ABSTRACT
The immunoregulatory protein B7-H3, a member of the B7 family, has been confirmed to be highly expressed in colon cancer. However, the exact influence of B7-H3 on the features and antitumor ability of γδT cells in colon cancer remains unknown. In the present study, we investigated that the proportions of B7-H3+ γδT cells were distinctly increased in the peripheral blood and tumor tissues of colon cancer patients. B7-H3 blockade or knockdown promoted proliferation, inhibited cell apoptosis and induced the expression of activation markers (CD25 and CD69) on Vδ2 T cells. In contrast, treatment with the B7-H3 agonist 4H7 had the opposite effect. Furthermore, B7-H3 suppressed IFN-γ expression by inhibiting T-bet in Vδ2 T cells. Moreover, B7-H3 mediated the inhibition of Vδ2 T cell cytotoxicity via the downregulation of IFN-γ and perforin/granzyme B expression. More importantly, blocking the B7-H3 function significantly enhanced the cytotoxicity of Vδ2 T cells against colon cancer cells in vivo. Therefore, the inhibition or blockade of B7-H3 is a potential immunotherapeutic approach for colon cancer.
Introduction
According to the 2018 Global Cancer Statistics report, colon cancer has become the third most common cancer worldwide.Citation1 Furthermore, the mortality of colon cancer has risen and is the second highest.Citation1 In China, the incidence and mortality of colon cancer have exhibited sustained growth over recent decades.Citation2,Citation3 Although improvements in screening programs and treatment patterns have been made, the five-year survival rate of colon cancer patients with distant metastases is only 10%.Citation4 For these patients, the standard treatment is surgical resection combined with radiotherapy or chemotherapy.Citation5,Citation6 However, the risk of recurrence and resistance to radiotherapy or chemotherapy results in poor clinical outcomes.Citation7,Citation8 New therapeutic methods have been proposed for colon cancer treatment, such as targeted therapy and immunotherapy.Citation9 Cancer immunotherapy, including active immunotherapy, passive immunotherapy, and immune checkpoint blockade, has become a new cancer treatment research direction and received significant attention.Citation10,Citation11 While much is known about the roles of natural killer (NK) cells and chimeric antigen receptor (CAR)–T cells in cancer immunotherapy,Citation12,Citation13 the role of gamma delta (γδ) T cells in colon cancer remains the least understood.
γδ T cells constitute approximately 5% of all circulating T cell populations and play a crucial role in innate and adaptive immune surveillance.Citation14,Citation15 Vγ9Vδ2 (Vδ2) T cells, the predominant human peripheral blood γδT cell subset (50-90%),Citation16 possess a high antitumor capability because they are without MHC-restricted antigen recognition and can produce abundant inflammatory cytokines, such as IFN-γ, TNF-a and IL-17.Citation17 Vδ2 T cells infiltrate several types of tumors, such as lung cancer, prostate cancer, melanoma, ovarian cancer, breast cancer, and colon cancer, and could serve as a prognostic factor.Citation18 Activated Vδ2 T cells were reported to kill various tumor cells in vitro.Citation19 However, several Vδ2 cell-based clinical adoptive immunotherapies for solid tumors have shown limited success.Citation20,Citation21 Therefore, an investigation is needed to determine why Vδ2 T cells do not effectively kill tumor cells in the solid tumor microenvironment.
As an important member of the B7 superfamily, B7-H3 (also known as CD276) is a type I membrane protein.Citation22 The extracellular domain of B7-H3 in mice contains one IgV domain and one IgC domain (2IgB7-H3 isoform), and two identical pairs of domains are found in human B7-H3 (4IgB7-H3 isoform).Citation23,Citation24 B7-H3 mRNA is broadly expressed by nonlymphoid and lymphoid organs, while the B7-H3 protein is expressed on immune cells, including dendritic cells (DCs), monocytes, natural killer (NK) cells, B cells, and T cells.Citation25 B7-H3 was shown to modulate the biological functions of immune cells, including macrophages,Citation22 NK cells,Citation26 CD4+ T cells,Citation23 and CD8+ T cells,Citation23,Citation27 and exerted a dual role in regulating the innate and adaptive immune responses.Citation22 However, no reports in the literature have addressed the potential contribution of B7-H3 to the regulation of γδ T cells.
In this study, the proportions of B7-H3+ γδT cells were distinctly increased in the peripheral blood and tumor tissues of colon cancer patients compared to healthy individuals. Furthermore, we investigated whether and how B7-H3 regulates the features and antitumor effect of γδT cells on colon cancer.
Materials and methods
Peripheral blood samples and tissue samples from colon cancer patients
To analyze the proportions of γδT cells in the peripheral blood of colon cancer patients, heparinized peripheral blood samples were collected from 18 healthy individuals and 49 colon cancer patients at the First Affiliated Hospital of Soochow University. In addition, to analyze the proportions of γδT cells in the tumors tissue of colon cancer patients, 9 pairs of colon cancer tissue samples and neighboring noncancerous tissue samples were obtained from patients who had undergone surgery at the First Affiliated Hospital of Soochow University. Healthy individuals were excluded from colon-related diseases and enrolled as controls. Both healthy individuals and colon cancer patients had no immune diseases. The Institutional Review Board of the First Affiliated Hospital of Soochow University approved the study protocol. Informed consent was obtained from healthy individuals and patients for experimentation. Detailed clinicopathological information is provided in Supplementary Table 1 and Supplementary Table 2.
Analysis of γδT cells in peripheral blood and tissue samples
We collected purified peripheral blood mononuclear cells (PBMCs) from the peripheral blood samples of healthy donors and colon cancer patients by using a Ficoll-Paque (Haoyang Biotec, #LTS1077) differential density gradient centrifugation process according to the manufacturer’s instructions. The proportions of γδT cells in PBMCs were determined by a flow cytometry analyzer (Beckman Coulter, Inc.).
Primary γδT cells were isolated from colon cancer tissue samples and neighboring noncancerous tissue samples by enzymatic digestion.Citation28,Citation29 Briefly, the tissue samples were stored in a 0.9% aqueous sodium chloride solution (Baxter Healthcare (Shanghai) Co., Ltd., #A6E1307) at 4°C, washed with PBS (HyClone, #SH30256.01B), cut into small fragments, and incubated for 2.5 h at 37°C in a 2 mg/mL type IV collagenase (Sigma-Aldrich, #V900893) solution. After digestion, disaggregated cells were filtered through a 40 μm strainer (SPL Life Sciences Co., Ltd., #93040) and harvested at 400 g for 5 min. The proportions of γδT cells among the disaggregated cells were determined by a flow cytometry analyzer (Beckman Coulter, Inc.).
Cell separation and culture
To expand Vδ2 T cells, we cultured PBMCs from healthy donors in Advanced RPMI 1640 medium (Gibco, #12633012) supplemented with 10% fetal bovine serum (FBS, Gibco, #10099141), 150 U/mL human recombinant IL-2 (PeproTech, #200-02), 1% penicillin-streptomycin (Beyotime Biotech, #C0222), 1% MEM nonessential amino acids (Gibco, #11140050), 1% l-glutamine (Gibco, #25030081), 50 μM β-mercaptoethanol (Sigma-Aldrich, #M3148-25 ml) and 5 μM zoledronate (Abcam, #ab141980) at a density of 1.5 × 106 cells per milliliter. The culture medium without zoledronate was replenished every 2–3 days. We used flow cytometry to evaluate the status and phenotype of γδT cells and used those data to consider the feasibility of further experiments. If more purified γδT cells were needed, the EasySepTM Human Gamma/Delta T cell Isolation Kit (StemCell Technologies, #18000) was used according to the manufacturer’s instructions. The purity of γδT cells was greater than 95%. For cell transfection with siRNAs, 2 × 105 cells/mL Vδ2 T cells were used in this study. In addition, for cell treatment with anti-human B7-H3 neutralizing antibody MIH35 (eBioscience, #16-5937-85, isotype: IgG2a) or B7-H3 agonists 4H7 (Suzhou Bright Scistar Biotechnology Co., Ltd., #XG-ABAB1158, isotype: IgG1), 1.5 × 106 cells/mL Vδ2 T cells were used in the current study.
Tumor cell culture
HCT116, RKO, and SW480 human colon cancer cell lines (ATCC, #CCL-247, #CRL-2577, #CCL-228) were cultured in Dulbecco’s modified Eagle medium (DMEM, HyClone, #SH30022.01) containing 10% FBS (Biological Industries, #04-001-1A) and 1% penicillin-streptomycin (Beyotime Biotech) at 37°C in a humidified atmosphere of 5% CO2.
Vδ2 T cell transfection
Human B7-H3 siRNA (5ʹ-GCUGUCUGUCUGUCUCAUUTT-3ʹ) and corresponding negative control (NC) siRNA were purchased from GenePharma Co., Ltd. (#A09004). Human T-bet siRNA (5ʹ-CCAAAGGATTCCGGGAGAA-3ʹ) and corresponding NC siRNA were purchased from Guangzhou RiboBio Life Science Co., Ltd. (#siB111213111548-1-5). Purified Vδ2 T cells (2 × 105 cells/mL) were transfected with B7-H3 siRNA, T-bet siRNA or NC siRNA using Lipofectamine 2000 (Invitrogen, #11668019) according to the manufacturer’s instructions. The transfection efficiency after 48 h of transfection was determined by flow cytometry and Western blotting.
Cell apoptosis assay
The apoptosis of Vδ2 T cells treated with MIH35 (10 μg/mL), 4H7 (10 μg/mL) or B7-H3 siRNA was evaluated using an Annexin-V-PE/7-AAD double staining apoptosis detection kit I (BD Biosciences, #559763) according to the manufacturer’s instructions. Annexin-V+/7-AAD− and Annexin-V+/7-AAD+ cells were considered apoptotic cells.
Proliferation assay
The proliferation of Vδ2 T cells was analyzed by CFSE assay. γδT cells were stained with CFSE (Selleck Chemicals, #S8269) for 10 min at room temperature and then cultured in 24-well culture plates for 3 days. The proliferation rate of γδT cells was tested by flow cytometry.
Flow cytometry
For γδT cell surface staining, γδT cells from PBMCs or tissue samples were incubated with specified mAb at 4°C for 20 min and analyzed by flow cytometry (Beckman Coulter). For intracellular staining, a Fixation/Permeabilization Solution Kit (BD Biosciences, #554714) was used to treat with γδT cells according to the manufacturer’s instructions. All antibodies used for flow cytometry analysis are listed in Supplementary Table 3. For supernatants, a Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Cytokine Kit (BD Biosciences, #560484) was used to test multiple cytokines (IL-4, IFN-γ, TNF-α, and IL-17) secreted from γδT cells according to the manufacturer’s instructions.
ELISA
The protein levels of human Perforin, Granzyme B, IFN-γ, IL-17, IL-4 or TNF-α in the supernatants of γδ T cells were examined with ELISA Kits (Arigo, #ARG80173 for Perforin, #ARG80171 for Granzyme B; NeoBioscience, #EHC102 g.48 for IFN-γ, #EHC170.48 for IL-17/IL-17A, #EHC006.48 for IL-4, #EHC103a.48 for TNF-α) according to the manufacturer’s instructions.
Real-time quantitative PCR (RT-qPCR)
Total cellular RNA was isolated by using RNAiso reagent (TaKaRa Bio, #9109) in accordance with the manufacturer’s instructions. In the Biometra TProfessional Standard Gradient Thermocycler (Biometra, #070-851), cDNA was synthesized with a PrimeScriptTM RT Master Mix (TaKaRa Bio, Inc., #RR036A) according to the manufacturer’s instructions. RT-qPCR was performed on a CFX96TM real-time system (Bio-Rad, Model No.#CFX96TM Optics Module) using SYBR Green Master Mix (Vazyme Biotech Co., Ltd., #Q121-02) per the manufacturer’s procedure. The specific primers used in this study were as follows: T-bet forward and reverse primers: 5′-GGTTGCGGAGACATGCTGA-3′ and 5′-GTAGGCGTAGGCTCCAAGG-3′, respectively; and human β-actin forward and reverse primers: 5′-CATGTACGTTGCTATCCAGGC-3′ and 5′-CTCCTTAATGTCACGCACGAT-3′, respectively. All gene expression was normalized to the level of human β-actin, which was used as an endogenous control.
Western blotting
Total protein was extracted from γδT cells and separated by 10% SDS-PAGE as previously described.Citation30 The antibodies used for Western blotting in this study were as follows: goat anti-human 4IgB7-H3 antibody (R&D Systems, #AF1027), rabbit anti-human T-bet/TBX21 antibody (Cell Signaling Technology, #30009), and mouse anti-human/mouse β-actin (CST, #3700).
Cytotoxicity assay
HCT116, RKO or SW480 cells (Target, T) pretreated with mitomycin (10 µg/mL, Sigma-Aldrich, #10107409001) were cocultured with Vδ2 T cells (Effector, E) at different E/T ratios in 96-well plates for 24 h. Subsequently, CCK-8 solution (Dojindo, #CK04) was added to each well and incubated for 4 h, and the optical density (OD) was measured at 450 nm. The cytotoxicity (%) of the Vδ2 T cells against the colon cancer cells was calculated using the following formula:Citation28 cytotoxicity (%) = 100 – 100× [OD value of Vδ2 T cells and colon cancer cells in combination – OD value of Vδ2 T cells alone (control)/OD value of colon cancer cells alone (control)]. In addition, the number and area of Vδ2 T cell cluster after Vδ2 T cell and colon cancer cell coculture were used to evaluate the cytotoxicity of γδT cells as previously described.Citation31 Clusters with an area over 300 μm2 were counted using ImageJ analysis software (National Institutes of Health). In some cases, the granzyme B inhibitor BCL-2 (1 μg/mL, R&D Systems, #827-BC-050), the perforin inhibitor concanamycin A (1 μg/mL, CMA, Dalian Meilun Biotechnology Co., Ltd., #MB0767), human anti-IFN-γ blocking antibody (10 μg/mL, BioLegend, #502509), recombinant human IFN-γ (0.05 ng/mL, PeproTech, #300-02-100), or recombinant human granzyme B (1 μg/mL, R&D Systems, #2906-SE-010) were individually added to the cocultures.
In vivo mouse experiments
Six-week-old female CB-17 SCID mice were purchased from Shanghai Lab. Animal Research Center (Shanghai, China). All mouse experiments were followed by the institutional guidelines for the use and care of laboratory animals of the Institutional Animal Care and Use Committee of Soochow University (Suzhou, China). 2 × 106 HCT116 cells were suspended in 100 μl PBS and subcutaneously injected into the right flank of each CB-17 SCID mouse. When the tumors were easily palpable, the diameter of each tumor was measured by vernier calipers every 2–3 days. The tumor volume was calculated by the following formula: Volume (mm3) = 0.5 × L (mm) × S2 (mm2), where S and L are the smallest and largest perpendicular tumor diameters, respectively.Citation24 At day 14, the tumors had grown to a size of 5–6 mm diameter, mice were randomly distributed into IgG2a, MIH35, γδT+IgG2a and γδT+MIH35 groups (n = 4 per group). IgG2a (4 mg/kg), MIH35 (4 mg/kg), Vδ2 T cells (1 × 107)+IgG2a (4 mg/kg) or Vδ2 T cells (1 × 107) +MIH35 (4 mg/kg) were administered via tail-vein injection every week, respectively. On day 28, all the mice were sacrificed. Then the tumor tissues were peeled off from each tumor-bearing mouse and weighed.
To investigate the anti-tumor effect of Vδ2 T cells in vivo, mice were injected via tail-vein with either Vδ2 T cells (1 × 107)+IgG2a (4 mg/kg) or Vδ2 T cells (1 × 107) +MIH35 (4 mg/kg). On days 1 following injection, blood samples and xenograft tumor samples were obtained from each group (n = 3). The proportions of IFN-γ+, Granzyme B+ and TNF-α+ γδT cells in mouse blood samples and xenograft tumor samples were analyzed by flow cytometry.
Statistical analysis
All statistical analysis was performed by GraphPad Prism 7.0. Two-tailed Student’s t-test was used to compare the significance of differences between the two groups. A one-way ANOVA test was used for multiple comparisons. Pearson correlation was performed to assess the correlation analysis of clinical data. All values are presented as the mean ± SD or SEM. Each experiment was repeated at least three times. *p < .05; **p < .01; ***p < .001 was considered statistically significant.
Results
γδT cells were reduced in CC patients
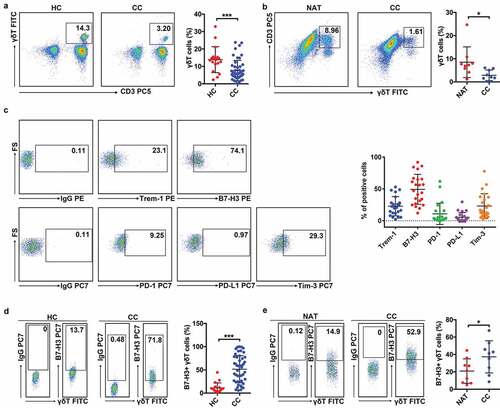
To determine the relationship between γδT cells and colon cancer, we detected the proportions of peripheral circulating γδT cells in healthy donors (n = 18) and colon patients (n = 49) by flow cytometry. Compared with healthy donors, the proportions of γδT cells were obviously reduced in the PBMCs of patients with colon cancer (). In addition, the proportions of infiltrating γδT cells were lower in tumor areas than in neighboring noncancerous areas in colon cancer patients (n = 9) (). These results suggested that γδ T cells exert a protective effect against the development of colon cancer.
Figure 1. The percentages of B7-H3+ γδT cells were increased in peripheral blood and tumor tissue samples from colon cancer patients
B7-H3+ γδT cells were increased in CC patients
Given that immune checkpoint molecules can control the biological functions of immune cells, including γδ T cells, in cancers,Citation32,Citation33 we detected the levels of a series of immune checkpoint molecules (Trem-1, B7-H3, PD-1, PD-L1, and Tim-3) on peripheral circulating γδT cells in colon patients (n = 23). The expression of B7-H3 on γδT cells was obviously increased compared to other molecules (), suggesting that B7-H3 is involved in the regulation of γδT cells. Next, we detected the proportions of B7-H3+ γδT cells in both the peripheral blood and tumor areas of colon cancer patients. The proportions of B7-H3+ γδT cells in the peripheral blood of colon cancer patients were higher than those in healthy donors (). Furthermore, the proportions of B7-H3+ γδT cells in the tumor areas of colon cancer patients were distinctly increased compared to neighboring noncancerous areas (). These data demonstrated that B7-H3 might have a key role in regulating the biological function of γδT cells in colon cancer.
Effect of B7-H3 on Vδ2 T cell proliferation, apoptosis, and activation marker expression
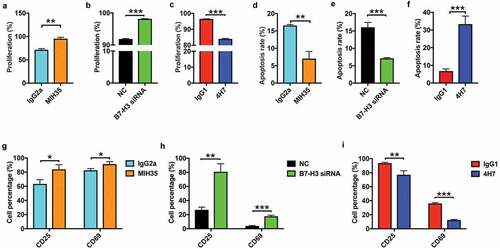
To assess the effect of B7-H3 on Vδ2 T cell proliferation, apoptosis, and activation marker expression, anti-human B7-H3 neutralizing antibody MIH35, B7-H3 siRNA or B7-H3 agonist 4H7 was used to treat Vδ2 T cells in vitro. MIH35 or B7-H3 siRNA treatment obviously reduced the proportions of B7-H3+ Vδ2 T cells (Supplementary Figure 1A and B), while 4H7 treatment increased the proportions of B7-H3+ Vδ2 T cells (Supplementary Fig. 1C). Both MIH35 and B7-H3 siRNA treatment significantly increased Vδ2 T cell activity ( and , Supplementary Fig. 2A and B), whereas 4H7 treatment suppressed Vδ2 T cell activity (, Supplementary Fig. 2 C). In vitro apoptosis experiments showed that both MIH35 treatment and B7-H3 siRNA transfection significantly decreased, while 4H7 treatment markedly increased the apoptosis rate of Vδ2 T cells (, Supplementary Fig. 2 D-F). Furthermore, the expression of activation markers CD25 and CD69 on Vδ2 T cells after treatment with MIH35, B7-H3 siRNA or 4H7 was analyzed. The expression of CD25 and CD69 was markedly increased on Vδ2 T cells after MIH35 or B7-H3 siRNA treatment ( and , Supplementary Fig. 2 G and H). In contrast, 4H7 treatment decreased the expression of CD25 and CD69 by Vδ2 T cells (, Supplementary Fig. 2I).
Figure 2. The effect of B7-H3 on the proliferation and apoptosis of Vδ2 T cells and activation marker expression on Vδ2 T cells
B7-H3 suppressed IFN-γ expression by Vδ2 T cells
To confirm the effect of B7-H3 on cytokine expression by Vδ2 T cells, ELISA assay and CBA assay were performed to detect the level of IL-4, IL-17, IFN-γ, and TNF-α. The results of ELISA assay and CBA assay showed that the expression of IFN-γ was significantly upregulated in Vδ2 T cells upon their treatment with MIH35 or B7-H3 siRNA ( and , Supplementary Fig. 3A), while it was obviously downregulated in Vδ2 T cells after treatment with 4H7 (, Supplementary Fig. 3B). Furthermore, intracellular IFN-γ levels in Vδ2 T cells were detected by flow cytometry, which indicated that both MIH35 and B7-H3 siRNA treatment significantly increased, while 4H7 treatment markedly decreased the proportions of IFN-γ+ Vδ2 T cells (, Supplementary Fig. 3 C-E). In addition, the levels of IFN-γ+ γδT cells were lower in the peripheral blood of cancer patients (n = 49) than in the peripheral blood of healthy donors (n = 18) (). Additionally, the proportions of IFN-γ+ γδT cells were obviously decreased in tumor areas compared with neighboring noncancerous areas (n = 9) (). Moreover, the proportions of B7-H3+ γδT cells were negatively correlated with the levels of IFN-γ+ γδT cells in the peripheral blood (n = 49) and cancer tissues (n = 9) of colon cancer patients ( and ). These results suggested that B7-H3 mainly regulates IFN-γ expression in Vδ2 T cells.
Figure 3. B7-H3 suppressed the production of IFN-γ in Vδ2 T cells via inhibiting T-bet
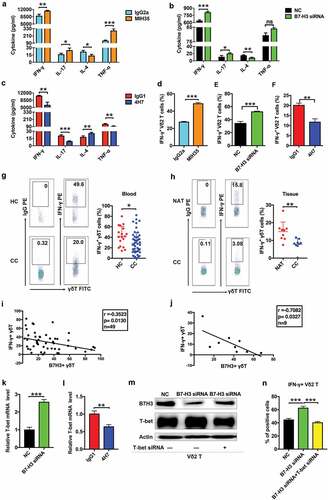
Previous studies have shown that T-bet, the main T-box transcription factor, is involved in regulating the expression of IFN-γ in immune cells.Citation34,Citation35 Importantly, T-bet is expressed by γδT cells.Citation36 Therefore, we hypothesized that the B7-H3-mediated inhibition of IFN-γ is T-bet dependent. RT-qPCR showed that B7-H3 siRNA treatment significantly increased, while 4H7 treatment markedly decreased the expression of T-bet in Vδ2 T cells ( and ). Additionally, the T-bet protein level was obviously increased in Vδ2 T cells treated with B7-H3 siRNA (). Moreover, T-bet siRNA, which significantly reduced T-bet protein expression, abrogated the B7-H3 siRNA-induced increase in the proportions of IFN-γ+ Vδ2 T cells ( and , Supplementary Fig. 3 F). These results indicate that B7-H3 can suppress IFN-γ expression by inhibiting the expression of T-bet in Vδ2 T cells.
B7-H3 inhibited the cytotoxic activity of Vδ2 T cells against colon cancer cells
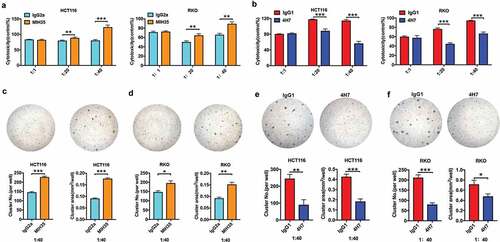
To investigate the impact of B7-H3 on Vδ2 T cell cytotoxic potential, Vδ2 T cells treated with MIH35, B7-H3 siRNA or 4H7 were cocultured with different colon cancer cells (HCT116, RKO, and SW480 cells) at E/T ratios of 1:1, 20:1, and 40:1. Both MIH35 and B7-H3 siRNA treatment significantly enhanced, while 4H7 treatment markedly suppressed the cytotoxic effect of Vδ2 T cells against colon cancer cell lines (HCT116 and RKO) at E/T ratios of 20:1 and 40:1 ( and , Supplementary Fig. 4A). Previous research has shown that the number and area of Vδ2 T cell clusters are effective indexes for estimating cytotoxic capacity.Citation31 Herein, Vδ2 T cells treated with MIH35 or B7-H3 siRNA formed more and larger clusters surrounding HCT116 and RKO cells than Vδ2 T cells treated with IgG2a or siRNA negative control ( and , Supplementary Fig. 4B and C), while Vδ2 T cells treated with 4H7 clearly displayed the opposite effects ( and ). In addition, B7-H3 siRNA treatment promoted, whereas 4H7 treatment suppressed the cytotoxic activity of Vδ2 T cells against SW480 cells (Supplementary Fig. 4D-G).
Figure 4. B7-H3 inhibited the antitumor cytotoxic potential of Vδ2 T cells
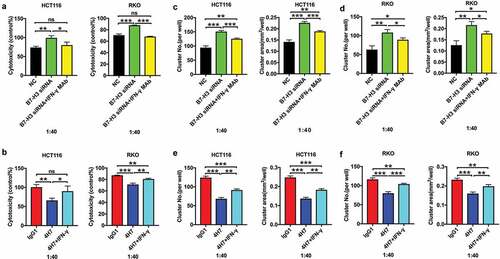
B7-H3 suppressed Vδ2 T cell cytotoxicity via controlling IFN-γ
One of the multiple mechanisms by which Vδ2 T cells kill tumor targets is through proinflammatory cytokines, such as IFN-γ. Anti-IFN-γ monoclonal antibody (IFN-γ mAb) and recombinant human IFN-γ were used to determine whether IFN-γ is essential for the B7-H3-mediated inhibition of Vδ2 T cell cytotoxicity. As shown in , IFN-γ mAb reversed the B7-H3 knockdown-induced increase in Vδ2 T cell cytotoxicity against HCT116 and RKO cells, whereas recombinant human IFN-γ abrogated the inhibitory effect of 4H7 treatment on the cytotoxicity of Vδ2 T cells against HCT116 and RKO cells (). These results were confirmed in parallel by Vδ2 T cell cluster analysis (, Supplementary Fig. 5A-D).
Figure 5. B7-H3 inhibited the cytotoxic effect of Vδ2 T cells against colon cancer cells by inhibiting IFN-γ
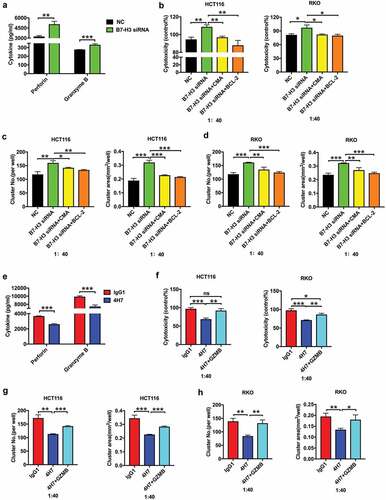
B7-H3 inhibited the killing functions of Vδ2 T cells via the perforin/granzyme pathway
A growing body of evidence indicates that activated γδ T cells exert cytotoxic effects against tumor cells through the granzyme B/perforin pathway.Citation37 We further explored whether the role of B7-H3 in modulating the cytotoxic potential of Vδ2 T cells is perforin/granzyme B pathway-dependent. ELISA showed that the expression levels of perforin and granzyme B were significantly increased in Vδ2 T cells after B7-H3 siRNA treatment (). Treatment with CMA, a V-H+-ATPase inhibitor that blocks perforin release, reversed the B7-H3 knockdown-induced increase in the killing functions of Vδ2 T cells (–, Supplementary Fig. 6A and B). Furthermore, BCL-2, a granzyme B inhibitor, also abrogated this effect (–, Supplementary Fig. 6A and B). Meanwhile, 4H7 treatment obviously downregulated the expression of perforin and granzyme B in Vδ2 T cells (). Moreover, the addition of recombinant human granzyme B significantly rescued the impaired antitumor ability of Vδ2 T cells stimulated with 4H7 (, Supplementary Fig. 6 C and D).
Figure 6. B7-H3 suppressed the cytotoxic effect of Vδ2 T cells against colon cancer cells by downregulating the perforin and granzyme pathway
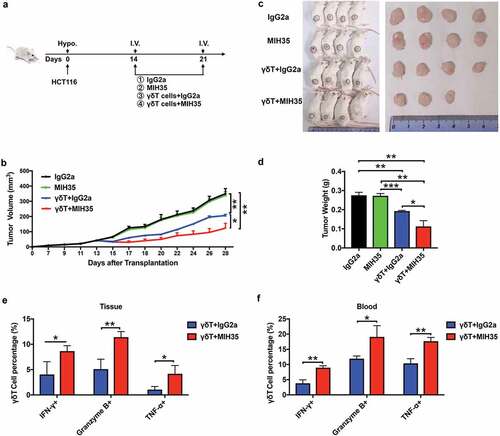
Blocking the B7-H3 function enhanced the cytotoxicity of Vδ2 T cells in vivo
To assess the effect of B7-H3 on the antitumor activity of Vδ2 T cells in vivo, SCID mice were used to establish subcutaneous colon cancer tumors with HCT116 cells, and Vδ2 T cells or MIH35 was administered at different time-points (). The tumor sizes, images, and weight indicated that the treatment of Vδ2 T cells could obviously inhibit the tumor growth as compared with IgG2a or MIH35 treatment (). More importantly, co-treatment with Vδ2 T cells and MIH35 had a more inhibitory effect on the tumor growth than Vδ2 T cells and IgG2a co-treatment (). Moreover, co-treatment with Vδ2 T cells and MIH35 significantly increased the proportions of IFN-γ+, Granzyme B+ and TNF-α+ γδT cells in the mouse PBMCs and xenograft tumor tissues as compared with Vδ2 T cells and IgG2a co-treatment ( and , Supplementary Fig. 7A and B).
Figure 7. B7-H3 blockade promoted the anti-tumor ability of γδT cells in vivo
Discussion
We focused our research on the regulatory effects of B7-H3 on the antitumor activities of Vδ2 T cells in the current study. B7-H3 can control the activities of multiple types of immune cells, such as cytotoxic T lymphocytes and NK cells.Citation26,Citation27 B7-H3 inhibition increased the cytotoxic function of NK and CD8+ T cells and reduced the growth of multiple tumors in mouse models.Citation27 In addition, a novel anti-CD3 × anti-B7-H3 bispecific antibody increased the cytotoxic effect of activated T cells against tumor cells and promoted IFN-γ, TNF-α and IL-2 secretion.Citation38 In this study, much more B7-H3 was expressed than other immune checkpoint molecules (Trem-1, PD-1, PD-L1, and Tim-3) on peripheral circulating γδT cells from colon patients. Although the proportions of γδT cells were obviously reduced in the PBMCs and tumor areas of patients with colon cancer, the proportions of B7-H3+ γδT cells was distinctly increased in colon cancer patients. These results suggested that B7-H3 serves as an important negative immune checkpoint molecule that regulates the activity and biological function of γδT cells in colon cancer.
Previous investigations showed that Vδ2 T cells, the most common subset of γδT cells among PBMCs, could be activated and expanded in vitro after combination treatment with zoledronic acid and IL-2.Citation39,Citation40 A growing number of studies have revealed that multiple immune checkpoint molecules or cytokines are involved in regulating the activation and expansion of Vδ2 T cells exposed to phosphoantigens and IL-2. For instance, PD-1/PD-L1 signaling blockade influenced the cytotoxicity and cytokine production of γδT cells in response to leukemia cells.Citation33 Julie et al. noted that the BTLA-HVEM interaction negatively regulated the phosphoantigen-mediated proliferation of Vδ2 T cells.Citation41 Furthermore, Vδ2 T cells that underwent triple stimulation with CD80, 4-1BB, and CD83 ligand showed long-term growth in low levels of IL-2 and displayed potent cytotoxic activities against tumor cells.Citation42 Additionally, TGF-β signaling augmented the cytotoxic effector activity and expression of CD54, CD103, IFN-γ, IL-9 and granzyme B in γδ T cells in the presence of IL-2 or IL-15.Citation43 In our current study, Vδ2 T cells treated with MIH35, a specific blocking antibody for B7-H3, or B7-H3 siRNA showed increased cellular activity, decreased apoptosis rates, and upregulated expression of the activation markers CD25 and CD69 in the presence of IL-2 and zoledronic acid. In contrast, B7-H3 agonist 4H7 treatment had the opposite effect. These results indicate that B7-H3 negatively impacts the biology of Vδ2 T cells.
IFN-γ signaling has been associated with antitumor immune responses via direct and indirect mechanisms.Citation44 Previous studies certified that B7-H3 signaling mediated the expression of IFN-γ in immune cells and affected the biological function of immune cells. Chapoval et al. reported that B7-H3 could selectively stimulate the expression of IFN-γ in T cells in the presence of T cell receptor signaling.Citation23 In contrast, bronchoalveolar lavage fluid from B7-H3-deficient mice contained higher IFN-γ concentrations than that from control mice.Citation45 In addition, soluble B7-H3 could block IL-2 and IFN-γ secretion from allogeneic T cells and further inhibit the allostimulatory capability of DCs.Citation46 Herein, we performed the ELISA assay and CBA assay to investigate the effect of B7-H3 on the cytokine profile of Vδ2 T cells and found that B7-H3 inhibited the production of IFN-γ in Vδ2 T cells. An intracellular IFN-γ assay also demonstrated this result. More importantly, there was a negative correlation between the proportions of B7-H3+ γδT cells and the levels of IFN-γ+ γδT cells in both the peripheral blood and cancer tissues of colon cancer patients. T-bet is involved in regulating the expression of IFN-γ in immune cells, such as CD4+ T cells and NK cells.Citation34,Citation35 We, therefore, assumed that the B7-H3-mediated inhibition of IFN-γ is T-bet-dependent. Our results indicated that B7-H3 negatively controlled T-bet expression in Vδ2 T cells. Moreover, T-bet siRNA abrogated the B7-H3 siRNA-induced increase in the proportions of IFN-γ+ Vδ2 T cells. These results suggest that B7-H3 suppresses IFN-γ expression via T-bet in Vδ2 T cells.
Various studies have indicated that ex vivo expanded Vδ2 T cells have cytotoxic effects on hematological and nonhematological malignancies. Ex vivo cultured γδT cells from the blood cells of patients with myeloma and lymphoma were able to kill tumor cells.Citation47 The in vitro expansion of Vδ2 T cells activated by bisphosphonate zoledronate substantially increased antitumor activities against human colon cancer stem cells.Citation48 However, cancer immunotherapy based on γδ T cells had only modest clinical results.Citation49 One of the reasons for these modest results could be the emergence of immune escape mechanisms. For instance, PD-1 was shown to be expressed by most follicular lymphoma-infiltrating γδ T cells and impair the antibody-dependent cellular cytotoxicity (ADCC) capacity of γδ T cells.Citation50 The checkpoint molecule Tim-3 limited the expansion of Vδ2 T cells following their culture with IL-21.Citation51 The targeting of BTN3A molecules with anti-BTN3A 20.1 monoclonal antibodies (mAbs) strongly enhanced the cytolytic functions of Vδ2 T cells against pancreatic ductal adenocarcinoma.Citation52 In this study, both MIH35 and B7-H3 siRNA treatment significantly enhanced, while 4H7 treatment markedly suppressed the cytotoxic effect of Vδ2 T cells on colon cancer cell lines. Furthermore, blocking the B7-H3 function by MIH35 could significantly enhance the cytotoxicity of γδT cells in vivo. These findings clearly demonstrate the important inhibitory effect of B7-H3 on the cytotoxicity of γδT cells against colon cancer cells.
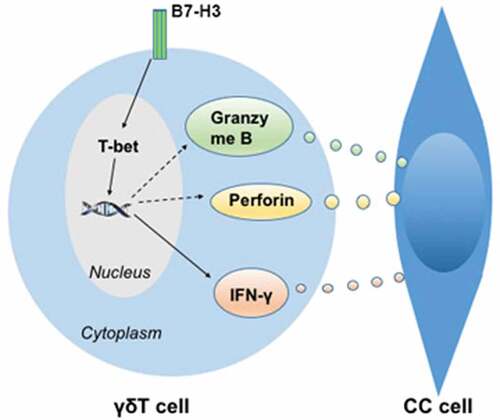
The antitumor effect of γδ T cells against tumor cells is largely achieved via several pathways, including pro-inflammatory cytokine (e.g., IFN-γ, TNF-α, and IL-4) secretion,Citation53 the apoptosis-inducing protein-ligand Fas-FasL pathway,Citation37 granzyme B and/or perforin production,Citation54 and antibody-dependent cellular cytotoxicity.Citation37 Given that B7-H3 suppressed IFN-γ expression in Vδ2 T cells, we determined whether IFN-γ is key for the inhibitory effect of B7-H3 on the cytotoxic effect of Vδ2 T cells on colon cancer cell lines. IFN-γ mAb reversed the B7-H3 knockdown-induced increase in Vδ2 T cell cytotoxicity against colon cancer cells, whereas recombinant human IFN-γ abrogated the inhibitory effect of 4H7 treatment on the cytotoxicity of Vδ2 T cells against colon cancer cells. In addition, we further investigated whether the role of B7-H3 in modulating the cytotoxicity potential of Vδ2 T cells is perforin/granzyme B pathway-dependent. B7-H3 negatively regulated the expression of perforin and granzyme B in Vδ2 T cells. Moreover, CMA or BCL-2 treatment reversed the B7-H3 knockdown-induced increase in the killing functions of Vδ2 T cells. Recombinant human granzyme B addition significantly rescued the impaired antitumor ability of Vδ2 T cells stimulated with 4H7. These results are consistent with earlier observations in follicular lymphoma; anti-CD20 monoclonal antibody dramatically enhanced the cytotoxic effect of Vδ2 T cells against tumor cells by upregulating perforin/granzyme and IFN-γ secretion.Citation55 These results indicate that B7-H3 contributes to the inhibition of Vδ2 T cell cytotoxicity via the downregulation of IFN-γ and perforin/granzyme B expression ().
Taken together, the results of the present study demonstrated that B7-H3 negatively impacts the proliferation, activation, and IFN-γ production of Vδ2 T cells. Moreover, B7-H3 mediates the inhibition of Vδ2 T cell cytotoxicity via the downregulation of IFN-γ and perforin/granzyme B expression. Hence, Vδ2 T cells combined with inhibiting or blocking B7-H3 represents a potential immunotherapeutic approach for colon cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download ()Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bray FI, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–13.
- Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21.
- Lu Y, Zhang H, Liang R, Xie Z, Luo H, Zeng Y, Xu Y, Wang L, Kong X, Wang K. Colorectal cancer genetic heterogeneity delineated by multi-region sequencing. PLoS One. 2016;11:3.
- Haggar F, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197.
- Kaur G, Sayegh ET, Larson AR, Bloch O, Madden M, Sun MZ, Barani IJ, James CD, Parsa AT. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16:628–636.
- Ilson DH. Adjuvant therapy in colon cancer: less is more. Lancet Oncol. 2018;19:442–443.
- Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425.
- Yu TC, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563.
- Kather JN, Halama N, Jaeger D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin Cancer Biol. 2018;52:189–197.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489.
- Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754.
- Velardi A. NK cell adoptive immunotherapy. Blood. 2005;105:3006.
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733.
- Bonneville M, Obrien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunolo. 2010;10:467–478.
- Fisher J, Anderson J. Engineering approaches in human gamma delta T cells for cancer immunotherapy. Front Immunol. 2018;9:1409.
- Willcox CR, Davey MS, Willcox BE. Development and selection of the human Vγ9Vδ2+ T-cell repertoire. Front Immunol. 2018;9:1501.
- Dar AA, Patil RS, Chiplunkar SV. Insights into the relationship between toll like receptors and gamma delta T cell responses. Front Immunol. 2014;5:366.
- Presti EL, Pizzolato G, Corsale AM, Caccamo N, Sireci G, Dieli F, Meraviglia S. γδ T cells and tumor microenvironment: from immunosurveillance to tumor evasion. Front Immunol. 2018;9:1395.
- Simoes AE, Lorenzo BD, Silvasantos B. Molecular determinants of target cell recognition by human γδ T cells. Front Immunol. 2018;9:929.
- Wu D, Wu P, Wu X, Ye J, Wang Z, Zhao S, Ni C, Hu G, Xu J, Han Y. Ex vivo expanded human circulating Vδ1 γδT cells exhibit favorable therapeutic potential for colon cancer. OncoImmunology. 2015;4:3.
- Fisher J, Flutter B, Wesemann F, Frosch J, Rossig C, Gustafsson K, Anderson J. Effective combination treatment of GD2-expressing neuroblastoma and Ewing’s sarcoma using anti-GD2 ch14.18/CHO antibody with Vγ9Vδ2+ γδT cells. OncoImmunology. 2016;5:1.
- Wang L, Kang F, Shan B. B7‐H3‐mediated tumor immunology: friend or foe? Int J Cancer. 2014;134:2764–2771.
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269–274.
- Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, Liu C, Zhang G, Wang Z, Wang R, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10(4):308.
- Sun J, Chen L, Zhang G, Jiang J, Zhu M, Tan Y, Wang H, Lu B, Zhang X. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–1171.
- Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–12645.
- Lee YH, Martinorozco N, Zheng P, Li J, Zhang P, Tan H, Park HJ, Jeong M, Chang SH, Kim BS. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–1045.
- Li X, Lu H, Gu Y, Zhang X, Zhang G, Shi T, Chen W. Tim-3 suppresses the killing effect of Vγ9Vδ2 T cells on colon cancer cells by reducing perforin and granzyme B expression. Exp Cell Res. 2020;386:111719.
- Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, Oo YH, Willcox BE. Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J Hepatol. 2018;69:654–665.
- Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, Shi T, Chen W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020;11:55.
- Zhou X, Gu Y, Xiao H, Kang N, Xie Y, Zhang G, Shi Y, Hu X, Oldfield E, Zhang X. Combining Vγ9Vδ2 T cells with a lipophilic bisphosphonate efficiently kills activated hepatic stellate cells. Front Immunol. 2017;8:1381.
- Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J Exp Med. 2019;216:1244–1254.
- Hoeres T, Holzmann E, Smetak M, Birkmann J, Wilhelm M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. OncoImmunology. 2019;8:1550618.
- Jogdand GM, Sengupta S, Bhattacharya G, Singh SK, Barik PK, Devadas S. Plasmodium berghei inducible costimulator expressing T cells promote parasitic growth during blood stage ANKA infection. Front Immunol. 2018;9:1041.
- Wang B, Zhou J, Chen Y, Wei H, Sun R, Tian Z, Peng H. A novel spleen-resident immature NK cell subset and its maturation in a T-bet-dependent manner. J Autoimmun. 2019;105:102307.
- Bhat SA, Vedpathak DM, Chiplunkar SV. Checkpoint blockade rescues the repressive effect of histone deacetylases inhibitors on γδ T cell function. Front Immunol. 2018;9:1615.
- Silvasantos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunolo. 2015;15:683–691.
- Ma J, Ma P, Zhao C, Xue X, Han H, Liu C, Tao H, Xiu W, Cai J, Zhang M. B7-H3 as a promising target for cytotoxicity T cell in human cancer therapy. Oncotarget. 2016;7:29480–29491.
- Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan P, Lam K, Peiris JSM, Lau YL, Tu W. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858–865.
- Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10(8):842–856.
- Gertnerdardenne J, Fauriat C, Orlanducci F, Thibult M, Pastor S, Fitzgibbon J, Bouabdallah R, Xerri L, Olive D. The co-receptor BTLA negatively regulates human Vγ9Vδ2 T-cell proliferation: a potential way of immune escape for lymphoma cells. Blood. 2013;122:922–931.
- Cho H, Kim S, Sohn D, Lee M, Park M, Sohn H, Cho H, Kim T. Triple costimulation via CD80, 4-1BB, and CD83 ligand elicits the long-term growth of Vγ9Vδ2 T cells in low levels of IL-2. J Leukoc Biol. 2016;99:521–529.
- Peters C, Meyer A, Kouakanou L, Feder J, Schricker T, Lettau M, Janssen O, Wesch D, Kabelitz D. TGF-β enhances the cytotoxic activity of Vδ2 T cells. Oncoimmunology. 2019;8:e1522471.
- Ikeda H, Old LJ, Schreiber RD. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109.
- Suh W, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia AEH. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906.
- Xu J, Huang B, Xiong P, Feng W, Xu Y, Fang M, Zheng F, Gong F. Soluble Mouse B7-H3 Down-Regulates Dendritic Cell Stimulatory Capacity to Allogenic T Cell Proliferation and Production of IL-2 and IFN-γ. Cell Mol Immunol. 2006;3:235–240.
- Saitoh A, Narita M, Watanabe N, Tochiki N, Satoh N, Takizawa J, Furukawa T, Toba K, Aizawa Y, Shinada S. Anti-tumor cytotoxicity of γδ T cells expanded from peripheral blood cells of patients with myeloma and lymphoma. Med Oncol. 2008;25:137–147.
- Todaro M, Dasaro M, Caccamo N, Iovino F, Francipane MG, Mg F, Meraviglia S, Orlando V, La Mendola C, Gulotta G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. Default J. 2009;182:7287–7296.
- Fournie J, Sicard H, Poupot M, Bezombes C, Blanc A, Romagne F, Ysebaert L, Laurent G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10:35–41.
- Rossi C, Gravelle P, Decaup E, Bordenave J, Poupot M, Tosolini M, Franchini D, Laurent C, Morin R, Lagarde J. Boosting γδ T cell-mediated antibody-dependent cellular cytotoxicity by PD-1 blockade in follicular lymphoma. OncoImmunology. 2019;8:1554175.
- Wu K, Zhao H, Xiu Y, Li Z, Zhao J, Xie S, Zeng H, Zhang H, Yu L, Xu B. IL-21-mediated expansion of Vγ9Vδ2 T cells is limited by the Tim-3 pathway. Int Immunopharmacol. 2019;69:136–142.
- Benyamine A, Loncle C, Foucher E, Blazquez J, Castanier C, Chretien A, Modesti M, Secq V, Chouaib S, Gironella M. BTN3A is a prognosis marker and a promising target for Vγ9Vδ2 T cells based-immunotherapy in pancreatic ductal adenocarcinoma (PDAC). OncoImmunology. 2018;7:1.
- Van Acker HH, Campillodavo D, Roex G, Versteven M, Smits E, Van Tendeloo V. The role of the common gamma-chain family cytokines in γδ T cell-based anti-cancer immunotherapy. Cytokine Growth Factor Rev. 2018;41:54–64.
- Chen Y, Zheng L, Aldarouish M, Zhou Z, Pan N, Liu J, Chen F, Wang L. Wnt pathway activator TWS119 enhances the proliferation and cytolytic activity of human γδT cells against colon cancer. Exp Cell Res. 2018;362:63–71.
- Braza MS, Klein B, Fiol G, Rossi J. γδ T-cell killing of primary follicular lymphoma cells is dramatically potentiated by GA101, a type II glycoengineered anti-CD20 monoclonal antibody. Haematologica. 2011;96:400–407.