miltenyi Biotec), according to the manufacturer’s protocol. Tumor-infiltrating T cells were then analyzed by flow cytometry. Single-cell suspensions (106 cells in a total volume of 100 μL) were pre-incubated with a Purified Rat Anti-Mouse CD16/CD32 monoclonal antibody (Fc block, BD Biosciences) and then stained with anti-CD3-APC and anti-CD8-FITC antibodies (BD Biosciences) at 4°C for 30 min. FACS analysis was performed on a BD FACS Verse system.
Enzyme-linked immunosorbent assay (ELISA) experiments
To study the T cell-mediated cytotoxicity after treatment, whole tumors were lysed in RIPA buffer, and total protein concentrations were determined using a BCA Protein Assay Kit (Pierce). Five hundred micrograms of total protein lysate from cells were used in ELISAs to detect IFNγ, TNFα, and Granzyme B (Simple Step ELISA Kits, Abcam), and protein expression was normalized to mg of total protein.
Statistics
Data were analyzed and graphs prepared using GraphPad Prism 7.0 (Graph Pad Software). Data are expressed as the median and range or mean + the standard error of the mean (SEM). Data were analyzed with unpaired, two-tailed Student’s t-tests and two-way analysis of variance (ANOVA). P < .05 was considered statistically significant (*P < .05, **P < .01, ***P < .001, ****P < .0001). Experiments were repeated three times to ensure reproducibility of the observations.
Results
High LAIR-1 expression correlates with poor prognosis of patients with cancer
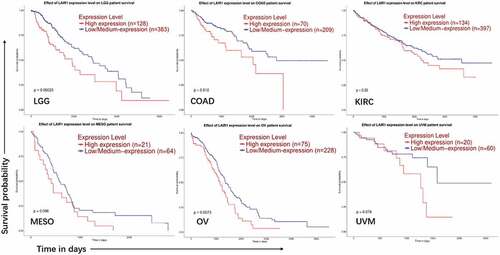
A previous study showed that the expression level of many known ITIM receptors inversely correlates with the overall survival of AML patients and that some of these receptors are crucial for the growth of human acute leukemia cells.Citation26 In this study using the GEPIA (Gene Expression Profiling Interactive Analysis) and TIMER (Tumor IMmune Estimation Resource) databases, we found upregulated transcriptional levels of LAIR-1 in a wide range of tumors when comparing with their adjacent normal tissues. The mRNA expression levels of LAIR-1 were significantly upregulated in patients with LGG: Brain Lower Grade Glioma; OV: Ovarian serous cystadenocarcinoma; PAAD: Pancreatic adenocarcinoma; SKCM: Skin Cutaneous Melanoma; STAD: Stomach adenocarcinoma; THYM: Thymoma; ESCA: Esophageal carcinoma; GBM: Glioblastoma multiforme; KIRC: Kidney renal clear cell carcinoma; KIRP: Kidney renal papillary cell carcinoma; LAML: Acute Myeloid Leukemia. (Figure S1). We also observed the LAIR-1 expression level was negatively related to the survival rates of some cancers including brain, colon, kidney, ovarian and intraocular cancers. The Kaplan-Meier log-rank survival curves demonstrated that the overall survival of patients of these cancers with high expression of LAIR-1 were significantly shorter than those in patients with low expression (). To investigate the relationship between LAIR-1 and the other immune related genes, we analyzed the correlations between LAIR-1 expression and immune marker genes in the TIMER databases, including CD8A, GZMA, TNF and IFNG. The results revealed that LAIR-1 expression level was positively correlated with T cells related immune genes (Figure S2). These analyzes suggest that targeting LAIR-1 could serve as a potential therapeutic treatment in both hematologic and solid malignancies.
Figure 1. Association of LAIR-1 mRNA expression levels with overall survival
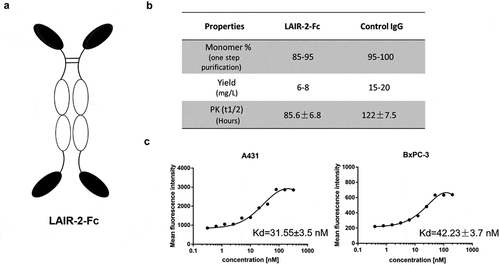
Construction and characterization of LAIR-2 fusion protein
Since LAIR-2 can function as a natural competitor for LAIR-1, we developed a novel recombinant protein to block LAIR-1-mediated immune suppression by fusing the human LAIR-2 with human IgG-Fc, according to the structure indicated in ). For LAIR-1 blocking experiments, the Fc fragment was mutated with N297A to prevent binding to Fc receptors. We further mutated the Fc domain with T250Q/M428 L mutations to increase the FcRn binding ability, and the resultant recombinant protein exhibited high yield, stability, and a longer serum half-life ()). With two human LAIR-2 fragments, the tetravalent protein is supposed to have a higher binding affinity to collagens. Therefore, we tested the capacity of LAIR-2-Fc fusion protein to bind tumor cell-expressed collagens on cancer cell lines. The binding affinity of the recombinant protein to collagen-expressing cell lines A431 and BxPC-3 was determined using flow cytometry, as KD of 31.55 nM (±3.5) and 42.23 nM (±3.7) ()), respectively.
Figure 2. Engineering and characterizing of LAIR-2-Fc recombinant protein. (a) Structural model of LAIR-2-Fc fusion protein generated in this study. The format is comprised of IgG-Fc linked to LAIR-2 fragments. (b) A summary of the properties of LAIR-2-Fc and the control IgG1. (c) Apparent binding affinities of the LAIR-2-Fc recombinant protein. Increasing concentrations of LAIR-2-Fc was incubated with collagen-expressing A431 and BxPC-3 cells. The binding of LAIR-2-Fc to both cell lines was assessed by flow cytometry. All data are presented as the mean ± SEM (n = 3) from one of three representative experiments
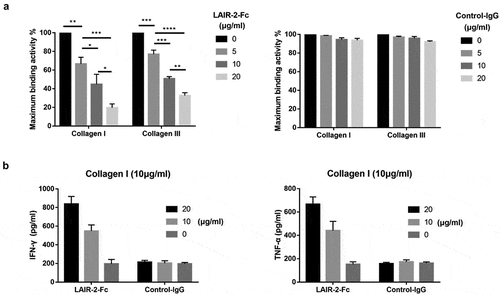
Reversal of LAIR-1-mediated immune suppression by LAIR-2 in vitro
First, we demonstrated that LAIR-2-Fc fusion protein inhibited collagens binding to LAIR-1–expressing U937 AML cells in a dose-dependent manner. LAIR-2–mediated inhibition of binding activity is shown as a percentage of maximum binding activity in ). Next, we investigated whether LAIR-2-Fc fusion protein could prevent immune suppression caused by LAIR-1 binding to collagen and promote antitumor immune activity. To this end, we stimulated T lymphocytes obtained from healthy individuals with anti-CD2, anti-CD3 and anti-CD28 in the presence or absence of collagen and LAIR-2-Fc fusion protein. As shown in ), binding of collagen I to human T cells reduced the production of IFN-γ and TNF-α. Preincubation of collagen with LAIR-2-Fc increased IFN-γ and TNF-α expression, suggesting that LAIR-2 reversed the collagen-mediated inhibition of T immune cell function in a dose-dependent manner.
Figure 3. LAIR-2 reversed immune suppression mediated by LAIR-1. (a) LAIR-2-Fc blocked the binding of collagen I/III to LAIR-1-expressing U937 AML cells in a dose-dependent manner. Ninety-six-well plates were coated with collagen I or III. Subsequently, collagen I or III was incubated with different concentrations of LAIR-2-Fc fusion protein or control IgG protein. CSFE-labeled U937 cells expressing hLAIR-1 were allowed to interact for 3 h. Percentage of adhering cells is shown. (b) Collagen-mediated suppression of IFN-γ and TNF-α production in human T cells is blocked by LAIR-2. T cells were stimulated with anti-CD2, anti-CD3 and anti-CD28 in the presence of collagen I (10 µg/mL), LAIR-2-Fc or control IgG. IFN-γ and TNF-α were measured in culture supernatants using IFN-γ- or TNF-α specific ELISA. Data represent the mean ± SEM (n = 3). *P < .05; **P < .01; ***P < .001; ****P < .0001
Antitumor activity of LAIR-2 fusion protein in vivo
To further examine the in vivo efficacy of blocking collagen-hLAIR-1 interaction, xenograft models of collagen-expressing human cancer cells were used. A431 or BxPC-3 cells, with high expression levels of collagens and capacity to bind human LAIR-1/LAIR-2,Citation27 were transplanted subcutaneously into T and B cell-deficient NOD/SCID mice to develop tumors. Once the tumors became palpable, the mice were injected intravenously with T lymphocytes from healthy human donors in the presence or absence of LAIR-2-Fc fusion protein, according to the schedule ()). As shown in ), injections of LAIR-2-Fc fusion protein and human T cells induced significant tumor regression in both A431 and BxPC-3 xenograft models compared with mice treated with a control.
Figure 4. Antitumor activity of LAIR-2 fusion protein in vivo. (a) Treatment plan of mouse xenograft model. NOD/SCID mice subcutaneously transplanted with A431 or BxPC-3 cells were treated with the indicated proteins (n = 8 per treatment group) when palpable tumors (100 ∼150mm3) were formed. Tumor-xenografted NOD/SCID mice were intravenously injected on day 7 and day 14 with human T cells (1 × 107 cells/mouse). LAIR-2-Fc (5 mg/kg) or IgG control were injected intravenously every three days. (b) Tumor volumes are plotted as the mean ± SEM. ***p < .001; ****p < .0001, p-values were derived from two-way ANOVA. (c) Tumor-infiltrating CD8 + T cells were analyzed by flow cytometry. A431 tumors were harvested on day 30. CD8 + T cells were gated on CD45+ CD3+ CD8+ populations. (d) TNF-α and IFN-γ protein concentrations measured in tumor cells isolated from A431 tumors after treatment (n = 6 biological replicates; **P < .01, and ****P < .0001 compared to PBS)
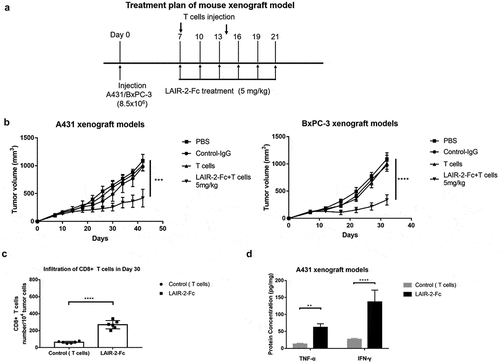
In addition, we sought to determine whether blocking collagen-hLAIR-1 interaction may induce infiltration of immune cells in the tumor microenvironment. Flow cytometric analysis of tumor-infiltrating immune cells isolated from LAIR-2-Fc fusion protein-treated A431 tumors on day 30 showed significantly more infiltration of human CD8+ cytotoxic T cells, compared to PBS-treated tumors ()). The strong control of tumor growth observed by LAIR-2-Fc fusion protein also coincided with increased antitumor immunity. Protein-expression signatures associated with productive IFNγ responses and TNFα responses were significantly enriched after therapy ()). Collectively, these results suggest that LAIR-2-Fc fusion protein can promote T cell recruitment and antitumor T cell responses in the TME that induce sustained tumor regression in mouse models of cancer.
LAIR-2 fusion protein and PD-1/PD-L1 inhibition synergized to control tumor growth
Based on the above findings, we hypothesized that blocking LAIR-1-collagen interaction would synergistically enhance anti-PD-1/PD-L1 immunotherapy. We subcutaneously injected collagen- and PD-L1-positive A431 cells and BxPC-3 tumor cells into NOD/SCID mice respectively. When the tumor volumes reached 100–150 mm3, the mice were injected intravenously with T lymphocytes from healthy human donors in the presence or absence of LAIR-2-Fc fusion protein and an anti-human PD-1 antibody (Pembrolizumab), alone or together ()). Mice treated with LAIR-2-Fc fusion protein or pembrolizumab alone exerted an effect on the inhibition of tumor growth, while remarkable tumor regression was observed in the combination-treatment group as measured by tumor volume. ()). Consistent tumor regression was also observed using the combination strategy against established BxPC-3 tumor model ()).
Figure 5. Targeting LAIR-1 synergized with anti-PD-1 blockade. (a) Treatment plan of the combination therapy. NOD/SCID mice subcutaneously transplanted with A431 or BxPC-3 cells (both collagens and PD-L1 are expressed) were treated with the indicated proteins (n = 8 per treatment group) when palpable tumors (100∼150mm3) were formed. Tumor-xenografted NOD/SCID mice were intravenously injected on day 7 and day 14 with human T cells (1 × 107 cells/mouse). LAIR-2-Fc (5 mg/kg) with or without anti-PD-1 mAb (10 mg/kg) were injected intravenously every three days. (b, c) Tumor volumes of A431 (b) and BxPC-3 (c) xenograft models are plotted as the mean ± SEM. (d) A431 cells were injected s.c. into mice (n = 10 mice/group). Antibodies were simultaneously administered injected during A431 cell implantation by i.v. injection. Tumor volumes are plotted as the mean ± SEM. ns mean non- significant, *P < .05 and ****P < .0001
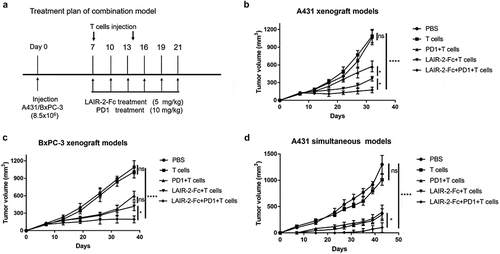
We then evaluated the efficacy of the combination strategy using an A431 tumor model in which LAIR-2-Fc and/or pembrolizumab were simultaneously injected during A431 cell implantation. The therapeutic efficacy of either single agent was limited, whereas the combination therapy exhibited synergistic effects and restrained the tumor progression ()). In conclusion, the results showed that combination therapy of blocking LAIR-1-collagen interaction and anti-PD-1/PD-L1 immunotherapy led to significant elimination of human tumor cells.
Tumor lysis by LAIR-2 fusion protein in immune competent mice
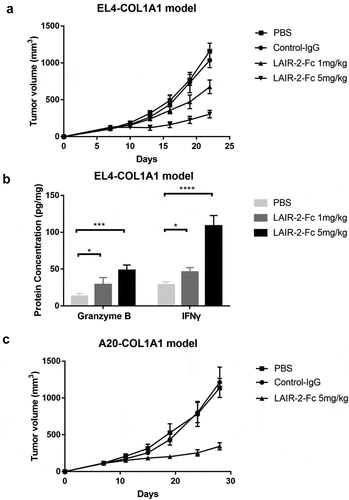
The mouse homologue of LAIR-1 (mLAIR-1) shares 40% sequence identity with hLAIR-1,Citation28,Citation29 and is known to bind collagens expressed by human tumor cells.Citation27 We also proved in vitro that LAIR-2-Fc fusion protein will block the interaction between human collagen I and mouse LAIR-1 (Figure S3). Therefore, we tested the antitumor activity of LAIR-2-Fc fusion protein in immune competent mice. EL4-COL1A1 cells, a murine lymphoma cell line (without expression of mouse LAIR-1 and collagens) transfected with human COL1A1 gene (collagen I) to facilitate tumor growth, were subcutaneously injected in C57BL/6 J mice. Mice were treated with LAIR-2-Fc fusion protein or IgG control when the tumor volumes reached 100–150 mm3. Proteins were administered by i.v. injection. As shown in ), we observed that mice treated with LAIR-2-Fc fusion protein had delayed tumor growth and smaller tumors than mice treated with a control. Consistent tumor regression was also observed in the A20-COL1A1 tumor model after the treatment of LAIR-2-Fc fusion protein ()). Additionally, in agreement, we confirmed that LAIR-2-Fc fusion protein promoted cytokine production and upregulation of markers associated with T cell cytotoxicity such as granzyme B, as T cells from LAIR-2-Fc treated mice expressed significantly more granzyme B and IFN-γ respectively than T cells from IgG control-treated mice ()).
Figure 6. Tumor lysis by LAIR-2 fusion protein in immune competent mice. (a) Effect of the LAIR-2 fusion protein on the growth of murine lymphoma cell line EL4 transfected with human COL1A1 gene in C57BL/6 J mice. EL4-COL1A1 cells were mixed in solubilized basement membrane matrix and injected subcutaneously into female mice (n = 6–8/group). Proteins were administered by i.v. injection on days 0, 4, and 8. Animals were monitored for tumor growth. Tumor volumes are plotted as the mean ± SEM. (b) IFNγ and granzyme B protein concentrations measured in T cells isolated from EL4-COL1A1 tumors after treatment (n = 5 biological replicates). (c) A20- COL1A1 cells were injected subcutaneously into female BALB/c mice (n = 6–8/group). LAIR-2 fusion protein or IgG control were injected intravenously on days 0, 4, and 8. Animals were monitored for tumor growth. Tumor volumes are plotted as the mean ± SEM. *P < .05, ***P < .001 and ****P < .0001
Discussion
Tumor cells use multiple strategies to avoid or block antitumor immune responses. One such mechanism involves hijacking normal immune regulatory mechanisms. Most cells of the immune system express multiple immune inhibitory receptors. Immunoreceptor tyrosine-based inhibition motif (ITIM) containing receptors have been shown to inhibit signaling from immunoreceptor tyrosine-based activation motif (ITAM) containing receptors, and the majority of these receptors are involved in tumor development and regulation of the immune system.Citation30 ITIM receptors and their downstream signaling molecules have been demonstrated as potential therapeutic targets to treat cancer, such as PD-1, CTLA-4, Siglec-15, and LILRB1.Citation31,Citation32
Our approach targeting a representative ITIM-receptor LAIR-1 identified a novel potential angle to combat cancer. Researches have showed LAIR-1 overexpression as a consequential factor in the development of kidney and ovarian cancers.Citation19,Citation33 Using bioinformatics analysis, we identified LAIR-1 overexpression in a broad range of tumor types, and in several tumors this expression significantly correlated with poor overall survival rates in patients. Taking advantage of the natural LAIR-2 regulatory system in humans, we established a recombinant LAIR-2-Fc fusion protein that can block the interaction between LAIR-1 and its ligand collagens. We observed a reversal of T cell immune suppression in vitro when LAIR-2-Fc fusion protein was added to the culture. Moreover, LAIR-2-Fc fusion protein administration delayed tumor growth in immune-deficient mice injected with human T cells and in C57BL/6J mice. In addition, antitumor activity of LAIR-2-Fc correlated with increased infiltration and function of CD8 + T cells in the TME, supporting our hypothesis that eliminating or blocking the binding of LAIR-1 to collagen can restore normal immune function and result in antitumor activity.
The inhibitory potential of LAIR-1 was initially demonstrated by cross-linking the molecule via mAb in vitro. In addition to T cells, crosslinking of LAIR-1 on human NK cells delivers a potent inhibitory signal that is capable of inhibiting target cell lysis by resting and activated NK cells.Citation10 LAIR-1 can also suppress neutrophil tissue migration and acts as a negative regulator of neutrophil-driven airway inflammation during lung diseases.Citation34 Furthermore, C1q is a functional ligand for LAIR-1 besides collagen, and C1q-LAIR-1 interactions inhibit DC (dendritic cell) differentiation and activation, which can be reversed by LAIR-2.Citation20 These studies suggest that, besides suppressing T cell immune responses in the TME, interactions between LAIR-1 and collagen or C1q may result in the suppression of various immune cells. Furthermore, eliminating these interactions is likely to restore the antitumor activities of T cells, DCs, macrophages, PMNs (polymorphonuclear neutrophils), etc. It will be important to investigate the underlying mechanisms by which LAIR-1 supports cancer progression and the consequences of blocking LAIR-1-collagen/C1q interaction.
ITIM receptors represent ideal targets for treating tumors, and strategies targeting these receptors have achieved great success, such as PD-1/PD-L1, CD47/SIRPα blockade therapies.Citation35,Citation36 However, to our knowledge, targeting LAIR-1 to treat tumors has not yet been reported. We not only showed that blocking LAIR-1 could inhibit tumor growth, but also indicated its potential to enhance the efficacy of PD-1 blockade by combination therapy via an improvement in the immune functions of T cells. Given the wide expression of LAIR-1 in multiple tumor subtypes, therapies blocking LAIR-1 could be applied in combination with other immune checkpoint blockers. Our study shows a potential target for a wide spectrum of tumors, and combination with conventional therapies may prove to be an effective strategy for the elimination of cancers that cannot be treated by present therapies.
Declarations of interest
The authors declare no competing interests.
Supplemental Material
Download ()Acknowledgments
We wish to thank all members of our laboratory for their helpful suggestions and support. This study was supported by grants from Key Scientific Research Projects of Universities in Henan Province (19B350002).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Vivier E, Daëron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18(6):286–9. doi:10.1016/S0167-5699(97)80025-4.
- Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7(2):283–290. doi:10.1016/S1074-7613(00)80530-0.
- Poggi A, Pella N, Morelli L, Spada F, Revello V, Sivori S, Augugliaro R, Moretta L, Moretta A. p40, a novel surface molecule involved in the regulation of the non-major histocompatibility complex-restricted cytolytic activity in humans. Eur J Immunol. 1995;25(2):369–376. doi:10.1002/()1521-4141.
- Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79(4):828–836. doi:10.1189/jlb.0705370.
- Poggi A, Tomasello E, Ferrero E, Zocchi MR, Moretta L. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur J Immunol. 1998;28(7):2086–2091. doi:10.1002/()1521-4141.
- Florian S, Sonneck K, Czerny M, Hennersdorf F, Hauswirth AW, Buhring H-J, Valent P. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61(9):1054–1062. doi:10.1111/all.2006.61.issue-9.
- Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224(1):11–43. doi:10.1111/imr.2008.224.issue-1.
- Meyaard L, Hurenkamp J, Clevers H, Lanier LL, Phillips JH. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol. 1999;162:5800–5804.
- Maasho K, Masilamani M, Valas R, Basu S, Coligan J, Borrego F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol Immunol. 2005;42(12):1521–1530. doi:10.1016/j.molimm.2005.01.004.
- Tomasello E, Revello V, Nanni L, Costa P, Moretta L, Poggi A, p40 molecule regulates NK cell activation mediated by NK receptors for HLA class I antigens and TCR-mediated triggering of T lymphocytes. Int Immunol. 1997;9(9):1271–1279. doi:10.1093/intimm/9.9.1271.
- Jansen CA, Cruijsen C, de Ruiter T, Nanlohy N, Willems N, Janssens-Korpela P-L, Meyaard L. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur J Immunol. 2007;37(4):914–924. doi:10.1002/()1521-4141.
- Saverino D, Fabbi M, Merlo A, Ravera G, Grossi CE, Ciccone E. Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum Immunol. 2002;63(7):534–546. doi:10.1016/S0198-8859(02)00409-3.
- Lebbink RJ, de Ruiter T, Kaptijn GJA, Bihan DG, Jansen CA, Lenting PJ, Meyaard L. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int Immunol. 2007;19(8):1011–1019. doi:10.1093/intimm/dxm071.
- Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203(6):1419–1425. doi:10.1084/jem.20052554.
- Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5(3):e9808. doi:10.1371/journal.pone.0009808.
- Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706. doi:10.1016/j.ceb.2010.08.015.
- Meyaard L. LAIR and collagens in immune regulation. Immunol Lett. 2010;128(1):26–28. doi:10.1016/j.imlet.2009.09.014.
- Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305). J Leukoc Biol. 2008;83(4):799–803. doi:10.1189/jlb.0907609.
- Jingushi K, Uemura M, Nakano K, Hayashi Y, Wang C, Ishizuya Y, Yamamoto Y, Hayashi T, Kinouchi T, Matsuzaki K, et al. Leukocyteassociated immunoglobulinlike receptor 1 promotes tumorigenesis in RCC. Oncol Rep. 2019;41(2):1293–1303. doi:10.3892/or.2018.6875.
- Lebbink RJ, van den Berg MCW, de Ruiter T, Raynal N, van Roon JAG, Lenting PJ, Jin B, Meyaard L. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J Immunol. 2008;180(3):1662–1669. doi:10.4049/jimmunol.180.3.1662.
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247.
- Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307.
- Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002.
- Li B, Xu L, Pi C, Yin Y, Xie K, Tao F, Li R, Gu H, Fang J. CD89-mediated recruitment of macrophages via a bispecific antibody enhances anti-tumor efficacy. Oncoimmunology. 2017;7(1):e1380142. doi:10.1080/2162402X.2017.1380142.
- Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs. 2011;3(5):422–430. doi:10.4161/mabs.3.5.16983.
- Kang X, Lu Z, Cui C, Deng M, Fan Y, Dong B, Han X, Xie F, Tyner JW, Coligan JE, et al. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat Cell Biol. 2015;17(5):665–677. doi:10.1038/ncb3158.
- Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol. 2011;49(1–2):402–406. doi:10.1016/j.molimm.2011.09.006.
- Lebbink RJ, de Ruiter T, Verbrugge A, Bril WS, Meyaard L. The mouse homologue of the leukocyte-associated Ig-like receptor-1 is an inhibitory receptor that recruits Src homology region 2-containing protein tyrosine phosphatase (SHP)-2, but not SHP-1. J Immunol. 2004;172(9):5535–5543. doi:10.4049/jimmunol.172.9.5535.
- Lebbink RJ, de Ruiter T, Kaptijn GJ, Meyaard L. Identification and characterization of the rat homologue of LAIR-1. Immunogenetics. 2005;57(5):344–351. doi:10.1007/s00251-005-0804-4.
- Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–177.
- Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, Zhang J, Song C, Zarr M, Zhou X, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25(4):656–666. doi:10.1038/s41591-019-0374-x.
- Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19(1):76–84. doi:10.1038/s41590-017-0004-z.
- Cao Q, Fu A, Yang S, He X, Wang Y, Zhang X, Zhou J, Luan X, Yu W, Xue J. Leukocyte-associated immunoglobulin-like receptor-1 expressed in epithelial ovarian cancer cells and involved in cell proliferation and invasion. Biochem Biophys Res Commun. 2015;458(2):399‑404. doi:10.1016/j.bbrc.2015.01.127.
- Kumawat K, Geerdink RJ, Hennus MP, Roda MA, van Ark I, Leusink-Muis T, Folkerts G, van Oort-jansen A, Mazharian A, Watson SP, et al. LAIR-1 limits neutrophilic airway inflammation. Front Immunol. 2019;10:842. doi:10.3389/fimmu.2019.00842.
- Jiang W, Kim YS, Zhang CC, Fu YX, Weissman IL.Feng M. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(8):568–586.
- Barclay AN, van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32(1):25–50. doi:10.1146/annurev-immunol-032713-120142.
