ABSTRACT
Background: Despite the improvement in therapeutic interventions, 5-year survival rates in Head and Neck Squamous Cell Carcinoma (HNSCC) are limited. HNSCC is an immunogenic cancer type for which molecular stratification markers are lacking. Tumor-infiltrating lymphocytes (TILs) have shown a favorable prognostic role in different cancer types. This study focused on the prognostic role of NK cells in HNSCC.
Methods: A systematic search was conducted in Pubmed/Medline and Embase. Articles that correlated the presence of intratumoral NK cells, activating/inhibiting receptors, death receptors, or their ligands with clinicopathologic characteristics or survival were included. A meta-analysis was performed that assessed the association between CD56+ and CD57+ and overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS).
Results: A pooled analysis indicated a favorable prognostic role of CD56+ and CD57+ NK cells for OS (HR 0.19 CI 0.11–0.35). NK cell markers NKp46 and Granzyme B (GrB) also have a favorable prognostic role. NK cell ligand Fas correlated with better survival and better characteristics. NK cell marker Fas-L, NK cell ligands CEACAM1, RCAS1, CD70 and TRAIL-R, and effector molecules of these ligands, FADD and FAP1, correlated to features of worse prognosis.
Conclusion: A favorable prognostic role of NK cells in HNSCC was found in this review. Some studies implied the opposite, indicating the fine balance between pro- and anti-tumor functions of NK cells. Future studies using homogeneous patient cohorts regarding tumor subsite and treatment modality, are necessary to further provide insight into the prognostic role of NK cells.
Introduction
The majority of Head and Neck Cancers (HNC) comprise squamous cell carcinomas arising from the stratified epithelium of the oral cavity (OC), nasopharynx (NP), hypopharynx (HP), oropharynx (OP) and larynx (L).Citation1 HNC is the sixth most common cancer worldwide with approximately 600,000 new cases per year.Citation2,Citation3 The mortality due to Head and Neck Squamous Cell Carcinoma (HNSCC) is 380.000 per year, making it the seventh most common cause of cancer-related mortality worldwide.Citation4 In recent decades, research has focused on identifying prognostic biomarkers in HNSCC to counteract major issues: late diagnosis and locoregional recurrence.
The archetypal patient with HNSCC is of male gender between the ages of 50–70 years, with a history of tobacco and alcohol use, both identified as risk factors.Citation5 Over the last decades another risk factor has been identified: the human papillomavirus (HPV). The most HPV positive HNSCC occurs in the oropharynx of a younger population.Citation6,Citation7
Roughly 60% of HNSCC patients have an advanced stage disease at the time of diagnosis, due to limited awareness and knowledge of alarm symptoms, and the characteristic rapid proliferation of HNSCC.Citation8 Currently, treatment methods are insufficient; locoregional recurrence occurs in 15-50% of patients,Citation9 and for all clinical stages combined, five-year survival rates are between 56%-62%.Citation10,Citation11 This calls for reliable prognostic biomarkers that enable clinicians to stratify patients in risk groups and treating them accordingly, improving personalized medicine. Cancer development is facilitated by the inability of the immune system to recognize and eliminate tumor cells. In HNSCC, an immunogenic cancer type, immune escape mechanisms are key to tumor initiation and progression. Since the host immune system plays an important role in the development of cancer, the scientific necessity arose to further employ the immune system for the treatment of HNSCC. Currently, cetuximab, an EGFR (endothelial growth factor receptor) inhibitor, is used in case a contradiction for chemotherapy exists.Citation12,Citation13 The antitumor effect of cetuximab is strengthened by binding the FcY receptor on NK cells enabling antibody-dependent cellular cytotoxicity (ADCC).Citation14
Immunological markers have shown to be prognostic indicators, even superior to the TNM staging system.Citation15 In lung, colorectal and breast cancer, studies have shown that tumor-infiltrating lymphocytes have a favorable prognostic role.Citation16–Citation18 A meta-analysis conducted by de Ruiter et al. (2017) reports findings in concordance with the latter for HNSCC.Citation19
NK cells are an essential component of the innate immune system as an early line of defense against tumor cells and act by killing them. They could, therefore, function as a prognostic biomarker in HNSCC. NK cell subpopulations can be defined based on CD56 expression on the surface of the cells, in which CD56bright cells express a relatively high density of CD56 and CD56dim cells express the relatively low density of CD56. Classically, NK cells are divided in CD56bright CD57− immune regulatory cells and CD56dim CD57+ cytotoxic cells.Citation20 In most studies CD56 is the archetypal phenotypic marker of NK cells.Citation21 Another marker, CD57, is a marker of differentiated and highly cytotoxic NK cells and is often used in studies, as it has been described as a phenotypically stable NK cell marker.Citation22
NK cells are regulated by activating membrane-bound receptors that enable lysis of target cells that fail to express sufficient levels of MHC class 1 (missing self hypothesis) and by inhibitory receptors that protect cells that do express MHC class 1. These inhibitory and activating receptors are killer-cell immunoglobulin like receptors (KIRs). Important activating receptors are NKG2D, that has MICA/B as a ligand, and NpK30, NpK44, and NpK46, that have a broad variety of ligands. Upon activation, NK cells release granules containing perforin and granzymes, and produce cytokines, causing cell death. NK cells also express death receptor ligands (Fas-L, TNF-α, TRAIL), that induce cell death upon binding death receptors (Fas, TRAIL-R (DR4, DR5)) on target cells.Citation23 These receptors can serve as potential markers for NK cells in HNSCC. In breast and colorectal cancer, reviews report that NK cells are of positive prognostic value.Citation24,Citation25
Objectives
This study was conducted to shed light on the prognostic role of NK cells and their ligands in HNSCC by systematically reviewing the literature. The main goal was to include all studies that assessed tumor infiltration with CD56+ and/or CD57+ lymphocytes, NK cell-activating/inhibiting receptors, death receptors or their ligands as prognostic biomarkers in HNSCC.
Results
Study selection
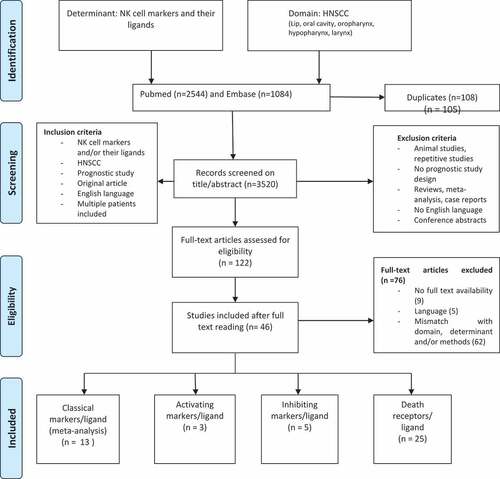
The initial search yielded 3520 references, after the removal of 180 duplicates. After title/abstract screening, reviewers SKB and ERU selected 122 articles, which were subjected to full-text reading. Of the selected 122 articles, 9 were not available, 4 were in Chinese, 1 in German, 1 had missing data and 61 did not comply with our inclusion criteria regarding the determinant, domain and/or methods. Of the 46 articles that were eligible for inclusion, 13 assessed classical NK cell markers.Citation26–Citation38 The activating markers group existed of three articles,Citation39–Citation41 the inhibiting markers group of 5Citation42–Citation46 and the death receptor group of 25.Citation47–Citation71 provides an overview of study selection.
Figure 1. Flow chart of the 3520 articles initially selected, 49 were included after full-text screening. (Moher et al. 2009. prisma flow chart).Citation98.
Critical appraisal
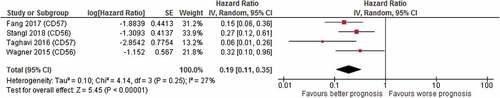
Critical appraisal for risk of bias was applied on the 13 articles of the classical markers group by use of the QUIPS criteria. All studies scored poorly in the study attrition domain, because none of the studies mentioned the patient’s loss to follow-up. This domain was therefore not considered when assessing the overall risk of bias. Because assessing survival analysis by hazard ratios is crucial for conducting a meta-analysis, studies that did not mention hazard ratios were excluded. Exclusion was therefore not based on total bias score. The quality assessment is shown in . The four articles that mentioned hazard ratiosCitation26–Citation29 were selected for meta-analysis after critical appraisal; the forest plot is shown in .
Table 1. Quality assessment of the classical marker studies. Total bias score excluded study attrition
Figure 2. Forest plot of prognostic value of CD56+/CD57+ NK cells on overall survival; high CD56/CD57 cell count correlated with better overall survival (HR 0.19, CI 0.11–0.35). (RevMan 2014).Citation99.
Classical markers as a predictor for survival
A total of six studies reported on CD56+ cells. The presence of CD56+ NK cells was associated with a better OS (Wagner et al.: HR 0.32, CI 0.10–0.96, p 0.04; Stangl et al.: HR 0.27, CI 0.12–0.60, p 0.001), local PFS (Stangl et al.: HR 0.35, CI 0.17–0.74, p 0.005) and distant metastasis-free survival (DMFS) (Stangl et al.: HR 0.27, CI 0.13–0.55, p 0.004). A total of seven studies reported on CD57+ cells. High numbers of CD57+ NK cells were associated with a better OS (Fang et al.: HR 0.15 CI 0.06–0.36 p < .001; Taghavi et al.: HR 0.06 CI 0.01–0.26 p < .001).Citation26–Citation30 The pooled meta-analysis showed an advantage for high CD56+/CD57+ NK cells regarding OS (pooled HR 0.19 CI 0.11–0.35) (). Some studies mentioned no correlation between CD56+ NK cell number and OS or recurrence-free survival (RFS) or between CD57+ NK cells and OS (de Carvalho Fraga et al.: OR 1.04,CI 0.44–2.46, p 0.93) or DFS.Citation31–Citation35 One study reported on CD16+ cells and found no correlation between CD16+ cells and DFS.Citation36
Classical markers as a predictor for clinicopathologic characteristics
Next, the correlation between classical markers and the known clinicopathologic characteristics that influence prognosis was investigated. One study found that high infiltration of CD57+ cells correlated with T3/T4 tumors and cervical metastasis (de Carvalho Fraga et al.: resp. OR 5.610, CI 1.516–20.763, p 0.010 and OR 3.401, CI 1.162–9.951, p 0.025). These results indicate that in this study CD57+ cells correlated with features of worse prognosis.Citation40 Contradictory, many studies mention the correlation of high CD57+ cell number with features of better prognosis; absence of lymph node metastasis, early clinical stages.Citation37,Citation38 Other studies have noted a trend toward features of better prognosis; fewer cases of nodal metastasis, advanced-stage disease, disease relapses, lower probability of local recurrence (LR) and death.Citation39,Citation45 One study mentioned a higher number of CD56+ NK cells in a study group without metastatic disease.Citation46 Another study found no correlation between CD16+ NK cells and tumor location, TNM stage, or recurrence of the disease.Citation44
Supplementary Table 2 mentions the characteristics of the studies that assessed classical markers.
Table 2. The outcomes of activating and inhibiting marker studies are summarized based on high expression of the markers in question. Oral Cavity (OC), Oropharynx (OP), Hypopharynx (HP), Larynx (L), Lip (Lip), Tongue (T). Overall survival (OS), Disease-free survival (DFS), Progression-free survival (PFS), Distant metastasis-free survival (DMFS), Disease-specific survival (DSS), Local recurrence (LR), Local-regional control (LRC)
Activating markers as predictors for survival and clinicopathologic characteristics
A total of two studies reported on NKp46+ NK cells; one study mentioned that NKp46+ NK cells alone were not associated with survival and the other study reported that NKp46+ NK cells were more abundant in low-grade tumors.Citation39,Citation40
One study investigated the prognostic role of tumoral CD70 expression. Tumoral CD70 expression was higher in poorly differentiated carcinomas. There was no correlation with TNM stage. High tumor CD70 expression correlated with a trend toward lower density of TIL’s.Citation41
Inhibiting markers as predictors for survival and clinicopathologic characteristics
A total of four studies reported on CEACAM1. Three studies mentioned that high CEACAM1 expression correlated with worse survival and features of worse prognosis; high tumor grade, local recurrence, lymph node metastasis, distant metastasis, and high clinical stage.Citation42–Citation45 One study mentioned contradictory results and found that high CEACAM1 expression correlates with better OS and DFS and features of better prognosis.Citation43
RCAS1 expression in tumor cells was investigated in one study, which found that it was associated with high-grade tumors and the presence of lymph node metastasis.Citation46
See for a summary of outcomes and supplementary Table 3 for study characteristics of the activating and inhibiting markers.
Table 3. The outcomes of death receptor studies are summarized based on high expression of the markers in question. Oral Cavity (OC), Oropharynx (OP), Hypopharynx (HP), Larynx (L), Lip (Lip), Tongue (T). Overall survival (OS), Disease-free survival (DFS), Progression-free survival (PFS), Distant metastasis-free survival (DMFS), Disease-specific survival (DSS), Local recurrence (LR), Local-regional control (LRC)
Death receptors as predictors for survival and clinicopathologic characteristics
A total of four studies reported on Fas or Fas-L and four other studies reported on both markers.
Fas expression in tumor cells correlated with negative lymph nodes (de Carvalho-Neto et al.: OR 5.02 CI 1.34–18.75 p 0.017), absence of LR, lower clinical stage, better OS, better disease-specific survival (DSS) (de Carvalho-Neto et al.: HR 0.268 CI 0.083–0.862 p 0.027).Citation49,Citation50,Citation53 Contradictory, one study mentioned Fas expression associated with a higher clinical stage.Citation47
Fas-L expression in the tumor is correlated with higher T stage, N stage, clinical stage, and worse DFS (de Carvalho-Neto et al.: HR 2.58 CI 1.03–6.46 p 0.044).Citation47,Citation50,Citation52,Citation57 One study mentioned that strong Fas-L expression in lymphoid cells was associated with lymph node metastasis, low DFS, and low DSS (Peterle et al.: resp. OR 5.39 CI 1.30–22.34 p 0.02, HR 2.24, CI 1.08–4.65 p 0.03 and HR 2.49 CI 1.04–5.99 p 0.041).Citation51 Some studies mention no correlation of Fas or Fas-L expression with OS, DFS, DSS, or clinicopathologic parameters.Citation48,Citation54-Citation56,Citation58
A total of eight studies investigated the prognostic role of FADD. High FADD expression correlated with the presence of lymph node metastasis, higher tumor grade, worse DSS, worse DFS (Chien et al.: HR 1.684 CI 1.209–2345 p 0.002; Fan et al.: HR 1.003 p 0.0006), shorter DMFS (Pattje et al.: HR 2.3 CI 0.96–5.7 p 0.062) and worse OS (Chien et al.: HR 1.387 CI 1.035–1.859 p 0.029; Fan et al.: HR 1.004 p 0.000; Rasamny et al.: OR 1.72 CI 1.14–2.60 p 0.01).Citation59–Citation63,Citation66 One study found that high FADD expression showed a trend toward better local-regional control (LRC) (Schrijvers et al.: HR 3.66 CI 0.85–15.66 p 0.081), but reported no correlation with clinicopathologic parameters or OS.Citation64 Another study found no correlation between FADD expression and clinicopathologic characteristics or survival.Citation65
Three studies reported on the prognostic role of TRAIL. The first one mentioned that high TRAIL expression correlated to worse OS.Citation67 The second study mentioned a positive correlation between tumor stage and TRAIL-R DR5 staining, although this correlation is on the edge of significance. TRAIL and other TRAIL-R were not associated with clinicopathologic characteristics and survival.Citation68 The third study mentioned that in their specimens, TRAIL staining was scarce. However, they investigated TRAIL-R and found that TRAIL-R DR5 positively correlated with tumor size.Citation69
One study investigated the prognostic role of Granzyme B (GrB) and found that there was a higher peritumoral density of Granzyme B in the non-metastatic study group in comparison with the metastatic study group. High peritumoral GrB correlated with better survival. GrB was higher in tumors with lower T stages, although not statistically significant.Citation70
Another study investigated the prognostic role of FAP-1 and found that FAP-1 negative cases showed a better OS than FAP-1 positive cases (Nariai et al.: HR 0.317 CI 0.108–0.931 p 0.0366).Citation71
See for outcomes and supplementary table 4 for study characteristics.
Discussion
To date, specific-validated prognostic biomarkers are an unmet need to improve HNSCC treatment. NK cells are of innate origin, but also carry out the functions of the adaptive immune system. The immune system is a key component in tumor growth and control, and as such NK cells act as good candidates for possible biomarkers, due to their ability to lyse tumor cells lacking sufficient levels of MHC class 1. In this review, we investigated the prognostic role of NK cell markers and their ligands in HNSCC. The studies included in this review largely stem from the pre-immunotherapy era and should be interpreted accordingly.
Firstly, the prognostic role of the classical NK cell markers CD56+, CD57+, and CD16+ was investigated. Our meta-analysis describes that high CD56+ or CD57+ NK cell count correlated to better OS. This result can be explained by the anti-tumor response of NK cells, as they are able to kill tumor cells without prior sensitization.Citation72 CD56dim CD57+ NK cell subtypes are important in anti-tumor immunity and also express CD16.Citation13,Citation73 CD16 is the activating FcY receptor for IgG, that enables antibody-dependent cell-mediated cytotoxicity (ADCC) when crosslinked to CD57.Citation74 One study investigated the prognostic role of CD16+ NK cells, and found no correlation with survival or clinicopathologic characteristics.
Important activating receptors are NKG2D, that has MICA/B as a ligand, and NKp30, NKp44, NKp46, that have a broad variety of ligands.Citation23 Tumor cells express NK activating receptor ligands de novo, making them susceptible to NK cell killing.Citation75 In this review we found that NKp46+ NK cells were more abundant in low-grade tumors, indicating a positive role of NK cells in tumor control.
One study, however, found contradictory results; high CD57+ NK cell number correlated to features of worse prognosis. This could be explained by the phenomenon of tumor escape mechanisms; escaping immune recognition by selective loss of NK activating receptor ligands, aberrant HLA types or induction of anergy in NK cells.Citation72,Citation76 Absence of MHC protects against T cell activation, but enables NK cell-mediated killing of tumor cells. In this regard, a great portion of HNSCC have resorted to expressing abnormal MHC types, that shield against both T cell and NK cell activation.Citation77 Korrer et al. (2017) mention the upregulation of NK cell inhibitory ligand NKG2A on tumor-associated NK cells in HNSCC as an immune escape mechanism.Citation78
The CD27-CD70 pathway enhances the cytotoxic effect of effector cells via the perforin dependent mechanism.Citation79 CD70 expression in the tumor should, therefore, promote NK cell killing. In this review CD70 expression correlated with features of worse prognosis, indicating the opposite. This could be explained by another hypothesis; that CD27-CD70 pathway is a mechanism of immune escape for tumor cells by inducing apoptosis in effector cells.Citation80
The inhibiting ligands CEACAM1 and RCAS1 correlated with features of worse survival and prognosis. CEACAM1 is expressed on tumor cells and NK cells, and an interaction inhibits tumor cell lysis by NK cells, as it is an inhibiting receptor/ligand.Citation81 It also inhibits the activating NKG2D signaling, thus a high expression of CEACAM1 inhibits NK cell antitumor function. RCAS1 plays a role in immune evasion of tumor cells by inducing apoptosis in NK cells, thus correlating with features of worse prognosis in HNSCC. This is in concordance with other studies, that mention a correlation with features of worse prognosis in pancreatic ductal cell carcinoma and shorter survival in esophageal squamous cell carcinoma.Citation82,Citation83
Lastly, NK cells also express death receptor ligands (Fas-L, TNFa, TRAIL), which mediate apoptosis by binding to death receptors (Fas, TRAIL-R (DR4, DR5)) on target cells.Citation16 These death receptors are also expressed on T cells; the results mentioned in the following paragraph need to be interpreted as a synergistic result of T cells and NK cell function. Regarding the direct relationship between NK cells and death receptors/ligands, expression of the latter on T cells is a confounding factor.Citation84
NK and T cell ligand Fas expressed on tumor cells, correlated to better survival and better clinicopathologic characteristics in HNSCC and this is in concordance with some other cancer types.Citation85,Citation86 However, one study found a correlation with features of worse prognosis. Tumor cells use escape mechanisms to become resistant to Fas mediated apoptosis, for instance by downregulation of FADD, causing loss of apoptotic signaling.Citation87 The classic function of FADD is inducing apoptosis in cells via stimulation of different death receptors. But the FADD oriented studies included in this review found that high expression of FADD correlated to a worse prognosis. Latter finding was previously reported by researchers in data from The Cancer Genome Atlas (TCGA).Citation88
It indicates a more complex and unresolved role of FADD, as it can also, for example, activate the NFkB pathway and induce cell survival.Citation63 Fas-L on NK and T cells mediates apoptosis when coupled to Fas on tumor cells, but one article mentioned Fas-L on NK and T cells correlated to worse survival and features of worse prognosis. Fas-L expression in tumor cells also correlated to worse survival, the hypothetical explanation for this effect is ‘tumor counterattack.’Citation89 By expressing Fas-L, the tumor induces apoptosis in lymphoid cells. TRAIL-R expression in the tumor correlated to worse prognosis, this might be explained by the use of decoy receptors to evade apoptosis induction.Citation69 FAP-1 is a regulating molecule blocking Fas-mediated apoptosis, thus explaining the result that low FAP 1 correlated to a better prognosis.Citation90
For some markers/ligands (GrB, NKp46, RCAS1, FAP1) the findings confirm the proposed pathophysiological mechanism as described in the literature. The other makers/ligands (CD56, CD57, CD70, CEACAM1, Fas, Fas-L, FADD, TRAIL) show bivalent results indicating more complex mechanisms underlying their function and prognostic role.
The findings of this review are bivalent, indicating a fine line between the anti- and pro-tumor function of NK cells. Previous studies identified this as an opportunity to use NK cells as a therapeutic strategy, tackling the unmet need for immunotherapy development. NK cells are good candidates; they recognize tumor cells by lack of sufficient MHC type 1 levels, subsequently activating NK cell stimulatory receptors, mediating NK cell killing. This distinctive method of NK cell killing avoids the conundrum T cell-based therapies face; dependability on presentation of specific antigens (e.g. tumor-specific antigens, tumor-associated antigens or differentiation antigens) on MHC in order to activate T cell killing. In HNSCC and various other tumor types, loss of tumoral MHC expression has been described, impairing T cell-mediated cytotoxicity.Citation91-Citation93
NK cells are known for their robust anti-tumor immunity, which is often impaired in cancers. NK cell immunotherapy, therefore, focuses on restoring the antitumor response, for example, by retargeting strategies with therapeutic antibodies or chimeric antigen receptors (CARs), by blocking KIRs with therapeutic antibodies (Lirilumab), by NK cell checkpoint inhibitors (Monalizumab: NKG2A inhibitor) or by NK cell-based adoptive immunotherapy.Citation87,Citation94,Citation95 Furthermore, TRAIL-R agonists propose as promising modulators in antitumor immunity.Citation96 Future research is needed to provide insight in the clinical translation of the NK cell immunotherapies.
The first general limitation of this study is heterogeneity in tumor subsite, among and within studies included in this review. In HNSCC, risk factors and pathophysiological mechanisms differ between different tumor subsites in the head and neck region, which could impact the prognostic role of NK cells among these subsites. Secondly, the studies showed heterogeneity in treatment modalities with different mechanisms of action, so the prognostic role of tumor-associated immune cells could also differ. The included studies had relatively small patients size, making it impossible to stratify patients according to treatment modality or tumor subsite. For some of the markers investigated in this review, pathophysiological mechanisms have not been clearly described yet, making interpretation of the prognostic role difficult. A limitation for the meta-analysis conducted for the classical markers was the reporting of statistical information, as only four studies provided hazard ratios. A trend in the selective reporting of p-values was observed, which might indicate reporting bias and eventually could lead to publication bias. Lastly, different NK cell subsets are known, but the majority of studies in the classical markers group only immunohistochemically stained CD56 or CD57. It would be beneficial to further characterize NK cell subsets, to provide a better understanding of the tumor microenvironment.
In conclusion, this systematic review and meta-analysis found an overall favorable prognostic role of NK cells, characterized by a different membrane and intracellular markers, in HNSCC. Some studies implied the opposite, indicating the fine balance between pro-and anti-tumor functions of NK cells. Future studies should use homogeneous cohort regarding tumor subsite and treatment modality, and use a standardized method of reporting, in order to further provide insight in the complex balance between NK cell functions.
Methods
Search strategy
The systematic search was conducted on the 7th of March 2019 in two databases: Pubmed/Medline and EMBASE. As domain, ‘head and neck squamous cell carcinoma’ and synonyms of this term were used as determinant, a variety of NK cell markers and their ligands were used (Supplementary Table 1).
In- and exclusion criteria
Studies were screened based upon title and abstract. The final selection was made by full-text reading of the selected articles. Studies were eligible for inclusion if they assessed the prognostic value of NK cell markers or their ligands in patients with HNSCC (lip, OC, OP, HP, LP) by a time-to-event analysis, described as overall survival (OS), disease-free survival (DFS), progression-free survival (PFS) or by correlation to clinicopathologic characteristics. Nasopharyngeal carcinomas were excluded due to the contributing role of epstein barr virus (EBV) in the tumorigenesis. Only original articles published in English were included. Animal studies, case reports, reviews, meta-analyses or repetitive studies were excluded. Conference abstracts and studies that used techniques other than immunohistochemistry were excluded. The title/abstract screening and full-text reading were conducted by two independent researchers (ERU and SKB) and any disagreements were resolved by discussion.
Data extraction
From the references selected by full-text screening the following data were extracted: author’s last name, year of publication, biomarkers, sample size, tumor subsite, tumor stage, HPV status, treatment modalities, median follow-up time, methods, scoring methods for the immunohistochemically stained samples, confounders, hazard ratios, confidence intervals, and p-values. These data were entered in a standardized form creating a synopsis of all relevant articles.
Outcome
NK cell markers and their ligands were organized in four groups: ‘classical,’ ‘activating,’ ‘inhibiting,’ and ‘death receptors.’ The classical group comprised CD56, CD57, CD16; the activating group NKp46, CD70; the inhibiting group CEACAM1, RCAS1; the death receptor group Fas, Fas-L, FADD, TRAIL, TRAL-R, FAP1. Our study focused on the classical markers as the primary outcome, strengthened by a meta-analysis. The other makers were evaluated in a narrative manner.
Critical appraisal
To assess the risk of bias of the prognostic studies included in the meta-analysis, the Quality in Prognosis studies (QUIPS) tool was used as described by Hayden et al. (2006).Citation97 The QUIPS tool comprises six items: study participation, attrition, prognostic factor measurement, outcome measurement, confounding and statistical analysis and reporting. For each of these items, the risk of bias was scored as low, moderate, or high. Taking into consideration our research question, we valued statistical analysis and reporting as most important when assessing the overall risk of bias, because the meta-analysis depended on the reporting of hazard ratios (HR).
Statistical analysis
In the meta-analysis, we used HR’s that were defined by high NK cells vs low NK cells. If the study mentioned HR’s as low NK cells vs high NK cells, we used the reciprocal. The meta-analysis was performed in review manager 5.3 by use of a random effect analysis.
Conflicts Of Interest
The authors report no conflict of interest.
Supplemental Material
Download ()Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website .
References
- Jou A, Hess J. Epidemiology and molecular biology of head and neck cancer. Oncology Res Treat. 2017;40(6):328–10. doi:10.1159/000477127.
- Rettig EM, D’Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin. 2015 Jul 1;24(3):379–396. doi:10.1016/j.soc.2015.03.001.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359–86. doi:10.1002/ijc.29210.
- Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer—Part 1: epidemiology, presentation, and preservation. Clin Otolaryngology. 2011 Feb;36(1):65–68. doi:10.1111/j.1749-4486.2010.02231.x.
- Ridge JA, Mehra R, Lango MN, Galloway T. Head and neck tumors. Cancer Manage. 2016 Jun 2.
- D’Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011 Oct;1(53):S5–11. doi:10.1016/j.ypmed.2011.08.001.
- Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010 Nov;16(11):1671. doi:10.3201/eid1611.100452.
- van Harten MC, de Ridder M, Hamming-Vrieze O, Smeele LE, Balm AJ. van den Brekel MW. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) patients in a dutch comprehensive cancer center. Oral Oncol. 2014 Apr 1;50(4):282–290. doi:10.1016/j.oraloncology.2013.12.018.
- Wu SY. Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Eur J Cancer. 2017 Feb;1(72):S109.
- Matta A, Ralhan R. Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma. Head Neck Oncol. 2009 Dec;1(1):6. doi:10.1186/1758-3284-1-6.
- Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EY. Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain. 2014 Oct 1;15(10):1015–1022. doi:10.1016/j.jpain.2014.07.003.
- Economopoulou P, Kotsantis I, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open. 2016 Dec 1;1(6):e000122.
- Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA, et al.. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016 Oct 20;1(17). doi:10.1172/jci.insight.89829.
- Faden DL, Concha-Benavente F, Chakka AB, Mcmichael EL, Chandran U, Ferris RL. Immunogenomic correlates of response to cetuximab monotherapy in head and neck squamous cell carcinoma. Head Neck. 2019 Apr;41(8):2591–2601. doi:10.1002/hed.25726.
- Bindea G, Mlecnik B, Fridman W-H GJ. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011 May;33(4):335–340. doi:10.1007/s00281-011-0264-x.
- Kadota K, Nitadori JI, Ujiie H, Buitrago DH, Woo KM, Sima CS, Travis WD, Jones DR, Adusumilli PS. Prognostic impact of immune microenvironment in lung squamous cell carcinoma: tumor-infiltrating CD10+ neutrophil/CD20+ lymphocyte ratio as an independent prognostic factor. J Thoracic Oncol. 2015 Sep 1;10(9):1301–1310. doi:10.1097/JTO.0000000000000617.
- Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Öberg Å, Rutegård J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011 May;24(5):671. doi:10.1038/modpathol.2010.234.
- Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, Goubar A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Annals Oncol. 2015 May 20;26(8):1698–1704. doi:10.1093/annonc/mdv239.
- de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology. 2017 Nov 2;6(11):e1356148. doi:10.1080/2162402X.2017.1356148.
- Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009 Apr;126(4):458–465. doi:10.1111/j.1365-2567.2008.03027.x.
- Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: more than a marker for cytotoxicity?. Front Immunol. 2017 Jul 24;8:892. doi:10.3389/fimmu.2017.00892.
- Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T .2012 Oct 1. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Seminars in immunology. Academic Press. Vol. 24. p. 331–341.
- Zingoni A, Fionda C, Borrelli C, Cippitelli M, Santoni A, Soriani A. Natural killer cell response to chemotherapy-stressed cancer cells: role in tumor immunosurveillance. Front Immunol. 2017 Sep;25(8):1194. doi:10.3389/fimmu.2017.01194.
- Stovgaard ES, Nielsen D, Hogdall E, Balslev E. Triple negative breast cancer–prognostic role of immune-related factors: a systematic review. Acta Oncol (Madr). 2018 Jan 2;57(1):74–82. doi:10.1080/0284186X.2017.1400180.
- Coppola A, Arriga R, Lauro D, Del Principe MI, Buccisano F, Maurillo L, Palomba P, Venditti A, Sconocchia G. NK cell inflammation in the clinical outcome of colorectal carcinoma. Front Med. 2015 May;26(2):33.
- Wagner S, Wittekindt C, Reuschenbach M, Hennig B, Thevarajah M, Würdemann N, Prigge ES, von Knebel Doeberitz M, Dreyer T, Gattenlöhner S et al.. CD 56‐positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer. 2016 May 1;138(9):2263–2273. doi:10.1002/ijc.29962.
- Stasikowska-Kanicka O, Wągrowska-Danilewicz M, Danilewicz M. Association of infiltrating cells with microvessel density in oral squamous cell carcinoma. Polish J Pathol. 2017 Jan 1;68(1):40–48. doi:10.5114/pjp.2017.67614.
- Ikeda T, Seki S, Fujiwara M, Matsuura M, Ozaki-Honda Y, Fujita S, Ikeda H, Umeda M, Asahina I. Low-risk population among patients with tumor-node-metastasis stage III/IV oral squamous cell carcinoma. Oncol Lett. 2017 Sep 1;14(3):3711–3716. doi:10.3892/ol.2017.6575.
- De Meulenaere A, Vermassen T, Aspeslagh S, Zwaenepoel K, Deron P, Duprez F, Ferdinande L, Rottey S. CD70 expression and its correlation with clinicopathological variables in squamous cell carcinoma of the head and neck. Pathobiology. 2016;83(6):327–333. doi:10.1159/000446569.
- Simonetti O, Lucarini G, Rubini C, Zizzi A, Aspriello SD, Di Primio R, Offidani AM. Correlation between immunohistochemical staining of CEACAM1 and clinicopathological findings in oral pre-neoplastic lesions and squamous cell carcinoma. Med Mol Morphol. 2018 Mar 1;51(1):41–47. doi:10.1007/s00795-017-0169-4.
- Dutsch-Wicherek M, Tomaszewska R, Lazar A, Wicherek L, Skladzien J. The association between RCAS1 expression in laryngeal and pharyngeal cancer and its healthy stroma with cancer relapse. BMC Cancer. 2009 Dec;9(1):35. doi:10.1186/1471-2407-9-35.
- Guler N, Uçkan S, Celik I, Oznurlu Y, Uckan D. Expression of Fas and Fas-ligand and analysis of argyrophilic nucleolar organizer regions in squamous cell carcinoma: relationships with tumor stage and grade, and apoptosis. Int J Oral Maxillofac Surg. 2005 Dec 1;34(8):900–906. doi:10.1016/j.ijom.2005.03.006.
- Nariai Y, Mishima K, Yoshimura Y, Sekine J. FAP-1 and NF-κB expressions in oral squamous cell carcinoma as potential markers for chemo-radio sensitivity and prognosis. Int J Oral Maxillofac Surg. 2011 Apr 1;40(4):419–426. doi:10.1016/j.ijom.2010.10.020.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151(4):264–269. doi:10.7326/0003-4819-151-4-200908180-00135.
- Taghavi N, Bagheri S, Akbarzadeh A. Prognostic implication of CD 57, CD 16, and TGF‐β expression in oral squamous cell carcinoma. J Oral Pathol Med. 2016 Jan;45(1):58–62. doi:10.1111/jop.12320.
- Stangl S, Tontcheva N, Sievert W, Shevtsov M, Niu M, Schmid TE, Pigorsch S, Combs SE, Haller B, Balermpas P et al.. Heat shock protein 70 and tumor‐infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German cancer consortium radiation oncology group (DKTK‐ROG). Int J Cancer. 2018 May 1;142(9):1911–1925. doi:10.1002/ijc.31213.
- Fang J, Li X, Ma D, Liu X, Chen Y, Wang Y, Lui VW, Xia J, Cheng B, Wang Z. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer. 2017 Dec;17(1):375. doi:10.1186/s12885-017-3317-2.
- Karpathiou G, Casteillo F, Giroult JB, Forest F, Fournel P, Monaya A, Froudarakis M, Dumollard JM, Prades JM, Peoc’h M. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017 Mar 21;8(12):19310. doi:10.18632/oncotarget.14242.
- Santos EM, de Matos FR, de Morais EF, Galvão HC. de Almeida Freitas R. Evaluation of Cd8+ and natural killer cells defense in oral and oropharyngeal squamous cell carcinoma. J Cranio-Maxillofacial Surg. 2019 Apr 1;47(4):676–681. doi:10.1016/j.jcms.2019.01.036.
- de Carvalho Fraga CA, de Oliveira MV, Domingos PL, de Carvalho Botelho AC, Guimarães AL, Teixeira-Carvalho A, Correa-Oliveira R, De Paula AM. Infiltrating CD57+ inflammatory cells in head and neck squamous cell carcinoma: clinicopathological analysis and prognostic significance. Appl Immunohistochem Mol Morphol. 2012 May 1;20(3):285–290. doi:10.1097/PAI.0b013e318228357b.
- Zancope E, Costa NL, Junqueira‐Kipnis AP, Valadares MC, Silva TA, Leles CR, Mendonça EF, Batista AC. Differential infiltration of CD8+ and NK cells in lip and oral cavity squamous cell carcinoma. J Oral Pathol Med. 2010 Feb;39(2):162–167. doi:10.1111/j.1600-0714.2009.00792.x.
- Sakakura K, Takahashi H, Kaira K, Toyoda M, Oyama T, Chikamatsu K. Immunological significance of the accumulation of autophagy components in oral squamous cell carcinoma. Cancer Sci. 2015 Jan;106(1):1–8. doi:10.1111/cas.12559.
- Schoenfeld JD, Gjini E, Rodig SJ, Tishler RB, Rawal B, Catalano PJ, Uppaluri R, Haddad RI, Hanna GJ, Chau NG et al.. Evaluating the PD-1 axis and immune effector cell infiltration in oropharyngeal squamous cell carcinoma. Intl J Radiation Oncology* Biology* Physics. 2018 Sep 1;102(1):137–145. doi:10.1016/j.ijrobp.2018.05.002.
- Lazaris AC, Segas JV, Nikolopoulos TP, Patsouris ES. Tissue detection of natural killer cells in laryngeal carcinoma. Neoplasma. 2007;54:379–382.
- Oliveira Maciel TA, Serpa MS, Mafra RP, Goes Gonzaga AK, De Souza LB, Pinto LP. Immunohistochemical analysis of natural killer cells and CD8+ T lymphocytes in lower lip squamous cell carcinoma. J Clin and Diagn Res. 2017 Dec 1;11(12):EC22–5.
- Ladányi A, Kapuvári B, Papp E, Tóth E, Lövey J, Horváth K, Gődény M, Remenár É. Local immune parameters as potential predictive markers in head and neck squamous cell carcinoma patients receiving induction chemotherapy and cetuximab. Head Neck. 2019 May;41(5):1237–1245. doi:10.1002/hed.25546.
- Lucarini G, Zizzi A, Re M, Sayeed MA, Di Primio R, Rubini C. Prognostic implication of CEACAM1 expression in squamous cell carcinoma of the larynx: pilot study. Head Neck. 2019 Jun 1. doi:10.1002/hed.25589.
- Wang N, Feng Y, Wang Q, Liu S, Xiang L, Sun M, Zhang X, Liu G, Qu X, Wei F. Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PLoS One. 2014 Feb 27;9(2):e89991. doi:10.1371/journal.pone.0089991.
- Shinozuka K, Uzawa K, Fushimi K, Yamano Y, Shiiba M, Bukawa H, Yokoe H, Tanzawa H. Downregulation of carcinoembryonic antigen-related cell adhesion molecule 1 in oral squamous cell carcinoma: correlation with tumor progression and poor prognosis. Oncology. 2009;76(6):387–397. doi:10.1159/000215580.
- Asensio C, Zapata AN, Garcia-Ahijado J, Gil B, Salvadores P, Schneider J. Fas expression is associated with a better prognosis in laryngeal squamous cell carcinoma. Anticancer Res. 2007 Nov 1;27(6B):4083–4086.
- de Carvalho-neto PB, Dos Santos M, de Carvalho MB, da Cunha Mercante AM, Dos Santos VP, Severino P, Tajara EH, Louro ID, da Silva-conforti AM. FAS/FASL expression profile as a prognostic marker in squamous cell carcinoma of the oral cavity. PLoS One. 2013 Jul 19;8(7):e69024.
- Muraki Y, Tateishi A, Seta C, Fukuda J, Haneji T, Oya R, Ikemura K, Kobayashi N. Fas antigen expression and outcome of oral squamous cell carcinoma. Int J Oral & Maxillofacial Surg: Oncol. 2000 Oct;29(5):360–365. doi:10.1016/S0901-5027(00)80053-3.
- Das SN, Khare P, Singh MK, Sharma SC. Fas receptor (CD95) & Fas ligand (CD178) expression in patients with tobacco-related intraoral squamous cell carcinoma. Indian J Med Res. 2011 Jul;134(1):54.
- Fang L, Sun L, Hu FF, Chen QE. Effects of FasL expression in oral squamous cell cancer. Asian Pacific J Cancer Prev. 2013;14(1):281–285. doi:10.7314/APJCP.2013.14.1.281.
- Peterle GT, Santos M, Mendes SO, Carvalho-Neto PB, Maia LL, Stur E, Agostini LP, Silva CV, Trivilin LO, Nunes FD et al.. FAS ligand expression in inflammatory infiltrate lymphoid cells as a prognostic marker in oral squamous cell carcinoma. Genet Mol Res. 2015 Sep 22;14(3):11145–11153. doi:10.4238/2015.September.22.8.
- Fujieda S, Sunaga H, Tsuzuki H, Fan GK, Ito T, Sugimoto C, Saito H. Expression of Fas (CD95) ligand is correlated with IL-10 and granulocyte colony-stimulating factor expression in oral and oropharyngeal squamous cell carcinoma. Cancer Lett. 2000 Dec 8;161(1):73–81. doi:10.1016/S0304-3835(00)00599-1.
- Bayazit Y. Significance of Fas protein in squamous cell carcinoma of the larynx. Acta Otolaryngol. 2000 Jan 1;120(4):557–561. doi:10.1080/000164800750046108.
- Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002 Oct 1;8(10):3137–3145.
- Jäckel MC, Mitteldorf C, Schweyer S, Füzesi L. Clinical relevance of Fas (APO‐1/CD95) expression in laryngeal squamous cell carcinoma. Head Neck. 2001 Aug;23(8):646–652. doi:10.1002/hed.1091.
- Tsuzuki H, Sunaga H, Ito T, Narita N, Sugimoto C, Fujieda S. Reliability of platelet-derived endothelial cell growth factor as a prognostic factor for oral and oropharyngeal carcinomas. Arch Otolaryngol–Head & Neck Surg. 2005 Dec 1;131(12):1071–1078. doi:10.1001/archotol.131.12.1071.
- Prapinjumrune C, Morita KI, Kuribayashi Y, Hanabata Y, Shi Q, Nakajima Y, Inazawa J, Omura K. DNA amplification and expression of FADD in oral squamous cell carcinoma. J Oral Pathol Med. 2010 Aug;39(7):525–532.
- Noorlag R, Boeve K, Witjes MJ, Koole R, Peeters TL, Schuuring E, Willems SM, van Es RJ. Amplification and protein overexpression of cyclin D1: predictor of occult nodal metastasis in early oral cancer. Head Neck. 2017 Feb;39(2):326–333. doi:10.1002/hed.24584.
- Chien HT, Cheng SD, Chuang WY, Liao CT, Wang HM, Huang SF. Clinical implications of FADD gene amplification and protein overexpression in Taiwanese oral cavity squamous cell carcinomas. PLoS One. 2016 Oct 20;11(10):e0164870. doi:10.1371/journal.pone.0164870.
- Fan S, Müller S, Chen Z, Lin P, Tighiouart M, Shin D, Khuri FR, Sun SY. Prognostic impact of Fas-associated death domain, a key component in death receptor signaling, is dependent on the presence of lymph node metastasis in head and neck squamous cell carcinoma. Cancer Biol Ther. 2013 Apr 1;14(4):365–369. doi:10.4161/cbt.23636.
- Pattje WJ, Melchers LJ, Slagter‐Menkema L, Mastik MF, Schrijvers ML, Gibcus JH, Kluin PM, Hoegen‐Chouvalova O, van der Laan BF, Roodenburg JL. van der Wal JE. FADD expression is associated with regional and distant metastasis in squamous cell carcinoma of the head and neck. Histopathology. 2013 Aug;63(2):263–270. doi:10.1111/his.12174.
- Rasamny JJ, Allak A, Krook KA, Jo VY, Policarpio-Nicolas ML, Sumner HM, Moskaluk CA, Frierson JHF, Jameson MJ. Cyclin D1 and FADD as biomarkers in head and neck squamous cell carcinoma. Otolaryngology–Head and Neck Surgery. 2012 Jun;146(6):923–931. doi:10.1177/0194599811435052.
- Schrijvers ML, Pattje WJ, Slagter-Menkema L, Mastik MF, Gibcus JH, Langendijk JA, van der Wal JE, van der Laan BF, Schuuring E. FADD expression as a prognosticator in early-stage glottic squamous cell carcinoma of the larynx treated primarily with radiotherapy. Intl J Radiation Oncology* Biology* Physics. 2012 Jul 15;83(4):1220–1226. doi:10.1016/j.ijrobp.2011.09.060.
- Wachters JE, Schrijvers ML, Slagter‐Menkema L, Mastik M, Langendijk JA, de Bock GH, Roodenburg JL, van der Laan BF, van der Wal JE, Schuuring E. Phosphorylated FADD is not prognostic for local control in T1‐T2 supraglottic laryngeal carcinoma treated with radiotherapy. Laryngoscope. 2017 Sep;127(9):E301–7. doi:10.1002/lary.26563.
- Carinci F, Monasta L, Rubini C, Stramazzotti D, Palmieri A, Melloni E, Knowles A, Ronfani L, Zauli G, Secchiero P. The negative prognostic value of TRAIL overexpression in oral squamous cell carcinomas does not preclude the potential therapeutic use of recombinant TRAIL. Invest New Drugs. 2012 Apr 1;30(2):810–818. doi:10.1007/s10637-010-9586-0.
- Erkul E, Kucukodaci Z, Pinar D, Gungor A, Alparslan Babayigit M, Kurt O, Cincik H. TRAIL and TRAIL receptors in patients with laryngeal cancer. Head Neck. 2016 Apr;38(S1):E535–41. doi:10.1002/hed.24035.
- Vigneswaran N, Baucum DC, Wu J, Lou Y, Bouquot J, Muller S, Zacharias W. Repression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but not its receptors during oral cancer progression. BMC Cancer. 2007 Dec;7(1):108. doi:10.1186/1471-2407-7-108.
- Costa NL, Alencar RD, Valadares MC, Silva TA, Mendonça EF, Batista AC. The clinicopathological significance of the expression of Granzyme B in oral squamous cell carcinoma. Oral Oncol. 2010 Mar 1;46(3):185–189. doi:10.1016/j.oraloncology.2009.11.016.
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, Raulet DH .2014 Jan 1. Recognition of tumors by the innate immune system and natural killer cells. Advances in immunology. Academic Press. Vol. 122. p. 91–128.
- Habif G, Crinier A, André P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. 2019 Mar;25:1.
- Stabile H, Fionda C, Gismondi A, Santoni A. Role of distinct natural killer cell subsets in anticancer response. Front Immunol. 2017 Mar;16(8):293.
- Romagné F, Vivier E Natural killer cell-based therapies. F1000 medicine reports. 2011; 3.
- Türkseven MR, Oygür T. Evaluation of natural killer cell defense in oral squamous cell carcinoma. Oral Oncol. 2010 May 1;46(5):e34–7. doi:10.1016/j.oraloncology.2010.02.019.
- Moy JD, Moskovitz JM, Ferris RL. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur J Cancer. 2017 May;1(76):152–166. doi:10.1016/j.ejca.2016.12.035.
- Korrer MJ, Kim Y. Natural killer cells from primary human head and neck squamous cell carcinomas upregulate NKG2A. J Immunol. 2017;198:1.
- Yamada S, Shinozaki K, Agematsu K. Involvement of CD27/CD70 interactions in antigen‐specific cytotoxic T‐lymphocyte (CTL) activity by perforin‐mediated cytotoxicity. Clin Exp Immunol. 2002 Dec;130(3):424–430. doi:10.1046/j.1365-2249.2002.02012.x.
- Held‐Feindt J, Mentlein R. CD70/CD27 ligand, a member of the TNF family, is expressed in human brain tumors. Int J Cancer. 2002 Mar 20;98(3):352–356. doi:10.1002/ijc.10207.
- Hosomi S, Grootjans J, Huang YH, Kaser A, Blumberg RS. New insights into the regulation of natural-killer group 2 member D (NKG2D) and NKG2D-ligands: endoplasmic reticulum stress and CEA-related cell adhesion molecule 1. Front Immunol. 2018;9.
- Kato H, Nakajima M, Masuda N, Faried A, Sohda M, Fukai Y, Miyazaki T, Fukuchi M, Tsukada K, Kuwano H. Expression of RCAS1 in esophageal squamous cell carcinoma is associated with a poor prognosis. J Surg Oncol. 2005 May 1;90(2):89–94. doi:10.1002/jso.20249.
- Giaginis C, Davides D, Zarros A, Noussia O, Zizi-Serbetzoglou A, Kouraklis G, Theocharis S. Clinical significance of tumor-associated antigen RCAS1 expression in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2008 Jun 1;53(6):1728. doi:10.1007/s10620-007-0035-7.
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. science. 1998 Aug 28;281(5381):1305–1308. doi:10.1126/science.281.5381.1305.
- Bębenek M, Duś D, Koźlak J. Prognostic value of the Fas/Fas ligand system in breast cancer. Contemp Oncol. 2013;17:120.
- Wu GZ, Pan CX, Jiang D, Zhang Q, Li Y, Zheng SY. Clinicopathological significance of fas and fas ligand expressions in esophageal cancer. Am J Cancer Res. 2015;5:2865.
- Li Y, Sun R. Tumor immunotherapy: new aspects of natural killer cells. Chinese J Cancer Res. 2018 Apr;30(2):173. doi:10.21147/j.1000-9604.2018.02.02.
- Eytan DF, Snow GE, Carlson S, Derakhshan A, Saleh A, Schiltz S, Cheng H, Mohan S, Cornelius S, Coupar J et al.. SMAC mimetic birinapant plus radiation eradicates human head and neck cancers with genomic amplifications of cell death genes FADD and BIRC2. Cancer Res. 2016 Sep 15;76(18):5442–5454. doi:10.1158/0008-5472.CAN-15-3317.
- Peter ME, Hadji A, Murmann AE, Brockway S, Putzbach W, Pattanayak A, Ceppi P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015 Apr;22(4):549. doi:10.1038/cdd.2015.3.
- Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with fas. Science. 1995 Apr 21;268(5209):411–415. doi:10.1126/science.7536343.
- Raulet DH .2006 Jun 1. Missing self recognition and self tolerance of natural killer (NK) cells. Seminars in immunology. Academic Press. Vol. 18. p. 145–150.
- Zamora AE, Crawford JC, Thomas PG. Hitting the target: how T cells detect and eliminate tumors. J Immunol. 2018 Jan 15;200(2):392–399. doi:10.4049/jimmunol.1701413.
- Yoo SH, Keam B, Ock CY, Kim S, Han B, Kim JW, Lee KW, Jeon YK, Jung KC, Chung EJ et al.. Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci Rep. 2019 May 22;9(1):7680. doi:10.1038/s41598-019-44206-2.
- Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol. 2019 Jan 10;40(2):142–158. doi:10.1016/j.it.2018.12.003.
- Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol. 2019;10:1205. doi:10.3389/fimmu.2019.01205.
- Trivedi R, Mishra DP. Trailing TRAIL resistance: novel targets for TRAIL sensitization in cancer cells. Front Oncol. 2015 Apr;2(5):69.
- Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013 Feb 19;158(4):280–286. doi:10.7326/0003-4819-158-4-201302190-00009.
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
