ABSTRACT
Systemic inflammation is a stage-independent marker of poor prognosis in colorectal cancer (CRC), activated in a complex, multifactorial process. It has been proposed that one of the main factors driving systemic inflammation may be tumor necrosis. Keratin 18 (KRT18) fragments are released from dead cells and their serum levels are markers for apoptotic and necrotic cell death. In CRC, high KRT18 levels associate with advanced disease, but their relationship with tumor necrosis and systemic inflammation is unknown. In this study, serum total soluble KRT18 (tKRT18) and apoptosis-related, caspase-cleaved fragment (aKRT18) levels were measured preoperatively from 328 CRC patients, and their difference was calculated to assess necrosis related KRT18 (nKRT18) levels. The relationships of these markers with tumor necrosis, clinicopathologic features, systemic inflammation markers (C-reactive protein, albumin, and 13 cytokines), and survival were analyzed. High serum tKRT18, aKRT18, and nKRT18 levels showed association with a higher extent of tumor necrosis, distant metastasis, and increased levels of several markers of systemic inflammation, including CXCL8. High serum tKRT18 (multivariable HR 1.94, 95% CI 1.28–2.95, p = .002) and nKRT18 (multivariable HR 1.87, 95% CI 1.24–2.82, p = .003) levels were associated with poor overall survival independent of potential confounding factors. Our results show that tumor necrosis in CRC contributes to serum levels of KRT18 fragments, and both necrosis and KRT18 levels associate with systemic inflammation. Moreover, we show that serum tKRT18 and nKRT18 levels have independent prognostic value in CRC. Our observations confirm the link between cell death and systemic inflammation.
Introduction
Colorectal cancer (CRC) is one of the most common causes of cancer deaths in the Western world,Citation1 and TNM staging is the major prognostic parameter in CRC.Citation2 Additional histopathologic features such as high tumor grade and lymphatic invasion can be used to further identify high-risk patients, and especially in stage II disease, to optimize treatment. Moreover, an increased area of necrosis, an uncontrolled process of cell death, and systemic inflammation have been reported to represent stage-independent markers of poor prognosis in CRC.Citation3–Citation7
Systemic inflammation is characterized by elevated levels of serum C-reactive protein (CRP) and several proinflammatory cytokines such as interleukin 1 receptor antagonist (IL1RN), IL6, and C-X-C motif chemokine ligand 8 (CXCL8, also known as IL8).Citation3,Citation8 Clinically important is that systemic inflammation contributes to the development of cachexia, a wasting syndrome linked to involuntary loss of body weight.Citation9 The activation of systemic inflammation in cancer is a complex, multifaceted process, which is currently not well understood.
Necrotic cell death may stimulate systemic inflammation, and high extent of tumor necrosis has been associated with increased serum IL6 and CRP levels in CRC.Citation10 Keratins such as keratin 18 (KRT18) are intracellular structural proteins, which are released from dead cells and can be used as serum biomarkers of cell death. KRT18 is widely expressed by a variety of single-layered epithelial cells, including gastrointestinal tract epithelium, hepatocytes, and CRC cells.Citation11,Citation12 During apoptotic cell death, caspase-cleaved KRT18 fragments (aKRT18) are released, whereas full-length KRT18 (nKRT18) is released during necrosis.Citation13,Citation14 Different forms of KRT18 are measured by specific enzyme-linked immunosorbent assays (ELISAs) using antibodies M30 and M65. The M30 antibody detects aKRT18, whereas the M65 antibody binds both aKRT18 and nKRT18, thus detecting total KRT18 (tKRT18).Citation15
Serum tKRT18 and aKRT18 levels have been shown to be elevated in various malignancies including colorectal, esophageal, head and neck, lung, and gastric cancer.Citation16–Citation19 In CRC patients, increased serum tKRT18 and aKRT18 levels have been associated with advanced stage.Citation20 Moreover, high tKRT18 or aKRT18 levels have been shown to be associated with poor prognosis in non-small-cell lung cancer, gastric cancer, pancreatic cancer, and CRC.Citation18,Citation20-Citation23 However, the studies evaluating CRC patients have been limited by small sample sizes and the lack of comparison of the prognostic performance of serum KRT18 levels to that of other relevant serum biomarkers.Citation20,Citation22
Therefore, the main objective of this study was to evaluate the prognostic significance of preoperative circulating KRT18 levels in a well-characterized cohort of 328 CRC patients. Based on two earlier studies, as well as findings on other cancers, we hypothesized that higher KRT18 levels would be associated with adverse outcome. As secondary analyses, we assessed the relationships between the circulating KRT18 levels and the extent of tumor necrosis, systemic inflammatory markers, and other clinicopathologic features. We hypothesized that necrotic cell death released KRT18 fragments would be associated with the extent of tumor necrosis, reflecting the release of KRT18 fragments from the dying tumor cells, and with markers of systemic inflammation, reflecting the activation of systemic inflammation by necrotic cell death and the release of intracytoplasmic fragments into the circulation.
Materials and methods
Patients and controls
The study was based on 328 newly diagnosed CRC patients operated in the Oulu University Hospital between 2006 and 2014, who had signed an informed consent to participate and were eligible for the study.Citation8,Citation24 The patients with earlier or simultaneously diagnosed other malignant diseases were excluded. Clinical data were collected from the clinical records and a questionnaire. The follow-up data were acquired from the clinical records and Statistics Finland,Citation25,Citation26 and study endpoints were cancer-specific survival (CSS; time from operation to cancer death) and overall survival (OS; time from operation to death from any cause).Citation24 The study was accepted by the Ethics Committee of the Oulu University Hospital (42/2005, 122/2009) and performed according to the principles of the Declaration of Helsinki. The study was designed in accordance with the REMARK guidelines.Citation27 Control serum samples were acquired from healthy cataract surgery patients (Oulu University Hospital; n = 50, age ≥ 65 y).
Histopathologic analysis
The tumors were staged according to TNM8 classification and graded according to the WHO2010 criteria.Citation2 Klintrup-Mäkinen Score of peritumoral inflammatory infiltrate was evaluated using hematoxylin and eosin (HE) stained sections.Citation28 The percentage of tumor tissue showing coagulative necrosis (characteristic necrosis appearance with increased eosinophilia and nuclear shrinkage, fragmentation, and disappearance in the HE-stained sections) was evaluated as described earlier.Citation7 Tumor necrosis index, an approximation of the total amount of necrosis in the tumor tissue, was obtained by multiplying the percentage of tumor tissue necrosis in the HE slides by the maximum tumor diameter.
Immunohistochemistry and immune cell counting
Tissue microarrays with 1–4 (median 3) cores of 3.0 mm diameter including both the invasive margin (IM) and the center of the tumor (CT) were constructed for immunohistochemical analysis.Citation29,Citation30 Immunohistochemistry was conducted on 3.5 µm sections cut from the tissue microarray paraffin blocks for six immune cell markers (CD3, CD8, CD68, FoxP3, mast cell tryptase, neutrophil elastase).Citation29 For immune cell counting, images were captured from the CT and the IM and the cell densities were counted using a computer-assisted cell counting methodCitation31 utilizing ImageJ, a freeware image analysis software.Citation32 Intraepithelial (CT-IEL) immune cells (CD3, CD8) were counted manually from the captured images due to the inadequacy of the automatic cell counting to segregate the intraepithelial cells from those in the stroma.Citation29 Mismatch repair (MMR) enzyme screening status for MLH1, MSH2, MSH6, and PMS2 was examined with immunohistochemistry, as described earlier.Citation24,Citation33,Citation34 BRAF V600E-specific VE1 immunohistochemistry was conducted.Citation24,Citation35 The fraction of proliferating cancer cells (MKI67 score) was evaluated with MKI67 immunohistochemistry.Citation7
Analysis of serum samples
Preoperative serum samples were collected in tubes without clot activator. The samples were centrifuged and stored at −70°C until the analysis. Blood leukocyte counts, serum CRP levels, and serum albumin levels were measured in the laboratory of Oulu University Hospital, and modified Glasgow Prognostic Score (mGPS) was calculated from CRP and albumin values as previously described.Citation36 Serum levels of 27 cytokines were measured by Bio-Plex Pro Human pre-manufactured 27-Plex Cytokine Panel (Bio-Rad, Hercules, CA, USA) from patients operated between April 2006 and January 2010.Citation8 Out of the 27 cytokines, 13 cytokines (IL1RN, IL4, IL6, IL7, CXCL8, IL9, IL12, IFNG, CXCL10, CCL2, CCL4, CCL11, and PDGF) with three (1.5%) or fewer values outside the assay working range were included in this study. Serum levels of aKRT18 were measured by M30Apoptosense® enzyme-linked immunosorbent assay (ELISA), and tKRT18 by M65® ELISA (Peviva AB, Bromma, Sweden). All the measurements were done in duplicate according to manufacturer’s instructions blinded to clinicopathologic data. Units were defined against a synthetic peptide standard (1 U/L = 1.24 pM). As the M65 ELISA measures both caspase-cleaved (apoptosis) and non-caspase cleaved KRT18 (necrosis), the level of KRT18 released during necrosis (nKRT18) was calculated as M65-M30.
Statistical analyses
The statistical analyses were conducted using IBM SPSS Statistics for Windows version 25.0 (IBM Corporation, Armonk, NY, USA) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), using the packages tidyverse (v.1.2.1), survival (v.2.42–6), survminer (v.0.4.3), and plotROC (v.2.2.1). The statistical significances of the associations between categorical and continuous variables were analyzed by Mann–Whitney test (comparing two classes) or Kruskal–Wallis test (comparing three or more classes). Correlations between two continuous variables were determined using Pearson correlation coefficients (r). Cytoscape 3.7.2 was used to create a correlation network with the Prefuse force directed algorithm weighted by the statistical significances of the associations between individual variables. Multiple linear regression analysis of the correlation of serum tKRT18, aKRT18, and nKRT18 levels with selected clinicopathological factors was conducted. Receiver operating characteristics (ROC) analysis was used to determine optimal cutoff values, with the shortest distance to the coordinate (0,1), in discriminating survivors from non-survivors (CSS). Univariate survival was performed according to the Kaplan–Meier method. Backward conditional stepwise Cox regression was used for multivariate survival modeling. Considering multiple hypothesis testing, a two-tailed p < .01 was considered statistically significant.
Results
Serum tKRT18, aKRT18 and nKRT18 levels in patients and controls
The characteristics of CRC patients and healthy controls are presented in Supplementary Table 1. In CRC patients, the median tKRT18, aKRT18, and nKRT18 levels (U/L) were 483.4, 185.9, and 298.9, and a strong correlation existed between circulating tKRT18 and aKRT18 levels (Pearson r = 0.865, p < .001). The median serum tKRT18, aKRT18 and nKRT18 levels were significantly higher in CRC patients ≥65 y compared to healthy controls (tKRT18: 483.7 vs. 195.1 U/L, p < .001; aKRT18: 177.6 vs. 112.2 U/L, p < .001; nKRT18 302.6 vs. 76.8 U/L, p < .001; Supplementary Table 1, Supplementary Figure 1).
Serum tKRT18, aKRT18, and nKRT18 levels in relation to basic clinicopathologic parameters
shows the relationships between serum levels of KRT18 molecules and clinicopathologic variables. Obese CRC patients (BMI>30) had increased tKRT18 and nKRT18 levels (p < .001). Higher tumor stages were associated with elevated tKRT18 (p = .002), aKRT18 (p < .001) and nKRT18 (p = .008) levels. Higher Klintrup-Mäkinen Scores indicating pronounced peritumoral inflammatory infiltrate were associated with decreased aKRT18 levels (p = .009), and activated systemic inflammation measured by mGPS associated with increased tKRT18 (p < .001) and nKRT18 levels (p < .001). There were no statistically significant associations between serum KRT18 levels and preoperative radiotherapy or chemoradiotherapy.
Table 1. Serum tKRT18, aKRT18, and nKRT18 levels in relation to clinical and pathological characteristics of CRCs.
Correlation of serum aKRT18, tKRT18 and nKRT18 levels and tumor necrosis index, systemic inflammation markers, MKI67 and tumor-infiltrating immune cells
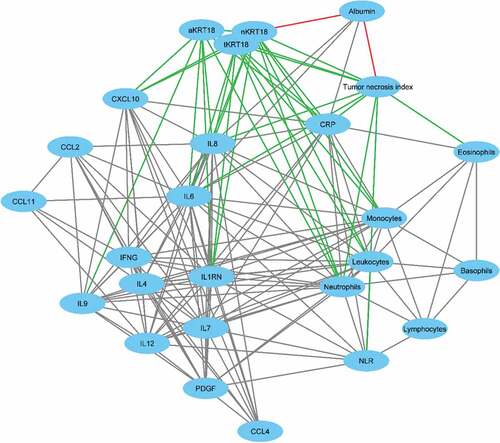
We hypothesized that serum KRT18 levels would reflect tumor necrosis and be associated with systemic inflammation. The correlations between serum tKRT18, aKRT18 and nKRT18 levels and tumor necrosis, the markers of systemic inflammation, cytokines, and proliferation marker MKI67 are presented in and . In support our hypothesis, tKRT18 (beta = 0.206, p = .001) and nKRT18 levels (beta = 0.165, p = .012, borderline statistical significance considering multiple hypothesis testing) were associated with tumor necrosis index. Moreover, tKRT18 levels associated with areal percentage of tumor necrosis (beta = 0.162, p = .006), whereas maximum tumor diameter showed no significant association with serum KRT18 levels. There were also positive correlations between serum KRT18 levels and several systemic inflammatory markers; tKRT18 showed positive multivariable-adjusted correlations with CRP (beta = 0.190, p = .001), blood monocyte count (beta = 0.158, p = .005), IL6 (beta = 0.385, p < .001), CXCL8 (beta = 0.522, p < .001), and CXCL10 (beta = 0.275, p < .001), while aKRT18 levels were associated with serum IL6 (beta = 0.275, p = .001), CXCL8 (beta = 0.397, p < .001), IL-9 (beta = 0.233, p = .003), and CXCL10 (beta = 0.280, p < .001). nKRT18 levels were associated with blood monocyte count (beta = 0.191, p = .001), IL6 (beta = 0.243, p = .004), CXCL8 (beta = 0.498, p < .001), and CXCL10 (beta = 0.231, p = .004). Serum KRT18 levels did not show statistically significant associations with tumor-infiltrating immune cells (Supplementary Table 2).
Table 2. Correlations between serum tKRT18, aKRT18, and nKRT18 level, the markers of systemic inflammation, cytokines, Ki-67, and tumor necrosis.
Figure 1. Correlation network of the interrelationships between serum keratin 18 levels, tumor necrosis index, blood immune cells, cytokines, CRP, and albumin. Individual variables are presented by nodes and their associations are presented by edges. Only the correlations with p < .01 are shown and the edge length illustrates the significance of the association. The correlations between tumor necrosis index and keratin 18 levels with other variables are presented by green (positive correlation) and red (negative correlation) edges, whereas the interrelationships between other variables are presented by gray edges.
Multivariate analysis
Multiple linear regression was used to further investigate the relative significance of potential determinants of serum KRT18 levels. Based on the univariate analyses, the factors included in the initial models were invasion through muscularis propria, nodal metastasis, distant metastasis, systemic inflammation (mGPS), BMI, Klintrup-Mäkinen Score of peritumoral inflammatory infiltrate, and tumor necrosis. Of these factors, the most prominent indicator of serum tKRT18 (beta = 0.250, p < .001) and aKRT18 (beta = 0.363, p < .001) levels was distant metastasis and the main predictor of serum nKRT18 level was mGPS (beta = 0.180, p = .002) (). However, when serum cytokines were considered for the inclusion in the models, CXCL8 was the major determinant of all KRT18 fragments, showing strong associations with tKRT18 (beta = 0.580, p < .001), aKRT18 (beta = 0.503, p < .001) and nKRT18 (beta = 0.520, p < .001) levels (Supplementary Table 3).
Table 3. Multiple linear regression model of tKRT18, aKRT18, and nKRT18 levels in colorectal cancer patients.
Survival analyses
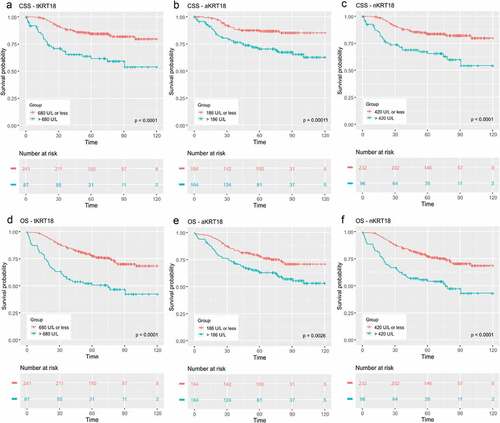
The primary aim of this study was to evaluate the prognostic significance of serum tKRT18, aKRT18, and nKRT18 levels. ROC analysis showed that serum tKRT18, aKRT18 and nKRT18 levels were capable of discriminating survivors from non-survivors (tKRT18: AUC = 0.656, 95% CI = 0.581–0.730, p < .001, aKRT18: AUC = 0.666, 95% CI = 0.591–0.741, p < .001, nKRT18: AUC = 0.626, 95% CI = 0.546–0.705, p = .001) (Supplementary Figure 2). Kaplan–Meier plots indicated that increased serum tKRT18 (>680 U/L), aKRT18 (>186 U/L) and nKRT18 (>420 U/L) were associated with worse CSS and OS (p < .001 for all) (). In multivariate Cox proportional hazard regression models, adjusting for tumor stage, systemic inflammation, and other clinicopathologic features, tKRT18 and nKRT18 were independent predictors of poor OS (tKRT18: HR 1.94, 95% CI 1.28–2.95, p = .002, nKRT18: HR 1.87, 95% CI 1.24–2.82, p = .003), whereas aKRT18 was not significantly associated with CSS or OS ().
Table 4. Cox regression model for the independent prognostic significance of serum tKRT18, aKRT18, and nKRT18 level.
Discussion
In this study, we found that all KRT18 fragments associated with advanced disease stage and systemic inflammation, and tKRT18 associated with the extent of tumor necrosis. In multivariate linear regression models, serum CXCL8 level was the main determinant of all three KRT18 forms. Serum levels of tKRT18 and nKRT18 predicted poor OS independent of disease stage, systemic inflammatory markers, and other clinicopathologic features. These results suggest that cell death and systemic inflammation are strongly connected in CRC patients, and the alterations in serum KRT18 levels in CRC reflect subsequent disease course.
During the past few decades, it has become evident that tumors can induce systemic changes long before gross metastatic disease appears,Citation37,Citation38 and many of these changes are involved in systemic inflammation. However, the factors underlying systemic inflammation in cancer have not been well characterized. Tissue necrosis may rapidly provoke a systemic inflammatory response required for the removal of dead tissues. The potential significance of tumor necrosis in CRC associated systemic inflammation has been highlighted by Richards et al.Citation39 and Guthrie et al.,Citation10 who reported that increasing amount of tumor necrosis in CRC is associated with higher mGPS and serum IL6 levels. Our present study adds to this by showing a strong link between serum cell-death-related KRT18 fragments and an assemblage of systemic inflammatory markers.
Tumor necrosis was associated with all three forms of serum KRT18 in univariate analyses, and it showed a statistically significant multivariable-adjusted association with tKRT18 (p = .001) and a tendency toward multivariable-adjusted association with nKRT18 levels (p = .012, borderline statistical significance considering multiple hypothesis testing). These findings support the hypothesis that tumor necrosis contributes to serum KRT18 levels in CRC. However, the associations between serum KRT18 levels and tumor necrosis were weaker than those between serum KRT18 levels and systemic inflammation. This may be related to the inadequacy of tumor necrosis percentage or tumor necrosis index, based on the evaluation of the primary tumor specimens, to estimate the necrotic tumor mass present in the body. In addition, KRT18 released from other epithelial tissues may be involved in activating systemic inflammation, as KTR18/KRT8 constitutes the predominant keratin pair of nonmalignant simple epithelial cells such as gastrointestinal tract epithelium,Citation40 in addition to being widely expressed by adenocarcinomas.
In particular, the liver is a potential source of the KRT18 fragments. KRT8/KRT18 represents the characteristic and only keratin pair of normal hepatocytes.Citation40 Indeed, an increase in KRT18 fragment levels is an established marker of liver injury.Citation41 Potential mechanism leading to liver injury in CRC may be related to changes in the extra-tumoral intestinal mucosa or tumor secreted or induced mediators, or metastasis. When the intestinal epithelial barrier is disrupted, and intestinal permeability is increased, bacteria or bacterial components can enter the liver via the portal circulation. Animal CRC models have shown a strong association between circulating IL6 levels and intestinal permeability.Citation42,Citation43 In the liver, gut-derived endotoxin, lipopolysaccharide (LPS) of gram-negative bacteria, induces Kupffer cell activation and liver injury that is reflected to circulating aKRT18 and tKRT18 and nKRT18 levels.Citation44 Thus, part of serum KRT18 in CRC patients may originate from the liver, representing a consequence rather than cause of systemic inflammation. Moreover, liver is the most common site of metastasis in CRC patients, and metastatic spread may result in cell damage in the metastatic site. Accordingly, in patients with metastatic CRC, alkaline phosphatase, and aspartate transaminase levels, markers of liver injury have been shown to correlate with tKRT18 and aKRT18.Citation20 All in all, multiple tissues may contribute to circulating KRT18 levels, and the potential sources besides necrotic tumor cells in CRC remain hypothetical.
Our study analyzed serum levels of 13 cytokines and identified that CXCL8 was the most important predictor of serum levels of all KRT18 forms. In the visualization of the systemic inflammatory marker network (), CXCL8, along with IL6, clustered at the center having a strong correlation with a high number of other systemic inflammatory markers. CXCL8 is a pro-inflammatory chemokine, which recruits neutrophils to the site of inflammation.Citation45 In addition, neutrophils synthesize and release CXCL8 in response to injury-released intracellular damage-associated molecular patterns (DAMPs).Citation46 These findings suggest that neutrophils and CXCL8 may represent a potential mechanistic link between cell death and systemic inflammation. Interestingly, serum CXCL8 levels are not statistically significantly associated with patient survival in multivariable models,Citation47 whereas this study indicates that serum tKRT18 and nKRT18 predict poor OS independent of disease stage, systemic inflammatory markers, and other clinicopathologic features. This supports the multifactorial background underlying the prognostic significance of serum KRT18 levels in CRC.
Improved prognostic parameters are needed to classify CRC into more homogenous, therapeutically relevant groups. The results of this study support the relevance of tKRT18 and nKRT18 as additional prognostic markers in CRC. This finding confirms the results of two previous studies.Citation20,Citation22 Relative to these studies, the major advantages of our study are a larger patient cohort and extensive evaluation of additional circulating biomarkers for comparison. Some of these markers, including mGPS, have been shown to be associated with patient outcome in a large group of independent studies.Citation48 In the present study, the serum tKRT18 and nKRT18 had stronger prognostic value than mGPS. However, our study was still limited by the lack of an independent validation cohort and by a relatively small number of patients in more specific patient subgroups, such as stage II patients. Therefore, further studies are required to evaluate, whether serum KRT18 levels could help to identify high-risk patients in these subgroups, in combination with other systemic inflammation-based markers such as mGPS, and whether these measurements could be used to guide the treatment of the patients.
In conclusion, elevated serum KRT18 levels in CRC are associated with adverse clinical outcome and systemic inflammation, especially higher serum CXCL8 levels. These results support a link between cell death, systemic inflammatory response, and patient prognosis in CRC.
Disclosure of potential conflict of interest
The authors declare that there is no conflict of interest.
Supplemental Material
Download ()Acknowledgments
We thank Ms. Riitta Vuento for her excellent assistance in the preparation of the study material. This work was supported by grants from Finnish Cancer Society, K. Albin Johansson Foundation, Kaarina ja Erkki Piippola Foundation.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–10. doi:10.3322/caac.21551.
- Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura SI. Carcinoma of the colon and rectum. In: Bosman F, Carneiro F, Hruban R, Theise N, et al., editors. WHO classification of tumours of the digestive system. Lyon, France: IARC Press; 2010. p. 134–146.
- McMillan DC. The systemic inflammation-based glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi:10.1016/j.ctrv.2012.08.003.
- Park JH, Watt DG, Roxburgh CSD, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome. Ann Surg. 2016;263(2):326–336. doi:10.1097/SLA.0000000000001122.
- Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, Langner C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41(12):1749–1757. doi:10.1016/j.humpath.2010.04.018.
- Komori K, Kanemitsu Y, Kimura K, Hattori N, Sano T, Ito S, Abe T, Senda Y, Misawa K, Ito Y, et al. Tumor necrosis in patients with TNM stage IV colorectal cancer without residual disease (R0 Status) is associated with a poor prognosis. Anticancer Res. 2013;33(3):1099–1105.
- Väyrynen SA, Väyrynen JP, Klintrup K, Mäkelä J, Karttunen TJ, Tuomisto A, Mäkinen MJ. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer. 2016;114(12):1334–1342. doi:10.1038/bjc.2016.128.
- Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig K-H, Näpänkangas J, Mäkelä J, Karttunen TJ, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. doi:10.1038/bjc.2012.456.
- Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2019;15(1):9–20. doi:10.1038/s41574-018-0123-0.
- Guthrie GJK, Roxburgh CSD, Richards CH, Horgan PG, McMillan DC. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. Br J Cancer. 2013;109(1):131–137. doi:10.1038/bjc.2013.291.
- Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother. 2005;59(Suppl 2):S359–S362. doi:10.1016/S0753-3322(05)80078-2.
- Omary MB, Ku N-O, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119(7):1794–1805. doi:10.1172/JCI37762.
- Caulín C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138(6):1379–1394. doi:10.1083/jcb.138.6.1379.
- Schutte B, Henfling M, Kölgen W, Bouman M, Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297(1):11–26. doi:10.1016/j.yexcr.2004.02.019.
- Kramer G, Erdal H, Mertens HJMM, Nap M, Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64(5):1751–1756. doi:10.1158/0008-5472.CAN-03-2455.
- Ozturk B, Coskun U, Sancak B, Yaman E, Buyukberber S, Benekli M. Elevated serum levels of M30 and M65 in patients with locally advanced head and neck tumors. Int Immunopharmacol. 2009;9(5):645–648. doi:10.1016/j.intimp.2009.02.004.
- Ulukaya E, Yilmaztepe A, Akgoz S, Linder S, Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007;56(3):399–404. doi:10.1016/j.lungcan.2007.01.015.
- Yaman E, Coskun U, Sancak B, Buyukberber S, Ozturk B, Benekli M. Serum M30 levels are associated with survival in advanced gastric carcinoma patients. Int Immunopharmacol. 2010;10(7):719–722. doi:10.1016/j.intimp.2010.03.013.
- Scott LC, Evans TRJ, Cassidy J, Harden S, Paul J, Ullah R, O’Brien V, Brown R. Cytokeratin 18 in plasma of patients with gastrointestinal adenocarcinoma as a biomarker of tumour response. Br J Cancer. 2009;101(3):410–417. doi:10.1038/sj.bjc.6605175.
- Greystoke A, Dean E, Saunders MP, Cummings J, Hughes A, Ranson M, Dive C, Renehan AG. Multi-level evidence that circulating CK18 is a biomarker of tumour burden in colorectal cancer. Br J Cancer. 2012;107(9):1518–1524. doi:10.1038/bjc.2012.416.
- Oven Ustaalioglu B, Bilici A, Ercan S, Orcun A, Seker M, Ozkan A, Ustaalioglu R, Gumus M. Serum M30 and M65 values in patients with advanced stage non-small-cell lung cancer compared with controls. Clin Transl Oncol. 2012;14(5):356–361. doi:10.1007/s12094-012-0808-0.
- Koelink PJ, Lamers CB, Hommes DW, Verspaget HW. Circulating cell death products predict clinical outcome of colorectal cancer patients. BMC Cancer. 2009;9(1):88. doi:10.1186/1471-2407-9-88.
- Dive C, Smith RA, Garner E, Ward T, George-Smith SS, Campbell F, Greenhalf W, Ghaneh P, Neoptolemos JP. Considerations for the use of plasma cytokeratin 18 as a biomarker in pancreatic cancer. Br J Cancer. 2010;102(3):577–582. doi:10.1038/sj.bjc.6605494.
- Väyrynen JP, Tuomisto A, Väyrynen SA, Klintrup K, Karhu T, Mäkelä J, Herzig K-H, Karttunen TJ, Mäkinen MJ. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8(1):1126. doi:10.1038/s41598-018-19572-y.
- Moilanen JM, Kokkonen N, Löffek S, Väyrynen JP, Syväniemi E, Hurskainen T, Mäkinen M, Klintrup K, Mäkelä J, Sormunen R, et al. Collagen XVII expression correlates with the invasion and metastasis of colorectal cancer. Hum Pathol. 2015;46(3):434–442. doi:10.1016/j.humpath.2014.11.020.
- Kantola T, Väyrynen JP, Klintrup K, Mäkelä J, Karppinen SM, Pihlajaniemi T, Autio-Harmainen H, Karttunen TJ, Mäkinen MJ, Tuomisto A. Serum endostatin levels are elevated in colorectal cancer and correlate with invasion and systemic inflammatory markers. Br J Cancer. 2014;111(8):1605–1613. doi:10.1038/bjc.2014.456.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387–391. doi:10.1038/sj.bjc.6602678.
- Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41(17):2645–2654. doi:10.1016/j.ejca.2005.07.017.
- Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109(7):1839–1847. doi:10.1038/bjc.2013.508.
- Väyrynen JP, Sajanti SA, Klintrup K, Mäkelä J, Herzig K-H, Karttunen TJ, Tuomisto A, Mäkinen MJ. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134(9):2126–2135. doi:10.1002/ijc.28533.
- Väyrynen JP, Vornanen JO, Sajanti S, Böhm JP, Tuomisto A, Mäkinen MJ. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. 2012;460(5):455–465. doi:10.1007/s00428-012-1232-0.
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with image. J Biophotonics Int. 2004;11:36–42.
- Väyrynen JP, Vornanen J, Tervahartiala T, Sorsa T, Bloigu R, Salo T, Tuomisto A, Mäkinen MJ. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int J Cancer. 2012;131(4):E463–E474. doi:10.1002/ijc.26435.
- Sajanti SA, Väyrynen JP, Sirniö P, Klintrup K, Mäkelä J, Tuomisto A, Mäkinen MJ. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virchows Arch. 2015;466(1):5–12. doi:10.1007/s00428-014-1683-6.
- Sajanti SA, Sirniö P, Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Ristimäki A, Mäkinen MJ. VE1 immunohistochemistry accurately detects BRAF V600E mutations in colorectal carcinoma and can be utilized in the detection of poorly differentiated colorectal serrated adenocarcinoma. Virchows Arch. 2014;464. doi:10.1007/s00428-014-1555-0.
- McMillan DC, Crozier JEM, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881–886. doi:10.1007/s00384-006-0259-6.
- Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25:4383–4404. doi:10.3748/wjg.v25.i31.4383.
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16(8):717–727. doi:10.1038/ncb3015.
- Richards CH, Roxburgh CSD, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99(2):287–294. doi:10.1002/bjs.7755.
- Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129(6):705–733. doi:10.1007/s00418-008-0435-6.
- Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18 - the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30(11–12):1103–1109. doi:10.1111/j.1365-2036.2009.04148.x.
- Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, Leclercq S, Van Hul M, Plovier H, Pachikian B, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9(26):18224–18238. doi:10.18632/oncotarget.24804.
- Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the ApcMin/+ mouse model of colon cancer cachexia. Biochim Biophys Acta – Mol Basis Dis. 2011;1812(12):1601–1606. doi:10.1016/j.bbadis.2011.08.010.
- Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. doi:10.3389/fphys.2012.00056.
- Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. doi:10.1158/1078-0432.CCR-07-4843.
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi:10.1038/nature08780.
- Väyrynen JP, Kantola T, Väyrynen SA, Klintrup K, Bloigu R, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer. 2016;139. doi:10.1002/ijc.30040.
- Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7(1):16717. doi:10.1038/s41598-017-16955-5.