ABSTRACT
Infiltrating tumor neutrophils and myeloid-derived suppressor cells represent major populations in the tumor microenvironment that contribute to tumor progression. However, the phenotype of circulating and tumor-associated neutrophils, and the impact of cancer patients’ metabolic state on neutrophil function need further characterization. Here we show that in kidney cancer patients, circulating neutrophils display an altered immature-like phenotype, and an activated/primed metabolic state. Circulating immature-like neutrophils acquire an activated phenotype upon migration into the tumor tissue, characterized by high expression of the immunosuppressive enzyme arginase-1, and active granule release. Interestingly, obesity and adipose tissue distribution were significantly associated with this activated phenotype of neutrophils, including the release of arginase-1 in the tumor tissue. These results provide a possible functional relationship between the metabolic status of the patients and disease progression, through an active immunosuppressive role of neutrophils within the kidney tumor microenvironment.
Introduction
Neutrophils constitute the most abundant subset of leukocytes in human blood, and their role in cancer is becoming widely appreciated.Citation1 Accumulating evidence suggests that neutrophil-derived myeloid suppressor cells (MDSCs) are importantly pro-tumorigenic, as they can modulate and suppress the function of innate and adaptive immune cells.Citation2–Citation4 The development of dysfunctional neutrophils, such as neutrophil-derived MDSCs, is reported to be related to both host-derived (body mass index-BMI-, and adipokines) and cancer-derived (granulocyte-monocyte colony stimulating factor, chemokines) factors, which skew neutrophil phenotype and effector functions toward an immunosuppressive state.Citation2,Citation3 In addition, patient adiposity and systemic metabolic state, and the dynamics of immune cell populations in the peripheral blood, are reported to be important clinical factors for predicting outcomes in cancer patients. In kidney cancer, a high blood neutrophil-to-lymphocyte ratio (NLR), increased numbers of tumor-associated neutrophils, and high BMI are independent negative prognostic factors for disease progression, survival, and therapeutic efficacy.Citation5–Citation9 Given that these clinical studies suggest a role for neutrophils in promoting kidney cancer progression, we characterized the phenotype and function of blood and tumor-associated neutrophils in patients. Moreover, we compared the metabolic profile of blood neutrophils in kidney cancer patients to that of healthy donors to examine metabolic pathways and substrate utilization differences at baseline and upon activation.
Methods
Human subjects
Kidney cancer patients undergoing partial or radical nephrectomy were recruited at the Emory University Hospital. Blood and tumor tissue were collected at the time of surgery, and blood was collected from healthy donors. Patients’ magnetic resonance imaging (MRI) or computed tomography (CT) imaging within 3 months prior to surgery was obtained and the cross-sectional image at the third lumbar vertebra was identified. Body composition was analyzed via Slice-O-Matic Software V4.3 (Tomovision), as detailed in the supplemental methods.Citation10 Samples were collected and this study was completed in accordance with an institutional review board approved protocol (IRB 00055316), and all patients provided informed consent.
Sample collection and processing
Blood was collected in K2-EDTA tubes by venipuncture from healthy donors and kidney cancer patients at the time of surgery. Briefly, blood cells were separated from plasma following 10 min, 400 g centrifugation at 4°C (minimal brake). Platelet-poor plasma was obtained following a second 10 min, 3000 g centrifugation at 4°C. Blood cells were washed and resuspended at the original blood volume using cold PBS-EDTA (2.5 mM) and used for downstream assays as detailed in Figure S1A. Intraoperative tumor tissue samples were harvested in Hank’s Balanced Salt Solution, cut into small pieces, digested using liberase enzyme cocktail (Roche), and homogenized into a single cell suspension using a MACS tissue dissociator (Miltenyi Biotec) for downstream assays (Fig. S1A). Isolation of cells for ex vivo immunosuppressive assay is described in the supplemental methods.
Flow cytometry
Blood and isolated tumor cells were resuspended in PBS-EDTA (2.5 mM) and stained as detailed in the supplemental methods. Samples were acquired on an LSR II cytometer (BD Biosciences) following bead-based machine calibration as previously described.Citation11 Fluorescence minus one (FMOs) controls for main outcome measures are shown in Fig. S3. Results were compensated and analyzed using FlowJo v9.9.5 (TreeStar).
Metabolic profiling
Real-time measurement of the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) of blood neutrophils was performed using the Seahorse XFp Extracellular Flux Analyzer (Agilent). Isolated blood neutrophils were plated on a Seahorse assay plate (supplemental methods) and exposed to four sequential injections:Citation1 glucose (10 mM),Citation2 PMA (100 nM) or Seahorse base media,Citation3 diphenyleneiodonium (DPI, 10 µM), andCitation4 2-deoxyglucose (2-DG, 0.1 M).
Soluble mediators
Arginase-1 was quantified in platelet poor plasma and in tumor supernatant by ELISA (RayBiotech), while presence of extracellular neutrophil elastase in platelet-poor plasma and in tumor supernatant was assessed via enzymatic activity by measuring the rate of digestion of a fluorescent probe (Cayman Chemical).
Statistical analysis
Data were compiled in Excel (Microsoft) and analyzed in JMP Pro13 (SAS Institute). Non-parametric tests were used, owing to the low numbers of subjects (<20) as described in the supplemental methods.
Results
Circulating neutrophils in kidney cancer patients display an immature-like phenotype
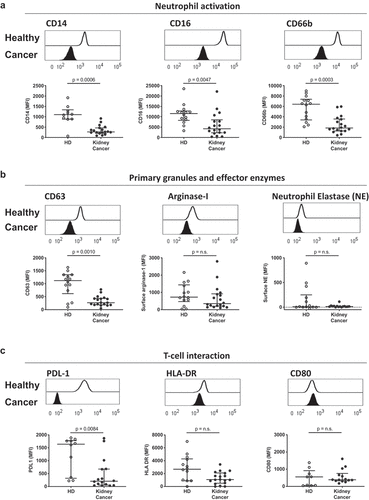
Previous reports associate neutrophil number and neutrophil-to-lymphocyte ratio as important predictors of overall survival and response to immunotherapy in kidney cancer.Citation6–Citation9 Based on these observations, we were interested in further characterizing the phenotype and key functional markers of circulating neutrophils in such patients. Blood was collected from 19 patients diagnosed with kidney cancer, of which 73.7% presented with clear cell renal cell carcinoma, and from 17 healthy donors (Fig. S1B). Characterization of circulating neutrophil phenotype via whole blood flow cytometry (Fig. S2A) showed a downregulation of surface CD62L and CD16 in kidney cancer patients compared to healthy donors (Fig. S2B, C), which differed from previously reported phenotypes of mature, band (immature), or CD62Ldim blood neutrophils. Furthermore, blood neutrophils from kidney cancer patients had reduced surface expression of the lipopolysaccharide receptor CD14, reduced surface expression of the phagocytic receptor CD16, and reduced surface expression of the secondary granule and maturation marker CD66b (). Similarly, patients’ circulating neutrophils also showed decreased CD63 expression, representing primary granule release. However, no significant difference was observed on the recapture of surface enzymes, such as arginase-1 and neutrophil elastase, that are usually sequestered within the primary granules of mature neutrophils (), or in the expression of antigen presentation-associated receptors human leukocyte antigen HLA-DR and CD80 (). Interestingly, circulating neutrophils from kidney cancer patients displayed lower surface expression of the immunomodulatory protein programmed death-ligand 1 (PD-L1) (). Together, this reveals that kidney cancer patients show an immature-like phenotypic profile in circulating neutrophils that differs from the mature phenotype seen in healthy donors. These results suggest that tumor-derived factors and stress signals may alter myeloid cell demand and promote the early release of immature neutrophils from the bone marrow.
Figure 1. Circulating neutrophils in kidney cancer patients display an immature-like phenotype. Blood leukocytes from 14 healthy donors (HD, open symbols) and 19 kidney cancer patients (closed symbols) were phenotyped by flow cytometry, and neutrophils were defined as CD45+, CD3−, CD66b+. (a) Neutrophil activation was measured by median fluorescence intensity (MFI) of surface CD14, CD16 and CD66b. (b) Primary granule release was quantified by surface CD63 expression and surface recapture of primary granule enzymes (arginase-1 and neutrophil elastase). (c) Immunomodulatory capacity of blood neutrophils was assessed by surface expression of PD-L1, HLA-DR and CD80. Differences between kidney cancer patients and healthy donors were determined using the Wilcoxon rank sum test
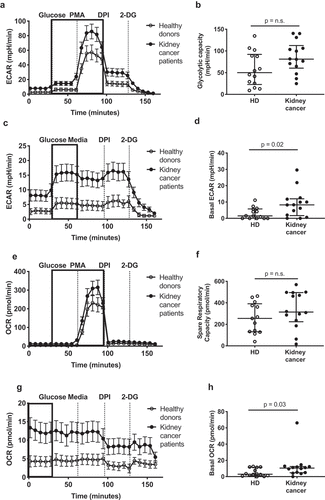
Circulating neutrophils have heightened metabolic activity in kidney cancer patients
Immature-like neutrophils are reported to have increased metabolic activity, with enhanced mitochondrial and glycolytic capacity.Citation12 Thus, we next sought to assess the metabolic profile and substrate utilization of neutrophils in the peripheral blood of kidney cancer patients. We determined maximal glycolytic capacity (, basal glycolytic activity (, and oxygen consumption rate (OCR) upon stimulation with phorbol myristate acetate (PMA) ( compared to baseline (. Interestingly, neutrophils from kidney cancer patients displayed increased glycolysis at resting state () and a trend toward increased maximal glycolytic capacity compared to healthy donor neutrophils (). Similarly, the OCR at baseline was increased in neutrophils from kidney cancer patients (); however, the difference was abrogated upon PMA stimulation (). Furthermore, the increase in OCR observed upon stimulation was dependent on nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation in both groups, as the administration of the NADPH oxidase inhibitor diphenyleneiodonium (DPI) abrogated the effect of PMA and brought the OCR back to baseline (). Moreover, the baseline activated state of circulating neutrophils from kidney cancer patients did not depend on a heightened pinocytic activity (Fig. S2D). Together, these results show that immature neutrophils from cancer patients circulate in an activated metabolic state, with enhanced basal oxidative and glycolytic capacities when compared to healthy donors. While this difference was lost at maximal metabolic activation, these metabolic changes suggest systemic adaptation to altered, potentially limiting substrate conditions.
Figure 2. Circulating neutrophils from kidney cancer patients show enhanced metabolic activity. Circulating neutrophils from healthy donors (HD, open symbols) and kidney cancer patients (closed symbols) were plated on Seahorse culture plates in Seahorse media (± glucose and PMA, depending on the condition) and analyzed for maximal (a-b) and basal (c-d) extracellular acidification rate (ECAR), for spare respiratory capacity (e-f) and basal oxygen consumption rate (OCR) (g-h) as determinants of glycolysis and oxidative phosphorylation/NADPH activity, respectively. Glycolytic capacity was defined as the difference between maximal ECAR upon PMA activation and average ECAR upon glucose injection (glycolysis). Differences between kidney cancer patients and healthy donors were determined using the Wilcoxon rank sum test
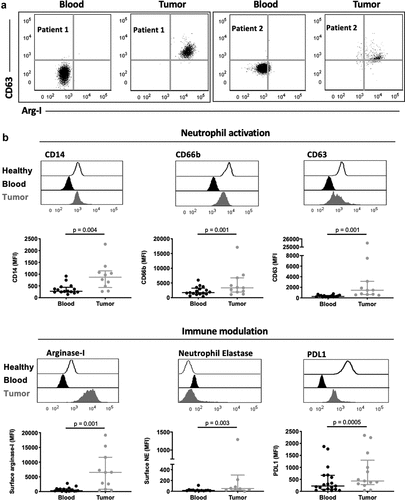
Tumor-associated neutrophils display an immunoregulatory phenotype
Our finding that neutrophils have an immature and metabolically active phenotype in kidney cancer prompted us to investigate if these systemic changes altered the phenotype and function of neutrophils within patients’ tumors. We phenotyped immune cells recovered from the resected tumors (Fig. S3A) and compared tissue-infiltrating neutrophils to circulating neutrophils from the same patient. Tumor neutrophils displayed active release of primary granules, as reflected by increased surface CD63 expression and concomitant recapture of arginase-1 (), an enzyme contained in the primary granules of neutrophils, at their surface. The increased release of primary granule content was accompanied by increased expression of the LPS receptor CD14, and increased release of secondary granules (based on CD66b expression, ). Moreover, tumor-associated neutrophils showed increased surface expression of immunoinhibitory mediators arginase-1, neutrophil elastase, and PD-L1 (), suggesting a potential role for tumor-infiltrating neutrophils in suppressing the anti-tumor T cell response. In contrast, no changes were observed in surface expression of the phagocytic receptor CD16 (Fig. S3B) or in the ability to present antigens through the major histocompatibility complex II HLA-DR (Fig. S3C). Finally, the co-stimulatory molecule CD80 was increased in tumor-associated compared to circulating neutrophils (Fig. S3D). These findings demonstrate that upon migration to the cancer tissue, neutrophils acquire phenotypic and functional changes associated with an immunosuppressive state.
Figure 3. Tumor-associated neutrophils from kidney cancer patients display an immunosuppressive phenotype. (a) Representative flow cytometry plots of CD63 and arginase-1 expression on matched circulating (blood) and tumor neutrophils from kidney cancer patients. Data are shown for two kidney cancer patients. (b) Comparison of neutrophil activation and expression of immunomodulatory surface receptors. Differences between circulating and tumor-associated neutrophils within kidney cancer patients were determined using the Wilcoxon matched-pairs signed rank test
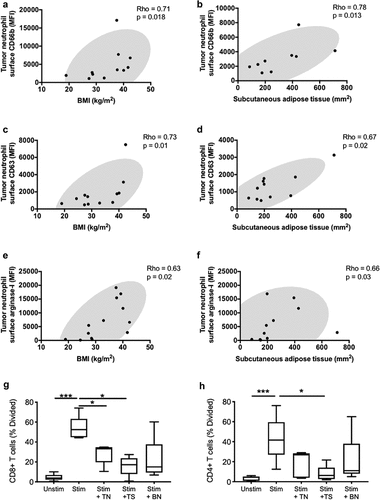
Systemic metabolic state correlates with active release of neutrophil-derived granules in the tumor
We were next interested in how clinical factors correlated with phenotype and function of tumor-infiltrating neutrophils. As BMI was previously identified as one of the main risk factors for developing several cancers, including kidney cancer, Citation13 we were interested in investigating the relationship between patient characteristics (BMI, age, sex, NLR) and neutrophils in the tumor tissue. While NLR, age and sex did not correlate with measures of neutrophil activation and granule release, increased adiposity (measured by BMI) correlated with primary and secondary granule release, as well as increase surface recapture of arginase-1 in the tumor. Interestingly, neutrophil granule release in the tumor tissue may also be related to adipose tissue distribution, as increased subcutaneous adipose tissue (SAT), but not visceral adipose tissue (VAT), positively correlated with arginase-1 and neutrophil elastase secretion in the tumor tissue. Neutrophil release of secondary granules, measured by surface CD66b, correlated with both BMI (Rho = 0.71, p = .018) and SAT (Rho = 0.78, p = .013) (). Likewise, both surface CD63 (primary granule release) and surface arginase-1 positively correlated with clinical measures of obesity-associated metabolic syndrome (). Interestingly, while neutrophil surface expression of arginase-1 differed between low and high patient BMI groups (Fig. S4A), neutrophil elastase, which is contained in the same granules, did not differ between the two obesity (overweight and obese) groups (Fig. S4B), suggesting the possibility of differential compartmentalization between arginase-1 and neutrophil elastase after their release from primary granules. Similar to surface neutrophil elastase, the free extracellular forms of both arginase-1 and neutrophil elastase did not differ between the two BMI categories. These results suggest that adipokines or other adipose-related factors may promote the release of granules containing active enzymes, such as arginase-1, that are known to suppress the T cell-mediated anti-tumor immune response. Given these results, we tested the ability of tumor neutrophils and the tumor microenvironment to suppress CD4+ and CD8+ T cells proliferation and activation ex vivo (Fig. S6 and S7). Both, neutrophils and the tumor supernatant decreased T-cell proliferation and activation ().
Figure 4. Systemic metabolic state correlates with degranulation by tumor-associated neutrophils. Correlations are shown between tumor-infiltrating neutrophil surface expression of granule release markers related to secondary granules (CD66b) (a-b), primary granules (CD63) (c-d) and the effector enzyme arginase-1 (e-f), and BMI or subcutaneous adipose tissue. BMI was calculated using the patients’ height and weight and subcutaneous adipose tissue area was calculated using an MRI dissection system as described in the methods. (g-h) Co-culture assay of T-cells with blood neutrophils (BN), tumor neutrophils (TN), and tumor supernatant (TS). Correlations were assessed using Spearman’s test, while comparison for the T cells assay was performed using the Friedman’s test with uncorrected Dunn’s test (* p < .05; *** p < .001)
Discussion
Neutrophils play an important role in predicting patient outcomes in kidney cancer, as they make up an important part of the most widely used prognostic score for overall survival, International Metastatic RCC Database Consortium (IMDC).Citation1,Citation5,Citation6 The findings presented herein show that, in addition to changes in ratios of circulating leukocytes, factors such as neutrophil phenotype and activation state may also contribute to disease progression in kidney cancer. Neutrophils in kidney cancer patients display an immature-like phenotype and a primed metabolic state, with enhanced basal glycolytic and oxidative capacities. The immature-like phenotype of circulating neutrophils in kidney cancer patients was characterized by downregulation of key surface molecules that are necessary for innate immune responses. The downregulation observed in the surface expression of the phagocytic receptor CD16 may be consistent with the advanced age of kidney cancer patients, Citation12,Citation14 though previous studies in elderly donors did not show modulation of other surface receptors (CD14, CD63, CD66b), suggesting that the phenotypic state of circulating neutrophils observed in this study is cancer-dependent. The immature-like phenotype in neutrophils from cancer patients may be due to an emergency hematopoiesis response in the bone marrow. This interpretation is further supported by the primed resting metabolic state of the neutrophils in kidney cancer patients. Our findings are consistent with previous reports, where circulating neutrophils from tumor-bearing mice displayed an immature-like phenotype and a primed metabolic state in a model of breast cancer (4T1).Citation15 Together, these results suggest that the tumor microenvironment promotes the release of immature-like neutrophils with a primed/activated metabolic state from the bone marrow into the circulation.
The activated metabolic phenotype observed in circulating neutrophils from kidney cancer patients prompted us to assess whether the patients’ systemic metabolic state was related with circulating or tumor-associated neutrophil function. Obesity, systemic metabolism, and patient fitness are emerging as influencing factors for cancer free-survival as both preemptive measures and post-operative disease progression.Citation16–Citation18 Obesity causes a state of chronic low-grade inflammation, where soluble factors, such as cytokines, chemokines, and growth factors are systemically modulated. This has been shown to give rise to immunosuppressive populations, notably MDSCs, as compensatory mechanisms to counteract obesity-associated diseases. Indeed, several research groups, including the International Agency for Research on Cancer, report a strong association between obesity and risk of developing kidney cancer, as well as cancer mortality.Citation13 Bhaskaran et al. (2014), reported a 1.25-fold increase in the hazard ratio for every 5 kg/m2 increase in BMI in a study of 1,900 patients in the United Kingdom. Our findings show a strong relationship between obesity and neutrophil release of an enzyme known to suppress T-cell function at the tumor site. Neutrophils from kidney cancer patients with higher BMIs had greater immune suppressive activity, with heightened release of primary and secondary granules, as well as surface expression of arginase-1. Importantly, depletion of arginine by arginase-1 has been reported to suppress CD8 anti-tumor responses in cancer models.Citation19–Citation22 Additionally, our results also show an association between adipose tissue distribution and primed/activated neutrophil phenotype in the tumor tissue. Given the known heterogeneity within adipose tissue, it is possible that subcutaneous adipose tissue produces local soluble factors/adipokines that support changes in neutrophil phenotype and function, which in turn promote disease progression. Future studies are needed to identify the function and mechanisms by which soluble molecules from specific adipose tissue sites modulate neutrophil behavior toward an immunosuppressive state in cancer.
In conclusion, this study highlights that circulating neutrophils from kidney cancer patients have phenotypically and metabolically distinct phenotypes from those of healthy donors. Additionally, we provide preliminary evidence that implicates systemic chronic low-grade inflammation and obesity in promoting an activated neutrophil phenotype with possible suppressive capacity in the tumor microenvironment. Further characterization of the mechanisms leading to release of immunomodulatory enzymes, such as arginase-1 and neutrophil elastase, by tumor-associated neutrophils would provide novel therapeutic targets and opportunities to enhance the efficacy of current immunotherapies, Citation23–Citation25 as both arginase-1 and neutrophil elastase have been shown to play a role in modulating tumor-infiltrating lymphocyte behavior.Citation19–Citation22
Competing interests
The authors declare no potential conflicts of interest.
Supplemental Material
Download ()Acknowledgments
We thank the Department of Urology at Emory University for supporting the study through the Faculty Scholars Award. We gratefully acknowledge support from the John W. Robinson Family Foundation, and the Christopher Churchill Foundation. We also thank the Emory University Pediatrics flow cytometry core for their cooperation.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019. doi:10.1038/s41571-019-0222-4.
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov R, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562–8. doi:10.1016/j.celrep.2014.12.039.
- Mollinedo F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol. 2019;40(3):228–242. doi:10.1016/j.it.2019.01.006.
- Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi:10.1158/0008-5472.CAN-08-1921.
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477–2490. doi:10.1056/NEJMra043172.
- Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27(28):4709–4717. doi:10.1200/JCO.2008.18.9498.
- Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015;5(4):e006404. doi:10.1136/bmjopen-2014-006404.
- Templeton AJ, Knox JJ, Lin X, Simantov R, Xie W, Lawrence N, Broom R, Fay AP, Rini B, Donskov F, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70(2):358–364. doi:10.1016/j.eururo.2016.02.033.
- Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, Akce M, Kissick HT, Carthon BC, Shaib WL, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–134. doi:10.1002/cncr.31778.
- Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. doi:10.1139/H08-075.
- Tirouvanziam R, Diaz D, Gernez Y, Laval J, Crubezy M, Makam M An integrative approach for immune monitoring of human health and disease by advanced flow cytometry methods. Advanced Optical Flow Cytometry: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. p. 333–362.
- Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, Nelson DJ. Aging and cancer: the role of macrophages and neutrophils. Ageing Res Rev. 2017;36:105–116. doi:10.1016/j.arr.2017.03.008.
- Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi:10.1016/S0140-6736(14)60892-8.
- Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O’Mahony D, Lord JM. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–886.
- Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, Patel NL, Palmieri EM, Weiss JM, Lee J-M, et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018;9(1):5099. doi:10.1038/s41467-018-07505-2.
- Siewierska K, Malicka I, Kobierzycki C, Paslawska U, Cegielski M, Grzegrzolka J, Piotrowska A, Podhorska-Okolow M, Dziegiel P, Wozniewski M, et al. The impact of exercise training on breast cancer. In Vivo. 2018;32(2):249–254. doi:10.21873/invivo.11231.
- Kim J, Choi WJ, Jeong SH. The effects of physical activity on breast cancer survivors after diagnosis. J Cancer Prev. 2013;18(3):193–200. doi:10.15430/JCP.2013.18.3.193.
- Graf C, Wessely N. Physical Activity in the Prevention and Therapy of Breast Cancer. Breast Care (Basel). 2010;5(6):389–394. doi:10.1159/000322650.
- Czystowska-Kuzmicz M, Sosnowska A, Nowis D, Ramji K, Szajnik M, Chlebowska-Tuz J, Wolinska E, Gaj P, Grazul M, Pilch Z, et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat Commun. 2019;10(1):3000. doi:10.1038/s41467-019-10979-3.
- Lu H, Xu S, Liang X, Dai Y, Huang Z, Ren Y, Lin J, Liu X. Advanced glycated end products alter neutrophil effect on regulation of CD4+ T cell differentiation through induction of myeloperoxidase and neutrophil elastase activities. Inflammation. 2019;42(2):559–571. doi:10.1007/s10753-018-0913-5.
- Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier G, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5(1):101. doi:10.1186/s40425-017-0308-4.
- Tateosian NL, Reiteri RM, Amiano NO, Costa MJ, Villalonga X, Guerrieri D, Maffía PC. Neutrophil elastase treated dendritic cells promote the generation of CD4+FOXP3+ regulatory T cells in vitro. Cell Immunol. 2011;269(2):128–134. doi:10.1016/j.cellimm.2011.03.013.
- Lalani AKA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, Duquette A, Bossé D, Bellmunt J, Van Allen EM, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. doi:10.1186/s40425-018-0315-0.
- Azuma T, Matayoshi Y, Nagase Y, Oshi M. Neutrophil number after interferon-alfa treatment is an independent predictive marker of overall survival in metastatic renal cell carcinoma. Clin Genitourin Cancer. 2012;10(3):180–184. doi:10.1016/j.clgc.2012.03.006.
- Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11(7):856–861. doi:10.1016/j.intimp.2011.01.030.
