ABSTRACT
Immunotherapy using immune checkpoint inhibitors has opened a new era for cancer management. In colorectal cancer, patients with a phenotype of deficient mismatch repair or high microsatellite instability benefit from immunotherapy. However, the response of rest cases to immunotherapy alone is still poor. Nevertheless, preclinical data have revealed that either ionizing irradiation or chemotherapy can improve the tumoral immune milieu, because these approaches can induce immunogenic cell death among cancer cells. In this regard, combination use of standard therapy plus immunotherapy should be feasible. In this review, we will introduce the specific roles of standard therapies, including radiotherapy, chemotherapy, antiangiogenic and anti-EGFR therapy, in improving therapeutic effect of immune checkpoint inhibitors on colorectal cancer.
1. Introduction
Colorectal cancer (CRC) is one of the top three most common cancers in the world. In China, both the incidence and mortality of CRC have been increasing in recent years. More medical sources have been paid to CRC patients, and a 5-year survival rate of 57% was reported in 2015.1 Nowadays, immune checkpoint inhibitors (ICIs) targeting programmed cell death protein-1 (PD-1), programmed cell death-ligand 1 (PD-L1) or cytotoxic T-lymphocyte associated protein-4 (CTLA-4) have become hot topics in the field of cancer treatment. In CRC, the cases having deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H) can directly benefit from ICI therapy,Citation2 but they only account for a small portion among all CRC cases. The rest cases with proficient MMR (pMMR), low microsatellite instability (MSI-L) or microsatellite-stable (MSS) phenotypes barely respond to ICI therapy.Citation3
In addition to dMMR or MSI-H, an ideal paradigm indicates that if tumors have massive tumoricidal T cells and high PD-L1 expression, they will shrink in response to anti-PD-1 therapy.Citation4 However, for patients without obvious infiltration of tumoricidal T lymphocytes, the immune milieu should be improved. Can the standard therapies for CRC become candidates in this process? The current clinical practice guidelines recommend chemotherapy plus antiangiogenic therapy for treating metastatic CRC with RAS or BRAFV600E mutation.Citation5,Citation6 Herein, antiangiogenic therapy enables vascular normalization to facilitate T cell infiltration into tumors, thus potentially synergizing with ICIs to control tumor progression.Citation7 In addition, radiotherapy, such as stereotactic body radiotherapy (SBRT), is apt to increase the production of tumor-associated antigens (TAA) and IFN-γ in tumor microenvironment (TME) along with upregulating the expression of PD-1 by T cells or major histocompatibility complex class-I (MHC-I) and PD-L1 by tumor cells.Citation8,Citation9 In this regard, some of the standard therapies have exhibited their potential in improving tumoral immune milieu, thus providing a platform for combination with ICI drugs.
In this review, we will introduce the immunosuppressive profile in tumors. Then, we will discuss the impacts of standard therapies on the host immune milieu. We hope that some opinions will shed new light on the combination use of standard therapies with ICIs in CRC treatment.
2. The suppressive immune microenvironment in tumors
In fact, it is observed that cancer patients at the same TNM stage differ in their prognosis.Citation10 The heterogeneity of cancer indeed is widely accepted, especially as the profiles of genetic mutations have been revealed among cancers, such as CRC,Citation11 gastric cancerCitation12 or lung cancer.Citation13,Citation14 Intrinsically, such mutations resist such processes as immune surveillance, recognition and clearance. In this case, although immune cells continue their migration into tumors, ‘cancer immunoediting’ enables tumoricidal processes to be weakened or even deprived.Citation4 Herein, the TME is dangerous in that it is able to thwart tumoricidal activities of tumor infiltrating lymphocytes (TIL) by inducing them to express molecules, such as PD-1 and CTLA-4.Citation15 Herein, interaction between PD-1 of T cells and PD-L1 of tumor cells can impair T cell survival and tumoricidal function.Citation15 Meanwhile, CTLA-4, a CD28 homologue but exhibiting higher affinities to CD80 and CD86 than CD28 molecule,Citation16 will antagonize the proliferation and activation of tumoricidal T cells if binding with co-stimulatory molecules,Citation15 a process of CTLA-4-induced dephosphorylation of T cell receptor (TCR) complex zeta chain.Citation17 Moreover, some cytokines contribute to immune suppression in TME as well (). For example, vascular endothelial growth factor (VEGF) is one of the chief criminals (). This cytokine exhibits potency in limiting the maturation and antigen-presenting functions of dendritic cells (DC) along with reducing the cytotoxic activity of CD8+ T cells.Citation7 Meanwhile, VEGF promotes the expansion of Treg cells and myeloid-derived suppressive cells (MDSC) and the phenotypic conversion of tumor-associated macrophages (TAM) from M1 to M2.Citation7 In line with these findings, other factors including angiogenin-2, indoleamine 2,3-dioxygenase (IDO), transforming growth factor-beta (TGF-β), prostaglandin E2 (PGE2), IL-6 and IL-10, along with some chemokines, also facilitate immunosuppressive eventsCitation7 (). In addition, these factors enable systemic immunity to be facilitated by the tumor by increasing the frequencies of suppressor cells (e.g., MDSCs or Treg cells) while decreasing the frequencies of effector cells (e.g., cytotoxic T or Th1) in the periphery.Citation7 In this process, the normal function of T cells will be equally challenged. In this case, exhaustion of T cells is the most critical event. Physically, T cells will become exhausted if undergoing persistent antigen exposure. Factors such as the inhibitory ligands of antigen-presenting cells or tumor cells and priming by PGE2, IL-10 or TGF-β also account for T cell exhaustionCitation18 (). In fact, exhausted T cells distinguish themselves from the subsets of memory or effector T cells in several aspects, including progressive loss of effector functions, abnormal responsiveness to the homeostatic cytokines (e.g., IL-7 or IL-15), sustained inhibitory receptor expression (e.g., PD-1, CTLA-4, T cell immunoreceptor with Ig and ITIM domains [TIGIT], T cell immunoglobulin and mucin domain containing molecule-3 [Tim-3] or lymphocyte-activation gene-3 [LAG-3]), metabolic alteration in terms of glycolysis and expression of exclusive transcriptional programs directing cell differentiationCitation18 (). In another route, tumor cells can secrete exosomes containing PD-L1 to manipulate distant T cell survival and function, which has been successfully tested in an experimental model of melanoma.Citation19 However, a recent study revealed that melanoma patients with high ratios of peripheral PD-1+CD8+ T cells to tumor burden exhibited better responses to anti-PD-1 therapy than those who did not have a high ratio.Citation20 Several newly published studies have identified that the cells that respond to anti-PD-1 therapy are pre-exhausted T cells in the peripheryCitation21,Citation22 rather than exhausted T cells pre-existing in tumors.Citation22,Citation23 However, there are still several approaches allowing for tumor escape from immune cell attack. For example, tumor cells can camouflage themselves by expressing CD47 on their surfaces, thus protecting against phagocytosis by macrophages.Citation24 Meanwhile, tumors will attract several facilitators (e.g., MDSCs, neutrophils, Treg cells or M2-like macrophages) and provide them with a context that induces them to reciprocally activate tumor cells. Together with this stroma maintenance, tumor cells establish a defensive network against immune cell attack (see details in Ref.Citation25).
Figure 1. Construction of immunosuppressive milieu by cancer cells. PD-L1 and VEGF expressed by cancer cells are critical in constructing the immunosuppressive milieu in tumors. Herein, PD-L1 is able to elicit T cell exhaustion, thus enabling them to be with poor response to IL-7 and IL-15 stimulations along with upregulating their expressions of PD-1, CTLA-4, TIGHT, LAG4 and Tim3. VEGF is potent in increasing interstitial pressure within the tumor by promoting angiogenesis. Moreover, VEGF is able to reverse the tumoricidal functions of immune cells, such as dendritic cell (DC), cytotoxic T lymphocyte (CTL) and tumor-associated macrophage (TAM), while promotes expansion of regulatory T cell (Treg) and myeloid-derived suppressive cell (MDSC). CAF: cancer-associated fibroblast; PD-1: programmed cell death-1; PD-L1: programmed cell death-ligand 1.
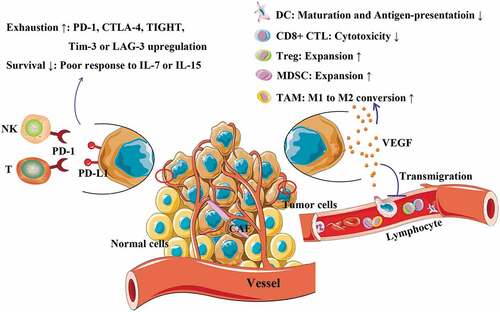
Table 1. Immunosuppresive cytokines and their cellular sources.
3. dMMR/MSI-H and ICI therapeutic responsiveness
Although various factors contribute to the immune suppression in TME, a portion of CRC cases are revealed to be inherently immune-privileged, presenting dMMR or MSI-H phenotypes in tumors.Citation11 As aforementioned, metastatic CRC cases with dMMR/MSI-H exhibit well responsiveness to ICI therapy.Citation2 As we know, microsatellites are regarded as short tandem repeats or simple sequence repeats, which consist of repeated sequences of 1 ~ 6 nucleotides in genome.Citation26 Normally, mismatch repair-associated proteins including MLH1, MSH2, MSH6 and PMS2 can protect against gene mutations.Citation27 However, deficiencies in mismatch repair-associated protein expressions can elicit MSI-H phenotypes, thus leading to excessive production of neoantigens associated with mutated genes.Citation27 If this is the case in CRC, tumoricidal lymphocytes will be attracted to TME after presentation of TAAs by DCs.Citation28 In fact, sustained exposure to foreign antigens serves as a route in eliciting T cell exhaustion.Citation18 In this context, ICI drugs can assist in protecting against this biological event, thus causing shrinkage of CRC tumors.Citation3 But from currently available data, it still can be found that not all of dMMR/MSI-H tumors can respond to ICI therapy.Citation3 Herein, activation of “Wingless/Integrated (Wnt)” signaling pathway has been reported to inhibit tumoricidal T cell infiltration into CRC tumors.Citation29 This case can be translated into dMMR/MSI-H tumors as well.Citation29 Meanwhile, it has been revealed that CRC cases with Wnt activation and pMMR/MSS phenotypes in tumors account for a large portion of all CRC cases,Citation11 but this does not mean that T infiltrates are inherently lacking in such tumors because CRC cases generally decline the amounts of cytotoxic and memory T cells in tumors as TNM stages increase.Citation30 Probably, this serves as a reason why metastatic CRC with pMMR/MSS phenotypes respond poorly to ICI therapy, although exact mechanisms remain elusive. In general, the amount of cytotoxic T plus memory T cells in the tumor is positively associated with CRC prognosis.Citation10 Moreover, such T infiltrates serve as main targets of ICI therapy.Citation3 In this situation, it is urgent to find a right approach to increase T infiltrates in advanced and metastatic CRC cases with pMMR/MSS phenotypes, thus enabling them to benefit from ICI therapy.
4. Standard therapy
Radiotherapy, chemotherapy or molecule-targeted therapy remain the standard of care for advanced or metastatic CRC cases. However, some cases will actually progress into refractory disease of metastases after multiple lines of therapy. At present, best supportive care can be selected as a resolution for these patients, but its low effectiveness commonly fails us. What could be upfronted after the later-line therapy? Combinational use of standard therapy plus immunotherapy appears to be feasible in this context.
4.1. Chemoradiotherapy
Radiotherapy plus concomitant 5-Fluorouracil (5-FU)/capecitabine remains the standard of care for local advanced rectal cancer (LARC) patients and serves as a neoadjuvant or adjuvant for enhancing the local-regional control rate of primary tumors. Herein, oxidative stress is one of the tumoricidal effects exerted by ionizing radiation. However, factors including nuclear factor kappa-B (NF-κB) activation,Citation31 ARG-1+ MDSC infiltration,Citation32 and overexpression of the chemokine (C-X-C motif) ligand 12 (CXCL12) along with its receptors chemokine (C-X-C motif) receptor 4 (CXCR4) and chemokine (C-X-C motif) receptor 7 (CXCR7) in primary tumors have been revealed to be positively associated with poor responses to neoadjuvant chemoradiotherapy (nCRT), thus resulting in the poor clinical outcomes of patients.Citation33–Citation35 As documented, 1.8 Gy ~ 2 Gy, the conventional fraction doses of radiotherapy, are sufficient for causing cell death among mature T cells in humans.Citation36 Providing that distant lymphocytes are recruited into tumors, most of them will undergo apoptosis during fractionated irradiation. But in fact, conventional radiotherapy alters tumoral immune profiles by inducing chemokine and cytokine upregulation, which can recruit the required immune cells into TME. For example, conventional radiotherapy is able to induce CD68+ TAMs to increase the activity of thymidine phosphorylase, which upregulates monocyte chemotactic protein-1 (MCP-1) production by TAMs, thus recruiting circulating monocytes into rectal tumors.Citation37 Likewise, tumoral CXCL12 is able to recruit CXCR4+ myeloid cells.Citation38 Innate immune cells attract adaptive immune cells into tumors by secreting chemoattractants. However, this is a comprehensive network that should not be limited to a certain cytokine (see details in Ref.Citation39). In another approach, ionizing radiation is a powerful tool for inducing immunogenic cell death (ICD).Citation40 In this context, DCs will migrate into peripheral lymph nodes to present TAAs to T cells, while such T cells will be recruited into tumors to perform their functions.Citation40 In the clinic, several retrospective studies have revealed that LARC tumors with a high density of CD3+ or CD8+ T cellsCitation41–Citation46 or with a low density of Treg cells responded well to nCRT.Citation43,Citation45 Moreover, nCRT has been shown to increase tumoral infiltration of CD8+ T cellsCitation42,Citation43,Citation45 but not Treg cells.Citation43,Citation45 Based on these results, several trials were designed to test the efficacy of nCRT plus ICI drugs in LARC (). The combination is expected to increase tumor remission, thus enabling the downstaging or the pathological complete response (pCR) of the primary tumor to be more efficient. In fact, pCR surely occurs in a certain portion of LARC patients after nCRT alone.Citation6 Herein, a phase II trial (VOLTAGE; NCT02948348) reported that after adding five cycles of nivolumab (an anti-PD-1 mAb) before surgery, the pCR rate was 30%,Citation47 which is higher than that seen when using nCRT alone, which approximates the situation of nCRT followed by multiple lines of chemotherapy.Citation48 In line with this, another phase II study (NSABP FR-2; NCT03102047) was designed to add Durvalumab (an anti PD-L1 mAb) during the period before surgery.Citation47 At the very least, such strategies are encouraging for enabling a certain portion of LARC patients to avoid surgery, although evidence suggesting that patient prognosis will be improved is lacking at present. As for the rationale behind such strategies, it should first be mentioned that PD-L1 expression can indeed be shared by multiple organs, such as normal mucosa,Citation49 tumor cellsCitation49 and immune cells.Citation50 Among a portion of LARC patients, it has been confirmed that nCRT is able to upregulate PD-L1 expression either by tumor cells or by stromal immune infiltrates.Citation49,Citation50 In fact, the expression of PD-L1 is associated with the increased expression of IFN-γ after nCRT.Citation49 Typically, CD8+ T and Th1 cells can produce IFN-γ, which serves as a strong inducer of PD-L1 expression by target cells.Citation19 In terms of PD-L1 expression and the density of immune infiltrates, another study revealed that a high level of PD-L1 was related to increased CD8+ T cell infiltration in tumors before and after nCRT.Citation50 In addition, favorable clinical outcomes were achieved among LARC patients with a high tumoral density of CD8+ T cells after nCRT.Citation46 Moreover, before nCRT, high PD-L1 expression by immune infiltrates can predict significant improvement of the disease-free survival (DFS) of LARC patients.Citation50 Tumors with a high density of CD8+ T cells commonly have a favorable prognosis.Citation10 In this regard, anti-PD-(L)1 therapy should be able to compensate for nCRT to overcome CD8+ T cell exhaustion.
Table 2. Advances in combinational use of radiotherapy plus ICI therapy in CRC.
4.2. SBRT
In contrast to conventional radiotherapy, SBRT has exhibited potential in combination with ICI therapy because a growing body of evidence suggests that SBRT has advantages over conventional radiotherapy in several aspects, including vascular normalization,Citation51 tumor cell lysis,Citation52 and sequential immune activation.Citation53 All of these aspects serve as the hallmark effects of SBRT. First, in contrast to the does needed for conventional radiotherapy, a single dose of 8 ~ 10 Gy can cause vascular dysfunction by increasing the activity of acid sphingomyelinase, which converts sphingomyelin into ceramide to induce endothelial apoptosis.Citation51 In addition, cancer stem cells (CSC) are considered critical components driving resistance to conventional radiotherapyCitation54 because CSCs manipulate the TME to instruct tumor responses to conform with the TME requirements in ‘health and disease’.Citation39,Citation55 Nevertheless, SBRT serves as a potential route to block the reciprocal interaction between CSCs and TME substratesCitation54 because SBRT can boost the equivalent biological dose to induce effective ICD among CSCs.Citation53 Due to the oncolytic effect, rapid release of TAAs potentially activates tumoricidal lymphocytes, probably leading to the regression of distant lesions, which share similar TAA profiles. Mechanistically, SBRT-induced increases in tumoral immunogenicity lead to the biological processes of CD8+ T cell activation, DC maturation and antigen presentation, IFN-γ upregulation, type 1 IFN responses and MHC-I upregulation by tumor cells.Citation56–Citation58 However, PD-L1 upregulation by tumor cells and PD-1 upregulation by CD8+ T cells also occur.Citation9 To overcome PD-L1-induced T cell exhaustion, the strategy of SBRT plus ICI therapy was designed in preclinical models, and it has been translated into clinical trials for patients with metastatic tumors to test the feasibility of this strategy.Citation53,Citation58,Citation59 In current clinical practice, SBRT has been recommended for treating CRC liverCitation5 and lung metastatic lesions.Citation60 Moreover, several trials using this combination mode for treating the metastases of refractory CRC are ongoing (). However, it is well known that if TCRs are specific for targetable cancer cell clones, such T cell subpopulations are impotent for killing other subclone cells that lack the same antigens. Therefore, a new opinion holds that SBRT should focus on all metastatic lesions to abandon its probable abscopal effect because TAA heterogenicity exists among different cancer cell subclones.Citation8
4.3. Chemotherapeutic agents
Oxaliplatin serves as an ICD inducer of CRC cells.Citation61 After ICD, the anticancerous functions of DCs and cytotoxic T cells will be activated.Citation62 Mechanistically, it was found that oxaliplatin could increase the serum levels of fms-like tyrosine kinase 3 ligand (Flt3 L), which serves as an indicator of the activation of tumor antigen-presenting DCs.Citation63 In a preclinical model, oxaliplatin was shown to upregulate PD-L1 expression by tumoral immune cells, thus prolonging the survival of CRC-bearing mice when combined with anti-PD-L1 therapy.Citation64 For 5-FU, the death of MDSCs is a valid effect exerted by this agent, whereas evidence suggesting an immune-supportive role of irinotecan has seldom been reported.Citation62 One observation is that the numbers of CD3+CD4+ and CD8+CD28+ cells in the peripheral blood of metastatic CRC patients increase after irinotecan intervention.Citation65 When combining 5-FU with oxaliplatin or irinotecan, a retrospective study reported that the percent of peripheral Treg cells was decreased in the periphery.Citation66 Due to the immunogenic properties of CRC chemotherapy, a phase Ib/II trial has been designed to investigate the safety and efficacy of chemotherapy plus durvalumab and tremelimumab (an anti-CTLA-4 antibody) in metastatic cases with an MSS phenotype (NCT03202758)Citation67 (). In this study, RAS mutation cases were also recruited. Herein, RAS mutations can elicit cell autophagy, which becomes a route of chemoresistance.Citation68 However, a phase III trial (IMblaze370) reported that atezolizumab (an anti-PD-L1 mAb) plus a RAF-mitogen activated protein kinase (MEK) inhibitor failed to improve the clinical outcomes of RAS-mutated metastatic CRC patients.Citation69 Therefore, will RAS mutations influence the efficacy of chemotherapy plus ICI therapy in CRC patients with an MSS or a pMMR phenotype? This question needs answers.
Table 3. Advances in combinational use of systematic therapy plus ICI therapy in CRC.
4.4. Antiangiogenic therapy
Not all CRC tumors respond to chemotherapy initially. Likewise, chemoresistance will occur in a certain number of CRC patients after multiple cycles of chemotherapy. Although several factors are involved in chemoresistance, vascular abnormality accounts for this event because high interstitial pressure potentially hampers drug delivery to tumor cells.Citation7 For example, bevacizumab is an antiangiogenic drug that elicits vascular normalization in tumors. In regard to its synergistic effect on chemotherapy, bevacizumab significantly improves the prognosis of metastatic CRC patients compared to chemotherapy alone.Citation70 Despite this superiority, a retrospective study revealed that the high expression of tumoral PD-L1 negatively impacted the survival of patients with metastatic CRC, irrespective of their receipt of neoadjuvant chemotherapy plus bevacizumab.Citation71 In this situation, it should be asked whether combining the current therapy with ICI therapy will potentially improve the clinical outcome of these patients. Similarly, although chemotherapy plus bevacizumab has been revealed to reduce the amount of MDSCs in the peripheral blood of metastatic CRC patients, MDSCs remain the predominant source of PD-L1, which leads to T cell exhaustion,Citation72 thus providing a rationale for the combination use of anti-PD-(L)1 therapy. In addition, chemotherapy plus bevacizumab appears to be insufficient for the treatment of a certain portion of CRC cases, such as those with high PD-L1 expression. Currently, many clinical trials have been designed to investigate the safety and efficacy of antiangiogenic therapy plus ICI therapy across several cancers, including CRCCitation7 (). Neutralization of VEGF enables bevacizumab to reduce the immunosuppressive functions of tumoral infiltrates, including MDSCs, Treg cells and M2-like TAMs.Citation7,Citation62 However, a preclinical model of breast cancer has revealed that deficiency in hypoxia inducible factor-1alpha (HIF-1α) or its target VEGF in CD8+ T cells can significantly limit their infiltration into tumors along with their cytotoxicity toward tumor cells.Citation73 For CRC, it is still unclear whether VEGF neutralization influences the tumoral amount of CD8+ T cells and their functions. Nevertheless, preclinical studies in CRC have confirmed the immune-supportive effects of antiangiogenic therapy on immunotherapy.Citation7 Thus, it is reasonable to expect that this strategy will benefit CRC patients.
4.5. Anti-epithermal growth factor receptor (EGFR) therapy
For metastatic CRC patients with wide-type RAS, BRAFV600E, PIK3CA and without HER-2 amplification, anti-EGFR therapy is recommended in combination with chemotherapy.Citation5 In terms of its anti-CRC role, it has been revealed that chemotherapy plus anti-EGFR therapy can increase the number of immune infiltrates, including memory T and cytotoxic T cells, in metastatic lesions.Citation74 Moreover, chemotherapy plus cetuximab is able to alter the TCR repertoire diversity of CD4+ T cells, while tumors bearing high TCR diversity in CD4+ T cells are more apt to shrink their sizes than those with low TCR diversity.Citation75 In addition, an in vitro study has revealed that irinotecan plus 5-FU is able to induce TAA production, including EGFR, calreticulin and heat shock protein 90 (HSP90), by colon cancer cells.Citation76 Thus, adding cetuximab to chemotherapy further influences several biological processes performed by DCs, such as inducing DC maturation and activation, enhancing tumor phagocytosis by DCs, and generating tumor-specific cytotoxic T cells that are cross-presented by DCs.Citation76 In fact, cetuximab exhibits immunocompetencies inherently because the Fc portion of its IgG1 backbone is able to bind with the Fc receptor on natural killer (NK) cells, activated macrophages and DCs. Via this action, NK cell-associated antibody-dependent cell-mediated cytotoxicity (ADCC) will be activated.Citation62 However, NK cells always become unable to kill tumor cells during gut carcinogenesis.Citation77 In this context, cetuximab-primed NK-mediated tumor cell lysis is deficient. To overcome this deficiency, several strategies have been developed, such as combinations with IL-21 to reverse NK cell exhaustion,Citation78,Citation79 adoptive transfer of in vitro-expanded tumoricidal NK cells to increase in vivo numbers,Citation80 and agonizing CD137 to induce EGFR-specific CD8+ T cell generation.Citation81 In a similar manner, due to the upregulation of Tim-3 and PD-1 by exhausted NK cells,Citation77 ICI therapy has been proposed as a candidate to prevent NK cell exhaustion. Theoretically, combination with cetuximab can further enhance the tumoricidal activity of NK cells partially due to the ADCC effect. Currently, several trials investigating the therapeutic efficacy of cetuximab plus ICI therapy in CRC are ongoing (). Critically, a newly published work has identified that anti-EGFR therapy truly elicits microsatellite instability emergence in CRC cells, further ensuring the feasibility of anti-EGFR therapy in combination with ICI therapy in CRC patients.Citation82
However, there remains a pitfall when combining ICI therapy with other types of therapy. For example, among CRC patients with operable metastatic lesions, it was found that anti-EGFR therapy plus chemotherapy was not able to induce the infiltration of tumoricidal T cells into all metastatic lesions at similar degrees, and a portion of metastatic lesions still lacked immune cells even after treatment.Citation74 This issue can also be extended to bevacizumab.Citation74 If so, combination uses of ICI drugs plus anti-EGFR or anti-angiogenic therapies will still not benefit all metastatic lesions in the same CRC patient. The situations in those lesions with low or no response to combination therapy are similar to the paradigms of PD-L1+/TIL− or PD-L1−/TIL− tumors.Citation4 As aforementioned, Wnt activation is associated with the low density of T cells in a CRC tumor.Citation29 In this context, the immune-boost therapy, such as SBRT, will be an available choice.
5. Conclusion
CRC patients with dMMR/MSI-H phenotypes in tumors can benefit from ICI therapy, but the response of tumors with pMMR/MSS phenotypes to ICI therapy is extremely poor. Moreover, a portion of CRC cases with pMMR/MSS phenotypes will become refractory after multiple lines of standard therapies. In these cases, radiotherapy, chemotherapy, antiangiogenic therapy or anti-EGFR therapy have been preclinically revealed to exhibit potencies in boosting tumoricidal immune milieus in CRC, thus providing a rational in combining with ICI drugs. On these bases, numerous trials investigating the therapeutic efficacies of standard therapies plus ICI therapy on refractory pMMR/MSS CRC are ongoing, and preliminary results from several trials indicate the combination strategies bring more benefits to CRC patients than ICI therapy alone does. Hence, standard therapies together with ICI therapy are expected to improve the prognosis of refractory cases in CRC.
Abbreviations
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| CRC | = | colorectal cancer |
| CSC | = | cancer stem cell |
| CTLA-4 | = | cytotoxic T-lymphocyte associated protein-4 |
| CXCL12 | = | chemokine (C-X-C motif) ligand 12 |
| CXCR4 | = | chemokine (C-X-C motif) receptor 4 |
| CXCR7 | = | chemokine (C-X-C motif) receptor 7 |
| DC | = | dendritic cell |
| DFS | = | disease-free survival |
| dMMR | = | deficient mismatch repair |
| EGFR | = | epithermal growth factor receptor |
| Flt3L | = | fms-like tyrosine kinase 3 ligand |
| 5-FU | = | 5-Fluorouracil |
| HIF-1α | = | hypoxia inducible factor-1alpha |
| HSP90 | = | heat shock protein 90; NK, nature killer cell |
| ICD | = | immunogenic cell death |
| ICI | = | immune checkpoint inhibitor |
| IDO | = | indoleamine 2, 3-dioxygenase |
| LAG-3 | = | lymphocyte-activation gene-3 |
| LARC | = | local advanced rectal cancer |
| MCP-1 | = | monocyte chemotactic protein-1 |
| MDSC | = | myeloid-derived suppressive cell |
| MSI-H | = | high microsatellite instability |
| MSI-L | = | low microsatellite instability |
| MSS | = | microsatellite stability |
| MHC-I | = | major histocompatibility complex class-I |
| MEK | = | RAF-mitogen activated protein kinase kinase |
| nCRT | = | neoadjuvant chemoradiotherapy |
| NF-Κb | = | nuclear factor kappa-B |
| pCR | = | pathological complete response |
| PD-1 | = | programmed cell death protein-1 |
| PD-L1 | = | programmed cell death-ligand 1 |
| PGE2 | = | prostaglandin E2 |
| Pmmr | = | proficient mismatch repair |
| SBRT | = | stereotactic body radiotherapy |
| TAA | = | tumor-associated antigen |
| TAM | = | tumor-associated macrophage |
| TCR | = | T cell receptor |
| TGF-β | = | transforming growth factor-beta |
| TME | = | tumor microenvironment |
| TIGIT | = | T cell immunoreceptor with Ig and ITIM domains |
| Tim-3 | = | T cell immunoglobulin and mucin domain containing molecule-3 |
| VEGF | = | vascular endothelial growth factor |
| Wnt | = | “Wingless/Integrated” |
Author’s contributions
LT, TW and CP wrote this paper; MS prepared figure and tables of this review; CP conceived this topic, designed the logic flow of this review.
Conflicts of interest
The authors claim no conflicts of interest.
Additional information
Funding
References
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–9. doi:10.1016/S0140-6736(17)33326-3.
- Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, et al. Durable clinical benefit with Nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi:10.1200/JCO.2017.76.9901.
- Emambux S, Tachon G, Junca A, Tougeron D. Results and challenges of immune checkpoint inhibitors in colorectal cancer. Expert Opin Biol Ther. 2018;18:561–573. doi:10.1080/14712598.2018.1445222.
- Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi:10.1016/j.cell.2018.09.035.
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi:10.1093/annonc/mdw235.
- Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D, ESMO Guidelines Committee. Rectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40. doi:10.1093/annonc/mdx224.
- Fukumura D, Klopper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiantiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi:10.1038/nrclinonc.2018.29.
- Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2018;16:123–135. doi:10.1038/s41571-018-0119-7.
- Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6:46. doi:10.1186/s40425-018-0361-7.
- Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al. Cancer classification using the immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi:10.1186/1479-5876-10-205.
- Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi:10.1038/nm.3967.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi:10.1038/nature13480.
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi:10.1038/nature11404.
- Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson D, et al. Molecular subtypes of small cell lung cancers: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–297. doi:10.1038/s41568-019-0133-9.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi:10.1126/science.aar4060.
- van der Merwe PA, Davis SJ. Molecular interaction mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi:10.1146/annurev.immunol.21.120601.141036.
- Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi:10.1126/science.282.5397.2263.
- McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi:10.1146/annurev-immunol-041015-055318.
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immune suppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi:10.1038/s41586-018-0392-8.
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi:10.1038/nature22079.
- Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, Kang B, Liu Z, Jin L, Xing R, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24(7):978–985. doi:10.1038/s41591-018-0045-3.
- Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. 2019;25(8):1251–1259. doi:10.1038/s41591-019-0522-3.
- Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer ALN, de Langen AJ, et al. Effect of Pembrolizumab After stereotactic Body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. Eput 2019 Jul 11;5(9):1276. doi:10.1001/jamaoncol.2019.1478
- Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi:10.1146/annurev-immunol-032713-120142.
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi:10.1126/science.aaa6204.
- Garrido-Ramos MA. Satellite DNA: an evolving topic. Genes. 2017;8:230. doi:10.3390/genes8090230.
- Boland PM, Yurgelun MB, Boland CR. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin. 2018;68:217–231. doi:10.3322/caac.21448.
- Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92. doi:10.1038/nrc.2017.24.
- Grasso CS, Giannakis M, Wells DK, Hamada T, Mu XJ, Quist M, Nowak JA, Nishihara R, Qian ZR, Inamura K, et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018;8:730–749. doi:10.1158/2159-8290.CD-17-1327.
- Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29:601–603. doi:10.1200/JCO.2010.32.9078.
- O’Neil BH, Funkhouser WK, Calvo BF, Meyers MO, Kim HJ, Goldberg RM, Bernard SA, Caskey L, Deal AM, Wright F, et al. Nuclear factor κ-light chain-enhancer of activated B cells is activated by radiotherapy and is prognostic for overall survival in patients with rectal cancer treated with preoperative fluorouracil-based chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;80(3):705–711. doi:10.1016/j.ijrobp.2010.02.063.
- Leonard W, Dufait I, Schwarze JK, Law K, Engels B, Jiang H, Van den Berge D, Gevaert T, Storme G, Verovski V, et al. Myeloid-derived suppressor cells reveal radioprotective properties through arginase-induced I-arginine depletion. Radiother Oncol. 2016;119:291–299. doi:10.1016/j.radonc.2016.01.014.
- Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Kawamoto A, Yasuda H, Inoue Y, Miki C, Kusunoki M, et al. Stromal CXCR4 and CXCL12 expression is associated with distant recurrence and poor prognosis in rectal cancer after chemoradiotherapy. Ann Surg Oncol. 2010;17:2051–2058. doi:10.1245/s10434-010-0970-y.
- D’Alterio C, Avallone A, Tatangelo F, Delrio P, Pecori B, Cella L, Pelella A, D’Armiento FP, Carlomagno C, Bianco F, et al. A prognostic model comprising pT stage, N status, and the chemokine receptors CXCR4 and CXCR7 powerfully predicts outcome in neoadjuvant resistant rectal cancer patients. Int J Cancer. 2014;135:379–390. doi:10.1002/ijc.28689.
- Samarendra H, Jones K, Petrinic T, Silva MA, Reddy S, Soonawalla Z, Gordon-Weeks A. A meta-analysis of CXCL12 expression for cancer prognosis. Br J Cancer. 2017;117:124–135. doi:10.1038/bjc.2017.134.
- Heylmann D, Rödel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846:121–129. doi:10.1016/j.bbcan.2014.04.009.
- Kim TD, Li G, Song KS, Kim JM, Kim JS, Yun EJ, Park JI, Park HD, Hwang BD, Lim K, et al. Radiation-induced thymidine phosphorylase upregulation in rectal cancer is mediated by tumor-associated macrophages by monocyte chemoattactant protein-1 from cancer cells. Int J Radiat Oncol Biol Phys. 2009;73:853–860. doi:10.1016/j.ijrobp.2008.07.068.
- Itatani Y, Kawada K, Inamoto S, Yamamoto T, Ogawa R, Taketo MM, Sakai Y. The role of chemokines in promoting colorectal cancer invasion/metastasis. Int J Mol Sci. 2016;17:pii: E643. doi:10.3390/ijms17050643.
- Barker HE, Paget JT, Khan AA, Harrington KJ. The tumor microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi:10.1038/nrc3958.
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi:10.1146/annurev-immunol-032712-100008.
- Schollbach J, Kircher S, Wiegering A, Seyfried F, Klein I, Rosenwald A, Germer C-T, Löb S. Prognostic value of tumour-infiltrating CD8+ lymphocytes in rectal cancer after neoadjuvant chemoradiation: is indoleamine-2,3-dioxygenase (IDO1) a friend or foe? Cancer Immunol Immunother. 2019;68:563–575. doi:10.1007/s00262-019-02306-y.
- Matsutani S, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Hirakawa K, Ohira M. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109:966–979. doi:10.1111/cas.13542.
- Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M, Yamamoto J, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiaotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414–21. doi:10.1245/s10434-014-3584-y.
- Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6:49. doi:10.1186/1748-717X-6-49.
- Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, Zhang J, Yu J. TILs, FOXP3, PD-L1 and CTLA-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–732.e1. doi:10.1016/j.trsl.2015.06.019.
- Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi:10.1158/1078-0432.CCR-13-2830.
- Carducci MA, Giblin J, Dottellis D. 2019 ASCO annual meeting proceedings. J Clin Oncol. 2019;37:192s.
- Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicenter, phase 2 trial. Lancet Oncol. 2015;16:957–966. doi:10.1016/S1470-2045(15)00004-2.
- Chiang SF, Huang CY, Ke TW, Chen TW, Lan YC, You YS, Chen WT, Chao KSC. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers imporved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol Immunother. 2019;68:283–296. doi:10.1007/s00262-018-2275-0.
- Ogura A, Akiyoshi T, Yamamoto N, Kawachi H, Ishikawa Y, Mori S, Oba K, Nagino M, Fukunaga Y, Ueno M, et al. Pattern of programmed death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur J Cancer. 2018;91:11–20. doi:10.1016/j.ejca.2017.12.005.
- Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi:10.1016/j.ccr.2005.07.014.
- Song CW, Kim MS, Cho LC, Dusenbery K, Sperduto PW. Radiobiological basis of SBRT and SRS. Int J Clin Oncol. 2014;19:570–578. doi:10.1007/s10147-014-0717-z.
- Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 2016;6:141. doi:10.3389/fonc.2016.00141.
- Baumann M, Krause M, Hill R. Exploring the roles of cancer stem cells in radioresistance. Nat Rev Cancer. 2018;8:545–554. doi:10.1038/nrc2419.
- Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24:41–53. doi:10.1016/j.stem.2018.12.009.
- Popp I, Grosu AL, Niedermann G, Duda DG. Immune modulation by hypofractionated stereotactic radiation therapy: therapeutic implications. Radiother Oncol. 2016;120:185–194. doi:10.1016/j.radonc.2016.07.013.
- Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front Oncol. 2014;4:1. doi:10.3389/fonc.2014.00001.
- Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, Mourtada F, Sims-Mourtada J. The impact of radiation on the tumor microenviroment: effect of dose and fractionation schedules. Cancer Growth Metastasis. 2018;11:1179064418761639. doi:10.1177/1179064418761639.
- Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, Al-Hallaq HA, Arina A, Khodarev NN, Janisch L, et al. Safety and clinical activity of Pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611–1618. doi:10.1200/JCO.2017.76.2229.
- Li J, Yuan Y, Yang F, Wang Y, Zhu X, Wang Z, Zheng S, Wan D, He J, Wang J, et al. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12(1):16. doi:10.1186/s13045-019-0702-0.
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi:10.1038/onc.2009.356.
- Duffy AG, Greten TF. Immunological off-target effects of standard treatments in gastrointestinal cancer. Ann Oncol. 2014;25:24–32. doi:10.1093/annonc/mdt349.
- Kalanxhi E, Meltzer S, Schou JV, Larsen FO, Dueland S, Flatmark K, Jensen BV, Hole KH, Seierstad T, Redalen KR, et al. Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favors survival without metastatic progression in high-risk rectal cancer. Br J Cancer. 2018;118(10):1322–1328. doi:10.1038/s41416-018-0085-y.
- Song W, Shen L, Wang Y, Liu Q, Goodwin TJ, Li J, Dorosheva O, Liu T, Liu R, Huang L, et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat Commun. 2018;9(1):2237. doi:10.1038/s41467-018-04605-x.
- Melichar B, Touskova M, Vesely P. Effect of irinotecan on the phenotype of peripheral blood leukocyte populations in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002;49:967–970.
- Maeda K, Hazama S, Tokuno K, Kan S, Meada Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011;31:4569–4574.
- Fumet JD, Isambert N, Hervieu A, Zanetta S, Guion JF, Hennequin A, Rederstorff E, Bertaut A, Ghiringhelli F. Phase Ib/II trail evaluating the safety, tolerability and immunological activity of durvalumab (MEDI4736) (anti-PD-L1) plus Tremelimumab (anti-CTLA-4) combined with FOLFOX in patients with metastatic colorectal cancer. ESMO Open. 2018;3:e000375. doi:10.1136/esmoopen-2018-000375.
- Serna-Blasco R, Sanz-Álvarez M, Aguilera Ó, García-Foncillas J. Targeting the RAS-dependent chemoresistance: the Warburg connection. Semin Cancer Biol. 2019;54:80–90. doi:10.1016/j.semcancer.2018.01.016.
- Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicenter, open-label, phase 3, randomized, controlled trial. Lancet Oncol. 2019;20:849–861. doi:10.1016/S1470-2045(19)30027-0.
- Botrel TEA, Clark LGO, Paladini L, Clark OAC. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2016;16:677. doi:10.1186/s12885-016-2734-y.
- D’Alterio C, Nasti G, Polimeno M, Ottaiano A, Conson M, Circelli L, Botti G, Scognamiglio G, Santagata S, De Divitiis C, et al. CXCR4-CXCL12-CXCR7 TLR2-TLR4, and PD-1/PD-L1 in colorectal cancer liver metastases from neoadjuvant-treated patients. Oncoimmunology. 2016;5:e1254313. doi:10.1080/2162402X.2016.1254313.
- Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, Boidot R, Végran F, Bonnefoy N, Vincent J, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-Bevacizumab drug treatment regimen. Cancer Res. 2016;76:5241–5252. doi:10.1158/0008-5472.CAN-15-3164.
- Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk I, et al. An HIF-1α/VEGF-A axis in cytotoxic t cells regulates tumor progression. Cancer Cell. 2017;32(5):669–683.e5. doi:10.1016/j.ccell.2017.10.003.
- Van den Eynde M, Mlecnik B, Bindea G, Fredriksen T, Church SE, Lafontaine L, Haicheur N, Marliot F, Angelova M, Vasaturo A, et al. The Link between the multiverse of immune microenviroments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34:1012–1026.e3. doi:10.1016/j.ccell.2018.11.003.
- Luo W, He WT, Wen Q, Chen S, Wu J, Chen XP, Ma L. Changes of TCR repertoire diversity in colorectal cancer after erbitux (cetuximab) in combination with chemotherapy. Am J Cancer Res. 2014;4:924–933.
- Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, Bestoso E, Apollinari S, Mannucci S, Marra M, et al. Cetuximab ± chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2012;130(7):1577–1589. doi:10.1002/ijc.26181.
- Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. 2017;153:980–987. doi:10.1053/j.gastro.2017.06.009.
- Seo H, Jeon I, Kim BS, Park M, Bae EA, Song B, Koh CH, Shin KS, Kim IK, Choi K, et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun. 2017;8:15776. doi:10.1038/ncomms15776.
- Steele N, Anthony A, Saunders M, Esmarck B, Ehrnrooth E, Kristjansen PE, Nihlén A, Hansen LT, Cassidy J. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. Br J Cancer. 2012;106:793–798. doi:10.1038/bjc.2011.599.
- Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, Mineno J, Yoshida N, Katada K, Kamada K, et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patient with gastric or colorectal cancer. Int J Cancer. 2018;142:2599–2609. doi:10.1002/ijc.31285.
- Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124(6):2668–2682. doi:10.1172/JCI73014.
- Russo M, Crisafulli G, Sogari A, Reilly NM, Arena S, Lamba S, Bartolini A, Amodio V, Magrì A, Novara L, et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science. 2019;366(6472):1473–1480. doi:10.1126/science.aav4474.
