ABSTRACT
Immunotherapy by chimeric antigen receptor (CAR)-modified T-cells has shown unprecedented clinical efficacy for hematological malignancies. Recently two CAR T-cell based therapeutics, Kymriah (Tisagenlecleucel) and Yescarta (Axicabtagene ciloleucel) were approved by the US Food and Drug Administration and by the European Medicines Agency. Despite the progress in treating hematological malignancies, challenges remain for the use of CAR T-cell therapy in patients with solid tumors. Barriers yet to overcome for achieving effective CAR T-cell therapy include antigenic heterogeneity of solid tumors, an immune‐suppressive microenvironment, and organ-specific properties that limit T-cell entry. This review will summarize available clinical data for CAR T-cell therapy in solid tumors, including present obstacles and promising strategies to advancement.
Introduction
The genetic engineering of T lymphocytes with chimeric antigen receptors (CARs) has rapidly advanced from preclinical tumor models to Food and Drug Administration (FDA) and European Medicines Agency approval (EMA) for hematologic malignancies, and clinical-grade production. To date, however, solid tumors are less susceptible to CAR therapies and instead have been treated more successfully with immune checkpoint inhibitors (ICIs)Citation1 or tumor-infiltrating lymphocyte (TIL) therapy.Citation2 The interactions between antigen-presenting cells and T-cells allow high precision host protection against pathogens and cancer cells. T-cells have unparalleled ability to not only recognize these antigens (Ag) but also to establish long-term memory, allowing rapid and robust response upon rechallenge against a given Ag. Tumors express Ags that are recognized by T-cells, whereby mutations of self-Ags or germline cancer Ags differ sufficiently from normal Ags, or those that are less easily detected, such as overexpressed self-Ags or differentiation Ags, expressed by the tumor-originating tissue.Citation3 Hence, tumors that are more similar to normal cells, and particularly those with highly immune-suppressive features, escape surveillance (i.e.via immune editing), which results in their uncontrolled growth. Technological advances have created opportunities to enhance the effector functions of T-cells against cancer through reeducation and intelligent design to overcome the immune evasion mechanisms established by solid tumors. Adoptive cell therapy (ACT) consists in ex vivo enrichment of autologous tumor-specific cells and expansion to large numbers, and subsequent reinfusion into the patient to specifically target and kill cancer cells. ACT is conducted via two methods: (1) naturally arising TILs can be directly expanded ex vivo from a tumor lesionCitation2 or (2) non-therapeutic host lymphocytes obtained from the peripheral blood can be artificially rendered tumor specific via genetic engineering with a T-cell receptor (TCR)Citation4 or a chimeric Ag receptor (CAR).Citation5 The CAR is a hybrid antigen receptor, part antibody and part TCR, and is composed of an extracellular Ag-binding domain and intracellular signaling domain(s).Citation5 Genetic modification of a T-cell with a CAR provides a new Ag-specificity through the single-chain variable fragment (scFv), which is derived from a tumor-specific antibody.Citation5 The scFv allows the T cell to bind a tumor Ag and the T-cell activation cascade is initiated through the intracellular domains, derived from CD3ζ ITAM domains.Citation6 To complete the genetic construct for the CAR, a hinge and a transmembrane domain (TM), commonly from CD8α or immunoglobulin, bridges the extracellular scFv and intracellular CD3ζ ITAM domains. Its first use by Kuwana et al. and Gross et al. in the late 1980s revealed that redirection of a T-cell with this receptor could induce Ag recognition through the scFv, as for a native Ig, without classical major histocompatibility complex (MHC) restriction required by a TCR recognizing Ag-derived peptide.Citation7,Citation8 These first-generation CAR T-cells had very limited persistence and antitumor efficacy in vivo.Citation9,Citation10 The modular nature of the CAR technology allows constant optimization, which is how first-generation CARs, containing only the CD3ζ portion of the TCR were replaced with second-generation CARs containing an added costimulatory element such as CD28 or 4–1BB. The specificity of a TCR is for only a short peptide (8–12 amino acids), so there is potential for cross-reactivity to similar sequences of amino acids.Citation11 TCR ligation of self Ag can lead to T-cell activation, autoimmunity, and even death. To minimize this risk, T-cells require at least two signals to fully activate.Citation12 Second-generation CARs contain the two-signal model of T-cell activation including a CD28 costimulatory domain in tandem with CD3ζ ITAM domain. This supports in vitro T-cell activation and killing, but more importantly efficient tumor killing and long-term T-cell persistencein vivo.Citation13 In addition, costimulatory domains other than CD28, such as CD27, 4–1BB, and OX40, provide similar improvement to CAR T-cell function and persistence in vivo.Citation14,Citation15
CAR-redirected T-cell therapies have been successful in hematologic malignancies but are less effective in treating the majority of patients with solid tumors to date. This review will summarize available data from completed clinical trials of CAR T-cells in solid tumors and discuss present obstacles and promising strategies to advancement.
Overcoming tumor heterogeneity: which target? At what price?
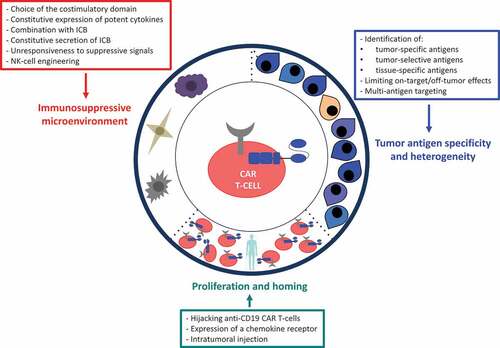
CAR T-cell-based therapy is an innovative anticancer approach based on the specific recognition of a tumor Ag by the patient’s own engineered T-cells. However, attempts to recapitulate the success achieved with CAR T-cells in B-cell malignancies for solid tumors have been disappointing. summarizes the clinical trials of CAR T-cells that have been completed to date in patients with solid tumors and reports the clinical outcome and the toxicity profile.Citation16–Citation33 The three main hurdles encountered for the application of CAR T-cell therapies to solid tumors are (1) the presence of tumor-associated Ags, which are generally cell-surface molecules not present on normal tissue, (2) the limited trafficking of adoptively transferred cells to tumor sites and (3) the immunosuppressive effect of tumor microenvironment ().
Table 1. Completed clinical trials of CAR T-cells in patients with solid tumors: target, outcome, and relevant toxicities.
Tumor-specific Ags, like the epidermal growth factor receptor variant IIICitation29 (EGFRvIII), are among the ideal targets in the sense that they are uniquely present on cancer cells. Therefore the CAR T-cell with engineered specificity toward EGFRvIII will attack only tumor cells, and normal tissue cells should theoretically be ignored. Additional attractive targets of tumor cells are represented by proteins resulting from unique post-transcriptional modifications such as alterations of the glycosylation patterns of MUC1, MUC16, TAG72 or B7-H3.Citation34–Citation36 Targeting tumor-selective Ag is an additional strategy: the Ag has to be expressed by tumor cells but at a much higher level than healthy cells, such as Human Epidermal growth factor Receptor 2 (HER2).Citation16 Consequently the effect on healthy cells should be negligible compared to that of tumor cells. A third class of Ag, represented by the Prostate Stem Cell Ag (PSCA),Citation37 is a tissue-specific Ag with very low expression in other tissues like pancreas or bladder. In this case, it is hoped that the side effects on healthy tissues will be minimal, as the elimination of PSCA-positive cells would not cause vital organ failure. Theoretically, even if the perfect Ag for a solid tumor could be identified and targeted, CAR T-cell therapies for solid tumors face further obstacles including poor trafficking to the tumor site,Citation38 as well as limited proliferation and persistence within the host.Citation39 Moreover, CAR T-cells can be functionally suppressed within the hostile tumor microenvironment.Citation40 These collective hurdles set solid tumor CAR-based therapies apart from liquid tumors.
Tumor heterogeneity is often major and makes it a crucial problem for CAR- T-cells.Citation41,Citation42 A difficulty with the principle of CAR T-cells lies in the fact that cytotoxicity is based on a single Ag, even an improved one. Indeed, the tumor tissue, whatever the primitive, is known to evolve over time but also in its different metastatic locations. One solution is to attack several tumor Ags concomitantly, as this should allow maintenance of cytotoxic activity despite loss of one of the target Ags. Several strategiesCitation43 are possible for targeting two Ags: the co-administration of two CAR T-cells each targeting a different Ag, the use of a bicistronic vector that leads to the expression of two distinct CARs on the same T-cell, the co-transduction of two vectors each encoding for one CAR, or the expression of a bispecific Tandem CAR.Citation44 Tandem CARs are constructed with two Ag specificities built in series in order to recognize two different tumor targets or to stimulate cytotoxicity with the second CAR recognizing a ubiquitous viral Ag such as CMV. Efficacy for bispecific CD19/CD22 CAR T-cells is under clinical evaluation for relapsed/refractory B-cell acute lymphoblastic leukemia.Citation45 In addition to increasing the specificity of the CAR to the tumor, this technique potentially minimize the “on-target/off-tumor” toxicity toward healthy cells with low-level single Ag expression.Citation46
Tumor heterogeneity over time also includes loss or down-regulation of expression of the Ag of interest, leading to “Ag-negative” relapse, while tumor heterogeneity in space leads to the risk of dissociated response between different metastases. Targeting Ag expressed by the cells of the tumor microenvironment, such as the fibroblast activation protein (FAP), particularly expressed on cancer associated fibroblasts (CAFs), seems an attractive option.Citation47,Citation48 Tran and colleaguesCitation49 showed in a mouse model that, despite anti-FAP CARs displayed specific degranulation and production of effector cytokines in response to Ag stimulation in vitro, they did not mediate an efficient antitumor response in vivo, and unexpectedly, anti-FAP CARs caused severe cachexia and lethal bone toxicities. The FAP protein is also expressed by multipotent bone marrow stromal cells (BMSCs), hence the observed toxicity is linked to their expression. Interestingly, Kakarla and colleagues,Citation48 using an anti-FAP CAR with a different scFv, demonstrated antitumor efficacy without toxicities in a mouse model of lung cancer. The safety concerns generated by the work of Rosenberg et al.Citation49 are likely related to the specificity and affinity of the scFv, given that the last two studies with CAR T-cells with different scFvs recognizing highly positive FAP cells have a good toxicity profile. Given the potential for multi-modal antitumor effects of FAP targeting, rational combinations for future immunotherapeutic approaches should include stroma-targeting CAR T-cells with either antitumor CAR T-cells or ICIs.
As a living therapy, CAR T-cells bear the potential for rapid and massive activation , which contributes to their therapeutic efficacy but simultaneously underlies their side effects. The most well-documented toxicity is called cytokine release syndrome (CRS), a systemic inflammatory response characterized by fever, hypotension and hypoxia. CRS is triggered by the activation of CAR T-cells and their subsequent production of pro-inflammatory cytokines including IFNγ, IL-6, and IL-2.Citation50 This is thought to result in additional activation of bystander immune and nonimmune cells (i.e. macrophages, endothelial and stromal cells) which further produce cytokines, including IL-10, IL-6, and IL-1beta and inflammatory mediators (i.e. ferritin).Citation51 The severity of CRS ranges from a mild fever to life-threatening, multi-organ failure.Citation52 Neurologic toxicity is another serious adverse event, which can occur alongside CRS.Citation53 Tocilizumab, a monoclonal IgG1 directed against the IL-6 receptor, is the current standard treatment for CRS.Citation54
It is important to highlight that lack of tumor Ag specificity increases the potential risk of significant on-target/off-tumor toxicity. This was the case for a patient with metastatic colon cancer, who received an infusion of HER2 (ERBB2)-targeting CAR T-cells and died 5 days later.Citation55 The cause of death was attributed to CAR T cytotoxicity against the pneumocytes, which express low levels of HER2. Another example of on-target, off-tumor toxicity has been described in a preclinical model with a high affinity anti-GD2 CAR for neuroblastoma, in which low levels of GD2 in the brain resulted in fatal encephalitis.Citation56 Thistlethwaite et al. also described a patient who developed acute respiratory distress due to the on-target/off-tumor effect of CEACAM5‑specific CAR T-cells exerting cytotoxicity against pneumocytes and lung-associated macrophages, and the trial was closed due to this severe and unexpected toxicity.Citation31 These fatal events underscore the importance of choosing a safe tumor-associated Ag, as even low level expression of the target Ag on normal tissues can result in severe toxicity. These acute responses also highlight that the binding affinity of a CAR is tightly linked to both safety and efficacy, and that higher affinity is not necessarily better. As an example, an in vivo study found that CAR T-cells targeting ICAM-1, a marker associated with many solid tumors including thyroid cancer (but also expressed on normal tissues as an adhesion molecule), was safer and more effective, when the CAR specificity for the Ag had only micromolar affinity.Citation57,Citation58
In order to specifically control CAR T-cell activity toward the Ag, several models of adapter-mediated CARs, also known as universal CARs (UniCAR), have been developed.Citation59–Citation61 A shared feature is their method of tumor recognition, which is achieved by linking an adaptor, a molecule recognized by the CAR, to an antibody or ligand that specifically recognizes the tumor Ag. While current clinically approved CARs are designed to be constitutively active, adapter-mediated CAR T-cells have the distinct advantage to only recognize and kill the Ag-expressing target cell when the adapter is administered, allowing for titratable and reversible control of the CAR T-cells. As an example, the UniCAR02-T associated with the CD123 Target Module is currently in phase I in patients with hematologic malignancies expressing CD123 (NCT04230265).Citation62
Improving expansion and homing
Trafficking to the tumor does not seem to be a major issue for hematologic tumors but is likely to be a challenge for CAR T-cells targeting solid tumors. The majority of solid tumors present with a fibrotic stromaCitation63 and may be more difficult for engineered T-cells to infiltrate (). Contrary to B-cell malignancies, CAR T-cells targeting solid tumors do not rapidly encounter their target once infused. This necessary time to migrate into the tumor certainly hinders the efficacy of CAR T-cells for solid tumors by limiting their proliferation and persistence. The high objective response rate observed with anti-CD19 CAR T-cells in refractory large B-cell lymphoma was found to be associated with CAR T-cell expansion following infusion.Citation64 Thus normal CD19 + B-cells act as an immediate and self-renewing source of Ag. A new immuno-oncology company proposed to tweak anti-CD19 CAR T-cells, thus making them able to recognize multiple different targets via the expression of fusion proteins while retaining their proliferation and persistence properties.Citation65 The fusion protein contains a CD19 extracellular domain and an anti-tumor antigen binding domain, thus it creates a bridge, which helps redirecting anti-CD19 CAR T-cells cytotoxicity against multiple tumor-associated Ags. This strategy seems attractive for the treatment of solid tumors by CAR-engineered T-cells.
Some studies have shown that modifying CAR T-cells to express a chemokine receptor (CCR2,Citation66 CCR4,Citation67 CXCR2Citation68,Citation69) matching to the chemokine secretion by the target tumor cells leads to improved T-cell homing into the tumor and enhanced antitumor efficacy in vivo. The enforced expression of a chemokine receptor such as CXCR1 or CXCR2 also augments intratumoral CAR T-cells persistence and tumor regression in xenograft mouse models of glioblastoma, ovarian, and pancreatic cancer.Citation70
Another way to solve this migration issue could be to inject CAR T-cells directly into the tumor. Several preclinical studies showed higher CAR T-cell activation, efficacy and persistence when a regional delivery is performed as compared to intravenous injection.Citation71–Citation73 This is particularly relevant for tumors localized within difficult-to-access niches, such as the central nervous system (CNS). Mulazzania et al.Citation74 used in vivo intracranial 2-photon microscopy to demonstrate that intracerebral injection of anti-CD19 CAR T-cells resulted in a deeper infiltration and an enhanced control of the tumor growth, than intravenous infusion in an orthotopic murine model of primary CNS lymphoma. Interestingly, 28 days after intracerebral injection, CAR T-cells were detected in distant non-draining lymph nodes. Anti-CD19 CAR T-cells persisted in the brain and the bloodstream for up to 159 days, even after complete regression of the CNS lymphoma.
Both intracranialCitation20 and intravenous routes are currently being tested in brain tumor clinical trials, but intracranial injection is a more risky procedure compared with intravenous infusion. Moreover, a recent publication reported on 8 patients with secondary CNS lymphoma treated with commercial tisagenlecleucel (anti-CD19 CAR T-cells containing a 4–1BB costimulatory domain) at a single institution.Citation75 CAR T-cells were administered as a single intravenous injection and the overall response rate was 50%. There was no increased rate of CRS or neurotoxicity. This retrospective analysis suggests that CAR T-cells can efficiently traffic to the CNS after intravenous injection, but larger studies are needed to clarify the optimal route of delivery. One remaining question could be whether a dose reduction of CAR T-cells is appropriate when injected directly into the tumor region.
Intratumoral injection of CAR T-cells has been tested in 6 patients presenting with a metastatic (accessible cutaneous or lymph node metastases) breast cancer.Citation30 The investigators used the previously published mRNA-transfected c-Met CAR T-cells,Citation18 whose transient expression of the c-Met CAR limits its possible on-target/off-tumor effect. The downside of this transient expression system is the rather rapid loss of the transgene, especially in proliferating cells, as the CAR-encoding RNA is not replicated during cell division. Despite an inflammatory response noted within the tumor, no objective clinical response was reported.
In conclusion, various approaches have been tested in preclinical models in order to enhance expansion, homing, and persistence of CAR T-cells in solid tumors. Some strategies have been evaluated in clinical studies, but more trials are needed to better assess their efficacy.
Overcoming the immunosuppressive microenvironment
Several solid tumors produce an immunosuppressive environment impairing the efficacy of ACT.Citation76 Multiple improvements of CAR T-cells have been proposed to allow their proliferation, persistence and cytotoxicity within an immunosuppressive environment. Regarding second-generation CAR T-cells, the choice of the costimulatory domain is certainly a key point and still a matter of debate. It has been shown, however, that UniCAR T-cells redirected to PSCA and harboring CD28 costimulation resist regulatory T cell (Treg) suppression, both in vitro and in vivo, via the secretion of Th1-related proinflammatory cytokines, in contrast to 4–1BB-based CARs, which are efficiently suppressed by Tregs.Citation77 Of note, 4–1BB costimulation is associated with an increased central memory differentiation and a prolonged persistence of the CAR T-cells.Citation78 Ideally, clinical trials should randomize CAR T-cells directed against the same target but bearing different costimulatory domains, and the optimal approach may be a defined ratio of CAR T-cells with different costimulatory domains.
One potential option for shaping the tumor microenvironment to enhance ACT efficacy is to induce the local release of stimulatory factors that promote antitumor immune responses.
The last generation of “armored” CAR T-cells, so called TRUCKs for T-cells redirected for universal cytokine killing, is particularly promising for the treatment of solid tumors associated with a suppressive microenvironment. These CAR T-cells are genetically modified to constitutively express potent cytokines. In this context IL-12 and IL-18 represent promising candidates to favorably remodel the tumor environment. In particular, IL-12 is a pro-inflammatory cytokine, able to improve T-cell activation and induce a Th1 CD4 + T-cell response, CD8+ clonal expansion, and effector function. It is also able to recruit NK-cells to the tumor site, reactivate anergic TILs, inhibit Tregs and the secretion of IL-10, IL-4 and transforming growth factor beta (TGFβ) by tumor-associated macrophages. IL-12 TRUCKs have shown efficacy in preclinical models of hematologicCitation79,Citation80 and solid tumors.Citation81–Citation83
Chmielewski and colleagues performed a cytokine screen, which identified IL-18 as inducing a T-Bethigh FoxO1low signature in CAR T-cells.Citation84 The authors engineered an IL-18 TRUCK, which improves the survival of immune-competent mice with advanced pancreatic cancer when compared to CAR T-cells without cytokine secretion. In addition, IL-18 CAR T-cell therapy induces a favorable remodeling of the tumor microenvironment. This model is of particular interest for pancreatic ductal adenocarcinoma, as it is one the most lethal human cancers, and its resistance to immune checkpoint inhibitors could be due to a predominance of immunosuppressive cells in the microenvironment.Citation85
Combining CAR T-cells with ICIs (such as programmed cell death protein 1 (PD-1) or its ligand PD-L1) is another obvious way to modify the tumor microenvironment. Preclinical data demonstrated that the administration of an anti-PD-1 antibody enhances the antitumor activity of CAR T-cells against HER2+ sarcoma and breast cancer cell lines.Citation86 The anti-PD-1 therapy acts on TILs as well as on the CAR T-cells themselves, whose PD-1 expression is often upregulated following Ag stimulation. In order to limit the toxicity related to systemic delivery of ICIs and to increase tumor concentration, Rafiq et al. proposed to “armor” CAR T-cells to secrete a PD-1 blocking scFv only in the local tumor site.Citation87 Interestingly, in a xenograft model of metastatic ovarian cancer, mice treated with this innovative strategy had improved survival compared with mice receiving the anti-MUC16ecto CAR T-cells plus an anti-PD-1 antibody.Citation87 This approach is promising to make CAR T-cells efficient in tumors with an immunosuppressive microenvironment.
Regarding PD-L1, CAR T-cells targeting carbonic anhydrase IX (CAIX) and engineered to secrete anti-PD-L1 antibodies have shown better control of the tumor growth than anti-CAIX CAR T-cells alone in a humanized mouse model of clear cell renal cell carcinoma.Citation88
To date, the clinical benefit of the combination of CAR T-cells with ICIs is not proven. In a phase 1 clinical trial, the administration of a PD-1 inhibitor together with anti-GD2 CAR T-cells did not improve antitumor responses of patients with neuroblastoma, although the number of treated patients was small.Citation89
Other research teams in the field have engineered CAR T-cells that are unresponsive to suppressive signals. For instance, genome editing has been used to remove the PD-1 receptor from CAR T-cells, making them inert to the PD-1/PD-L1 inhibitory pathway.Citation90–Citation92 One can also express a dominant-negative form of some receptors (like TGFβCitation93 or PD-1Citation94,Citation95) rendering CAR T-cells unresponsive to inhibitory signals. Nonetheless, such strategies raise the risk of uncontrolled CAR T-cell activation, as the suppressive pathways are essential to modulate T-cell effector functions. Special attention should be paid to the toxicity profile of such approaches, which should be monitored cautiously.
Very recently, Porter et al. published the combination of an oncolytic virus armed with a bispecific tumor-targeted T-cell engager (BiTE) molecule specific for CD44v6 plus IL-12 plus an anti-PD-L1 antibody (so called CAdTrio) with anti-HER2 CAR T-cells.Citation96 They showed that the association of both the CAdTrio and the CAR T-cells leads to a more sustained control of an orthotopic head and neck squamous cell carcinoma model than any component alone. Albeit a bit futuristic, this strategy that employs both intratumoral and intravenous routes, may be able to counteract both Ag heterogeneous expression and immune suppression by the solid tumor microenvironment.
NK-cells belong to the innate immune system and mediate cytotoxic functions against cancer cells through a complex network of activating and inhibitory receptors.Citation97 Interestingly, the density, phenotype and functions of tumor-infiltrating NK-cells have been associated with a favorable outcome in various solid tumorsCitation98-Citation101 but the microenvironment can impair their natural properties.Citation102 NK-cell based immunotherapy encompass multiple promising approaches, including CAR engineering,Citation103 which is under preclinicalCitation104-Citation106 and clinicalCitation102,Citation107 development for treating solid tumors. NK-cells can also be engineered to overcome the suppressive effect of the tumor microenvironment on their function. Interestingly, Parihar et al. produced modified NK-cells with a chimeric NKG2D receptor comprising the extracellular domain of the native NKG2D fused to the intracellular ζ-chain of the TCR (NKG2D.ζ), instead of the physiological DAP10 that is commonly downregulated by suppressive factors secreted by the microenvironment, such as TGFβ.Citation108 They showed that NKG2D.ζ NK-cells, but not unmodified NK-cells, killed NKG2D ligand-expressing myeloid-derived suppressor cells (MDSCs) in a xenograft model of MDSCs-containing neuroblastoma and enhanced infiltration and antitumor activity of co-injected anti-GD2 CAR T-cells.Citation108 Finally we are experiencing a new and exciting era almost resembling a science fiction movie, where the engineering of CAR T-cells seems to have no limit to overcome the evasion mechanisms of solid tumors. It is now time to assess if all these preclinical data will translate into clinical benefit for patients with aggressive solid tumors.
Conclusions
In the past few years, CAR T-cells made a huge breakthrough in the treatment of B-cell malignancies. Second generation CAR T-cells encompass one costimulatory domain (commonly CD28 or 4–1BB) and are now commercialized for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma. This proof of concept generated great interest for the development of CAR T-cells directed against solid tumors. Unfortunately, clinical trials evaluating second generation CAR T-cells in solid tumors have shown disappointing results. While a few complete responses have been observed, the duration of response is still limited. This anecdotal success is due to several hurdles encountered with solid tumors, including the heterogeneous and nonspecific expression of tumor-associated Ag, the homing capacity, and the immunosuppressive tumor microenvironment. summarizes the solutions that have been proposed to face these challenges. Because no single CAR T-cell modality will likely defeat all evasion mechanisms of solid tumors, including plasticity of tumor Ag expression and active immune suppression by the tumor environment, “armored” CAR T-cells strategy (TRUCKs) is likely to increase the breadth, potency and duration of antitumor activity of second generation CAR T-cells. This last generation of CAR T-cells has demonstrated promising results in preclinical studies.
In addition, as CAR-related toxicities often arise acutely, control mechanisms should ideally allow fast control over CAR T-cell activity. Permanent elimination of CAR T-cells could abrogate their long-term antitumor effect, and many methods therefore aim at reversible, ligand-enabled control, allowing to swiftly turn off the CAR T-cells when toxicities occur, such as with the design of adapter CAR T-cells. In addition, the use of boolean logic gates and tumor selectivity strategies is under intense investigation to generate autonomous CARs with a higher target specificity and tissue selectivity, capable of better distinguishing tumor from healthy cells.Citation109,Citation110 In the future, the choice of CAR T-cell should also be tailored to the tumor-type targeted, as tissue-specific vascularization can hinder adequate CAR T-cell biodistribution, concentration, and persistance in the involved organs. Positive results from clinical trials are now awaited to hold the promise of this emerging category of cell-based therapy.
Declaration of interest statement
The authors have no conflict of interest to disclose.
Acknowledgments
We wish to thank Kim Margolin, City of Hope, for insightful comments on the manuscript.
Supplementary
Supplemental data for this article can be accessed on the publisher’s website.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–10. doi:10.1158/2159-8290.CD-18-0367.
- Geukes Foppen MH, Donia M, Svane IM, Haanen JB. a. G. tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol Oncol. 2015;9(10):1918–1935. doi:10.1016/j.molonc.2015.10.018.
- Vigneron N. Human tumor antigens and cancer immunotherapy. BioMed Res Int. 2015;2015:948501. doi:10.1155/2015/948501.
- D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, et al. Antitumor activity associated with prolonged persistence of adoptively transferred ny-eso-c259 t cells in synovial sarcoma. Cancer Discov. 2018;8(8):944–957. doi:10.1158/2159-8290.CD-17-1417.
- Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi:10.1038/nrc971.
- Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64(5):891–901. doi:10.1016/0092-8674(91)90314-O.
- Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987;149(3):960–968. doi:10.1016/0006-291X(87)90502-X.
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. U. S. A. 1989;86(24):10024–10028. doi:10.1073/pnas.86.24.10024.
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. U. S. A. 1993;90(2):720–724. doi:10.1073/pnas.90.2.720.
- Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009;21(2):215–223. doi:10.1016/j.coi.2009.02.009.
- Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015;33(1):169–200. doi:10.1146/annurev-immunol-032414-112334.
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–126. doi:10.1038/nri727.
- Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, Seliger B, Abken H. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol Baltim Md 1950. 2001;167:6123–6131.
- Imai C, Mihara K, Andreansky M, Nicholson IC, Pui C-H, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi:10.1038/sj.leu.2403302.
- Song D-G, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119(3):696–706. doi:10.1182/blood-2011-03-344275.
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. Human epidermal growth factor receptor 2 (her2) –specific chimeric antigen receptor–modified t cells for the immunotherapy of her2-positive sarcoma. J. Clin. Oncol. 2015;33(15):1688–1696. doi:10.1200/JCO.2014.58.0225.
- Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et al. HER2-specific chimeric antigen receptor–modified virus-specific t cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094. doi:10.1001/jamaoncol.2017.0184.
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-specific chimeric antigen receptor mrna-engineered t cells induce antitumor activity in solid malignancies. Cancer Immunol. Res. 2014;2(2):112–120. doi:10.1158/2326-6066.CIR-13-0170.
- Brown CE, Badie B, Barish ME, Weng L, Ostberg LR, Chang WC, Naran A, Starr R, Wagner R, Wright C, et al. Bioactivity and safety of il13r 2-redirected chimeric antigen receptor cd8+ t cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21(18):4062–4072. doi:10.1158/1078-0432.CCR-15-0428.
- Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of glioblastoma after chimeric antigen receptor T-Cell Therapy. N. Engl. J. Med. 2016;375(26):2561–2569. doi:10.1056/NEJMoa1610497.
- Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, Han W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 2016;59(5):468–479. doi:10.1007/s11427-016-5023-8.
- Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, Yang Q, Wang Y, Han W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018;9(10):838–847. doi:10.1007/s13238-017-0440-4.
- Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA, et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer. 2017;5(1):22. doi:10.1186/s40425-017-0222-9.
- Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo ASY, Abedi M, Davies RA, Cabral HJ, Al-Homsi AS, et al. Phase I trial of anti-psma designer car-t cells in prostate cancer: possible role for interacting interleukin 2-t cell pharmacodynamics as a determinant of clinical response: car-t cells plus il2 in prostate cancer. Prostate. 2016;76(14):1257–1270. doi:10.1002/pros.23214.
- Katz SC, Burga RA , McCormack E, Wang LJ, Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified t-cell therapy for cea+ liver metastases. Clin. Cancer Res. 2015;21(14):3149–3159. doi:10.1158/1078-0432.CCR-14-1421.
- Lamers CHJ, Klaver Y, Gratama JW, Sleijfer S, Debets R. Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells–a completed study overview. Biochem. Soc. Trans. 2016;44(3):951–959. doi:10.1042/BST20160037.
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al. Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi:10.1182/blood-2011-05-354449.
- Louis CU, Straathof K, Bollard CM, Ennamuri S, Gerken C, Lopez TT, Huls MH, Sheehan A, Wu M-F, Liu H, et al. Adoptive transfer of ebv-specific t cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33(9):983–990. doi:10.1097/CJI.0b013e3181f3cbf4.
- O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984. doi:10.1126/scitranslmed.aaa0984.
- Tchou J, Zhao Y, Levine BL, Zhang PJ, Davis MM, Melenhorst JJ, Kulikovskaya I, Brennan AL, Liu X, Lacey SF, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (car) t cells in metastatic breast cancer. Cancer Immunol. Res. 2017;5(12):1152–1161. doi:10.1158/2326-6066.CIR-17-0189.
- Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW, Sharma SK, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 2017;66(11):1425–1436. doi:10.1007/s00262-017-2034-7.
- You F, Jiang L, Zhang B, Lu Q, Zhou Q, Liao X, Wu H, Du K, Zhu Y, Meng H, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci. China Life Sci. 2016;59(4):386–397. doi:10.1007/s11427-016-5024-7.
- Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, et al. Phase I escalating-dose trial of car-t therapy targeting cea + metastatic colorectal cancers. Mol Ther. 2017;25(5):1248–1258. doi:10.1016/j.ymthe.2017.03.010.
- Hanson RL, Hollingsworth MA. Functional consequences of differential o-glycosylation of muc1, muc4, and muc16 (downstream effects on signaling). Biomolecules. 2016;6(3):34. doi:10.3390/biom6030034.
- Chen J-T, Chen CH, Ku KL, Hsiao M, Chiang CP, Hsu TL, Chen MH, Wong CH. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc. Natl. Acad. Sci. U. S. A. 2015;112(42):13057–13062. doi:10.1073/pnas.1516991112.
- Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015;10(1):473–510. doi:10.1146/annurev-pathol-012414-040438.
- Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: A jekyll and hyde molecule? Clin. Cancer Res. 2010;16(14):3533–3538. doi:10.1158/1078-0432.CCR-09-3169.
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14(11):1264–1270. doi:10.1038/nm.1882.
- Gargett T, Yu W , Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID , Brenner MK, Brown MP. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by pd-1 blockade. Mol. Ther. J. Am. Soc. Gene Ther. 2016;24(6):1135–1149. doi:10.1038/mt.2016.63.
- Scarfò I, Maus MV. Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother Cancer. 2017;5(1):28. doi:10.1186/s40425-017-0230-9.
- McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613–628. doi:10.1016/j.cell.2017.01.018.
- Yuan Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb. Perspect. Med. 2016;6(8):a026583. doi:10.1101/cshperspect.a026583.
- Shah NN, Maatman T, Hari P, Johnson B. Multi targeted car-t cell therapies for b-cell malignancies. Front Oncol. 2019;9:146. doi:10.3389/fonc.2019.00146.
- Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, et al. TanCAR: A novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther - Nucleic Acids. 2013;2:e105. doi:10.1038/mtna.2013.32.
- Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, Han X, Liu Y, Zhang W, Wang C, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol J Hematol Oncol. 2020;13(1):30. doi:10.1186/s13045-020-00856-8.
- Li D, Hu Y, Jin Z, Zhai Y, Tan Y, Sun Y, Zhu S, Zhao C, Chen B, Zhu J, et al. TanCAR T cells targeting CD19 and CD133 efficiently eliminate MLLleukemic cells. Leukemia. 2018;32(9):2012–2016. doi:10.1038/s41375-018-0212-z.
- Schuberth PC, Hagedorn C, Jensen SM, Gulati P, van den Broek M, Mischo A, Soltermann A, Jüngel A, Marroquin Belaunzaran O, Stahel R, et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J. Transl. Med. 2013;11(1):187. doi:10.1186/1479-5876-11-187.
- Kakarla S, Chow KK, Mata M, Shaffer DR, Song X-T, Wu M-F, Liu H, Wang LL, Rowley DR, Pfizenmaier K, et al. Antitumor effects of chimeric receptor engineered human t cells directed to tumor stroma. Mol. Ther. 2013;21(8):1611–1620. doi:10.1038/mt.2013.110.
- Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CCR, Restifo NP, Rosenberg SA. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013;210(6):1125–1135. doi:10.1084/jem.20130110.
- Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel t cell-engaging therapies. Cancer J. 2014;20(2):119–122. doi:10.1097/PPO.0000000000000035.
- Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018;24(6):731–738. doi:10.1038/s41591-018-0041-7.
- Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019;25(4):625–638. doi:10.1016/j.bbmt.2018.12.758.
- Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–1419. doi:10.1158/2159-8290.CD-17-0698.
- Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy. 2019;11(10):851–857. doi:10.2217/imt-2019-0074.
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther J Am Soc Gene Ther. 2010;18(4):843–851. doi:10.1038/mt.2010.24.
- Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, Barrett DM, Grupp SA, Milone MC. High-Affinity GD2-Specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol. Res. 2018;6(1):36–46. doi:10.1158/2326-6066.CIR-17-0211.
- Min IM, Shevlin E, Vedvyas Y, Zaman M, Wyrwas B, Scognamiglio T, Moore MD, Wang W, Park S, Park S, et al. CAR T therapy targeting ICAM-1 eliminates advanced human thyroid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23(24):7569–7583. doi:10.1158/1078-0432.CCR-17-2008.
- Park S, Shevlin E, Vedvyas Y, Zaman M, Park S, Hsu YMS, Min IM, Jin MM. Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Sci. Rep. 2017;7(1):14366. doi:10.1038/s41598-017-14749-3.
- Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell DJ. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72(7):1844–1852. doi:10.1158/0008-5472.CAN-11-3890.
- Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of t cell responses. Cell. 2018;173(6):1426–1438.e11. doi:10.1016/j.cell.2018.03.038.
- Lee YG, Chu H, Lu Y, Leamon CP, Srinivasarao M, Putt KS, Low PS. Regulation of CAR T cell-mediated cytokine release syndrome-like toxicity using low molecular weight adapters. Nat. Commun. 2019;10(1):2681. doi:10.1038/s41467-019-10565-7.
- Bachmann M. The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol Lett. 2019;211:13–22. doi:10.1016/j.imlet.2019.05.003.
- Piersma B, Hayward M-K, Weaver VM. The fibrotic tumor stroma. Biochim Biophys Acta Rev Cancer. 2020;(2):188356. doi:10.1016/j.bbcan.2020.188356.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447.
- Rennert P, Dufort F, Su L, Wu L, Birt A, Lobb R, Ambrose C. Hijacking CAR19 T cells to target diverse hematologic and solid tumors. SITC. 2018; abstract #11102.
- Moon EK, Carpenito C, Sun J, Wang LCS, Kapoor V, Predina J, Powell DJ, Riley JL, June CH, Albelda SM, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17(14):4719–4730. doi:10.1158/1078-0432.CCR-11-0351.
- Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G, Savoldo B, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi:10.1182/blood-2009-03-209650.
- Whilding LM, Halim L, Draper B, Parente-Pereira AC, Zabinski T, Davies DM, Maher J. CAR T-Cells Targeting the Integrin αvβ6 and Co-Expressing the Chemokine Receptor CXCR2 Demonstrate Enhanced Homing and Efficacy against Several Solid Malignancies. Cancers. 2019;11(5):674. doi:10.3390/cancers11050674.
- Liu G, Rui W, Zheng H, Huang D, Yu F, Zhang Y, Dong J, Zhao X, Zhao X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur J Immunol. 2020; doi:10.1002/eji.201948457.
- Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, Dyson KA, Grippin AJ, Deleyrolle LP, Zhang W, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 2019;10(1):4016. doi:10.1038/s41467-019-11869-4.
- Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi:10.1126/scitranslmed.3010162.
- Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, Chang W-C, Ostberg JR, Neman J, Jandial R, et al. Regional delivery of chimeric antigen receptor–engineered t cells effectively targets her2+breast cancer metastasis to the brain. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24(1):95–105. doi:10.1158/1078-0432.CCR-17-2041.
- Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, Foreman NK, Warren KE, Lee DW. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer. 2018;6(1):30. doi:10.1186/s40425-018-0340-z.
- Mulazzani M, Fräßle SP, von Mücke-heim I, Langer S, Zhou X, Ishikawa-Ankerhold H, Leube J, Zhang W, Dötsch S, Svec M, et al. Long-term in vivo microscopy of CAR T cell dynamics during eradication of CNS lymphoma in mice. Proc. Natl. Acad. Sci. U. S. A. 2019;116(48):24275–24284. doi:10.1073/pnas.1903854116.
- Frigault MJ, Dietrich J, Martinez-Lage M, Leick M, Choi BD, DeFilipp Z, Chen Y-B, Abramson J, Crombie J, Armand P, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–866. doi:10.1182/blood.2019001694.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Kegler A, Koristka S, Bergmann R, Berndt N, Arndt C, Feldmann A, Hoffmann A, Bornhäuser M, Schmitz M, Bachmann MP, et al. T cells engrafted with a UniCAR 28/z outperform UniCAR BB/z-transduced T cells in the face of regulatory T cell-mediated immunosuppression. Oncoimmunology. 2019;8(9):e1621676. doi:10.1080/2162402X.2019.1621676.
- Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Patel PR, Guedan S, Scholler J, Keith B, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T Cells. Immunity. 2016;44(2):380–390. doi:10.1016/j.immuni.2016.01.021.
- Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi:10.1182/blood-2011-12-400044.
- Pegram HJ, Purdon TJ, van Leeuwen DG, Curran KJ, Giralt SA, Barker JN, Brentjens RJ. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29(2):415–422. doi:10.1038/leu.2014.215.
- Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017;7(1):10541. doi:10.1038/s41598-017-10940-8.
- Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi:10.4161/2162402X.2014.994446.
- Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18(6):1672–1683. doi:10.1158/1078-0432.CCR-11-3050.
- Chmielewski M, Abken H. CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017;21(11):3205–3219. doi:10.1016/j.celrep.2017.11.063.
- Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, Olive D. Pancreatic ductal adenocarcinoma: a strong imbalance of good and bad immunological cops in the tumor microenvironment. Front. Immunol. 2018;9:1044. doi:10.3389/fimmu.2018.01044.
- John LB, Devaud C, Duong CPM, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19(20):5636–5646. doi:10.1158/1078-0432.CCR-13-0458.
- Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018;36(9):847–856. doi:10.1038/nbt.4195.
- Suarez ER, Chang D-K, Sun J, Sui J, Freeman GJ, Signoretti S, Zhu Q, Marasco WA. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341–34355. doi:10.18632/oncotarget.9114.
- Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, Liu H, Wu MF, Mei Z, Gee A, et al. CAR T cells administered in combination with lymphodepletion and pd-1 inhibition to patients with neuroblastoma. Mol. Ther. J. Am. Soc. Gene Ther. 2017;25(9):2214–2224. doi:10.1016/j.ymthe.2017.05.012.
- Choi BD, Yu X, Castano AP, Darr H, Henderson DB, Bouffard AA, Larson RC, Scarfò I, Bailey SR, Gerhard GM, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7(1):304. doi:10.1186/s40425-019-0806-7.
- Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother CII. 2019;68(3):365–377. doi:10.1007/s00262-018-2281-2.
- Hu B, Zou Y, Zhang L, Tang J, Niedermann G, Firat E, Huang X, Zhu X. Nucleofection with Plasmid DNA for CRISPR/Cas9-mediated inactivation of programmed cell death protein 1 in cd133-specific CAR T Cells. Hum. Gene Ther. 2019;30(4):446–458. doi:10.1089/hum.2017.234.
- Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, Maus MV, Fraietta JA, Zhao Y, June CH, et al. Dominant-negative TGF-β receptor enhances psma-targeted human car t cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018;26(7):1855–1866. doi:10.1016/j.ymthe.2018.05.003.
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126(8):3130–3144. doi:10.1172/JCI83092.
- Chen N, Morello A, Tano Z, Adusumilli PS. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2017;6(2):e1273302. doi:10.1080/2162402X.2016.1273302.
- Porter CE, Rosewell Shaw A, Jung Y, Yip T, Castro PD, Sandulache VC, Sikora A, Gottschalk S, Ittman MM, Brenner MK, et al. Oncolytic adenovirus armed with bite, cytokine, and checkpoint inhibitor enables car t cells to control the growth of heterogeneous tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2020;28(5):1251–1262. doi:10.1016/j.ymthe.2020.02.016.
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19. doi:10.1038/nrc.2015.5.
- Habif G, Crinier A, André P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. 2019;16:415–422. doi:10.1038/s41423-019-0224-2.
- Muntasell A, Rojo F, Servitja S, Rubio-Perez C, Cabo M, Tamborero D, Costa-Garcia M, Martinez-Garcia M, Menéndez S, Vazquez I, et al. NK cell infiltrates and hla class Iexpression in primary HER2+breast cancer predict and uncouple pathological response and disease-free survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25(5):1535–1545. doi:10.1158/1078-0432.CCR-18-2365.
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018;24(8):1178–1191. doi:10.1038/s41591-018-0085-8.
- Ménard C, Blay J-Y, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taieb J, Delahaye NF, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69(8):3563–3569. doi:10.1158/0008-5472.CAN-08-3807.
- Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the tumor microenvironment on nk cell function in solid tumors. Front Immunol. 2019;10:3038. doi:10.3389/fimmu.2019.03038.
- Rezvani K. Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. 2019;54(S2):785–788. doi:10.1038/s41409-019-0601-6.
- Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. doi:10.1016/j.stem.2018.06.002.
- Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, Victor A, Meisen WH, Kim S-H, Grandi P, et al. CAR-engineered nk cells targeting wild-type egfr and egfrviii enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015;5(1):11483. doi:10.1038/srep11483.
- Ao X, Yang Y, Li W, Tan Y, Guo W, Ao L, He X, Wu X , Xia J, Xu X, et al. Anti-αFR CAR-engineered NK-92 Cells Display Potent Cytotoxicity Against αFR-positive Ovarian Cancer. J Immunother Hagerstown Md 1997. 2019;42:284–296.
- Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, Jin Q, Su L, Liu X, Wang K, et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. J. Am. Soc. Gene Ther. 2019;27(6):1114–1125. doi:10.1016/j.ymthe.2019.03.011.
- Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, Gottschalk SM, Rooney CM. NK Cells Expressing a Chimeric Activating Receptor Eliminate MDSCs and Rescue Impaired CAR-T Cell Activity against Solid Tumors. Cancer Immunol. Res. 2019;7(3):363–375. doi:10.1158/2326-6066.CIR-18-0572.
- Roybal KT, Rupp L, Morsut L, Walker W, McNally K, Park J, Lim W. Precision tumor recognition by t cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–779. doi:10.1016/j.cell.2016.01.011.
- Srivastava S, Salter AI, Liggitt D, Yechan-Gunja S, Sarvothama M, Cooper K, Smythe KS, Dudakov JA, Pierce RH, Rader C, et al. Logic-gated ror1 chimeric antigen receptor expression rescues t cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell. 2019;35(3):489–503.e8. doi:10.1016/j.ccell.2019.02.003.