ABSTRACT
Amid controversial reports that COVID-19 can be treated with a combination of the antimalarial drug hydroxychloroquine (HCQ) and the antibiotic azithromycin (AZI), a clinical trial (ONCOCOVID, NCT04341207) was launched at Gustave Roussy Cancer Campus to investigate the utility of this combination therapy in cancer patients. In this preclinical study, we investigated whether the combination of HCQ+AZI would be compatible with the therapeutic induction of anticancer immune responses. For this, we used doses of HCQ and AZI that affect whole-body physiology (as indicated by a partial blockade in cardiac and hepatic autophagic flux for HCQ and a reduction in body weight for AZI), showing that their combined administration did not interfere with tumor growth control induced by the immunogenic cell death inducer oxaliplatin. Moreover, the HCQ+AZI combination did not affect the capacity of a curative regimen (cisplatin + crizotinib + PD-1 blockade) to eradicate established orthotopic lung cancers in mice. In conclusion, it appears that HCQ+AZI does not interfere with the therapeutic induction of therapeutic anticancer immune responses.
Introduction
The current epidemic of coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requires curative interventions.Citation1 Hydroxychloroquine (HCQ) inhibits SARS-CoV-2 replication in vitro,Citation2 presumably through a nonspecific action on acidic organelles.Citation3 This in vitro observation spurred clinical evaluation of this antimalarial drug, alone or in combination with macrolide antibiotics (in particular azithromycin, AZI), which are often used for the treatment of respiratory infections. The oral administration of HCQ, alone or (more so) in combination with AZI, yielded encouraging results in uncontrolled observational studies.Citation4,Citation5 However, HCQ alone failed to reduce SARS-CoV-2 replication in vitro in susceptible animal species including ferretsCitation6 and non-human primates (https://www.researchsquare.com/article/rs-27223/v1). Moreover, HCQ alone or in combination with AZI failed to show clinical activity against COVID-19 in randomized trials.Citation7,Citation8
The polemics around the therapeutic use of HCQ+AZI is not closed. However, in the absence of other therapeutic options, the clinical trial “ONCOCOVID” (accession No. NCT04341207 at www.clinicaltrials.com) was launched at Gustave Roussy Cancer Campus on April 10, 2020. The objective of this trial is (i) to determine the prevalence and the 3-months incidence of SARS-CoV-2 in cancer patients and (ii) to evaluate the Covid-19 disease-specific mortality rate in cancer patients treated by HCQ+AZI. Indeed, the underlying conditions (aging, obesity, smoking, … ), co-morbidities (metabolic syndrome, arterial hypertension, cardiopathy, immunodeficiency, …) and nosocomial exposure predispose oncological patients to infection by SARS-CoV-2 and the development of severe COVID-19,Citation9 calling for therapeutic interventions against the virus that do not interfere with the clinical management of cancer patients.
The success of most if not all antineoplastic treatments depends on the induction of a mostly T lymphocyte-mediated anticancer immune response. While this appears obvious for so-called immunotherapies, it also applies to successful chemotherapies and targeted therapies.Citation10-Citation12 For this reason, we investigated whether the HCQ+AZI combination would be compatible with the treatment of two cancers with immunogenic chemotherapy or a combination chemo-immunotherapy.
Results
The combination of hydroxychloroquine and azithromycin is compatible with the success of chemotherapy
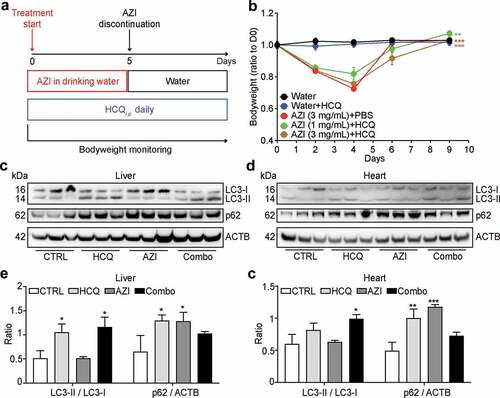
C57Bl/6 mice housed in specific pathogen-free conditions were treated with HCQ (injected intraperitoneally, i.p. at a daily dose of 50 mg/kg) or AZI (given orally with the drinking water at 1 to 3 mg/mL for 5 days), alone or in combination ()). At this dose, AZI caused a reduction in bodyweight that was not aggravated by HCQ ()). As to be expectedCitation13-Citation15 HCQ caused a partial blockade in autophagic flux, as indicated by an increase in the lipidated form (LC3-II) of microtubule-associated proteins 1A/1B light chain 3B (hereafter referred to as LC3) and in the abundance of sequestosome-1 (SQSTM1, best known as p62). These alterations were observed in the liver and in the heart ()). Thus, both HCQ and AZI were used at concentrations that affected whole-body physiology.
Figure 1. Effects of hydroxychloroquine plus azithromycin on naïve mice. Tumor-free mice were treated interperitoneally (i.p.) with hydroxychloroquine (HCQ), orally fed with azithromycin (AZI, supplied at different concentrations in the drinking water), or their combination (Combo) as illustrated in the scheme (a). The bodyweight of the mice was monitored every 2 ~ 3 days and the ratios to the 1st measurement at different time points are summarized as line chart (b), mean ± SEM, n = 10 mice/group; **P < .01, and ***P < .001, two-way ANOVA test, compared to the Water group). Livers and hearts were excised from 3 mice of each group at 4 hours after the second injection of HCQ and subjected to protein extraction for SDS–PAGE and immunoblot (c, d). β-Actin (ACTB) was measured as a loading control. Band intensities were measured to quantify the ratio of LC3-II to LC3-I (LC3-II/LC3-I) and p62 to ACTB (p62/ACTB). Data are expressed as means ± SEM of three mice (e, f). Statistical significance is indicated as *P < .05, **P < .01, and ***P < .001 as compared with untreated control (CTRL) (Student’s t-test).
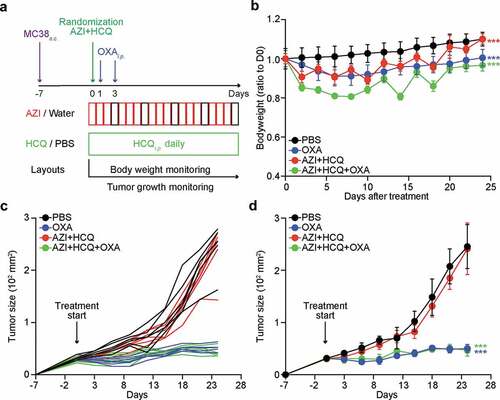
In the next step, we determined whether the combination treatment with HCQ+AZI would affect the chemotherapeutic response of MC38 colon cancers to oxaliplatin (OXA) ()), the antitumor effect of which is known to entirely rely on the active contribution of T lymphocytes.Citation16-Citation19 Systemic OXA-based therapy alone (given twice i.p. on days 8 and 10 post-implantation of MC38 cells under the skin) caused a similar weight loss as did the treatment with HCQ+AZI, and both treatments exhibited additive toxicities ()). The OXA-based chemotherapy led to a prolonged tumor growth control that lasted beyond treatment discontinuation and that was not affected by the simultaneous treatment with HCQ+AZI. Thus, the HCQ+AZI combination is compatible with the success of chemotherapy.
Figure 2. Influence of hydroxychloroquine plus azithromycin on the efficacy of anticancer chemotherapy. Subcutaneous MC38 colorectal cancers were established and interperitoneally (i.p.) treated with oxaliplatin (OXA) or an equivalent volume of PBS, either alone or in combination with hydroxychloroquine (HCQ, i.p.) plus azithromycin (AZI) in drinking water as illustrated in the scheme (a). The bodyweight of mice was monitored every 2 days and ratios to 1st measurement at different time points are summarized as line charts (b) mean ± SEM). Tumor size was measured every 3 days. Growth curves of individual tumors (c) and different groups (d) mean ± SEM) are shown. Statistical differences are expressed as ***P < .001 as compared to the PBS group (two-way ANOVA test, n = 6 mice/group).
The combination of hydroxychloroquine and azithromycin is compatible with successful chemo-immunotherapy
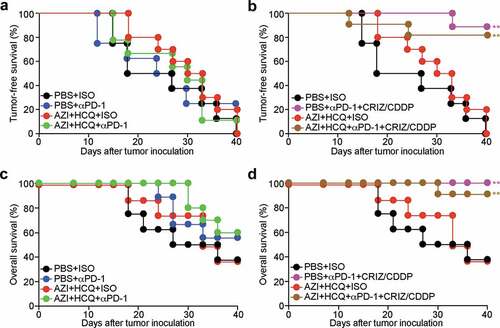
Recently, we developed a protocol for the eradication of established non-small cell lung cancers (NSCLC) consisting of a combination of cis-diamminedichloridoplatinum (II) (CDDP, best known as cisplatin, a classical cytotoxicant targeting DNA), high-dose crizotinib (a tyrosine kinase inhibitor) and PD-1 blockade. This combination treatment can be applied to mice bearing bioluminescence-detectable, luciferase-expressing TC1 lung cancers and induces full resolution of the disease in the majority (80–90%) of cases.Citation20,Citation21 We combined this therapeutic regimen with HCQ+AZI ()) without observing additive toxicities ()). We then monitored the (pre-)clinical evolution of NSCLC by imaging technology ()). PD-1 blockade alone limited tumor growth, and this effect was abolished by HCQ+AZI. However, the combination treatment (CDDP+crizotinib+PD-1 blockade) led to a major reduction of the growth of all tumors, and this effect was compatible with HCQ+AZI co-treatment (). Accordingly, HCQ+AZI had no significant negative effect on the curative effects of the combination treatment (around 80% for CDDP+crizotinib+PD-1 blockade), defined as the complete disappearance of the luciferase-dependent bioluminescence signal ()). Moreover, 90% of the mice survived after combination therapy with CDDP+crizotinib+PD-1 blockade irrespective of the co-treatment with HCQ+AZI ()). Thus, HCQ+AZI does not interfere with therapeutic efficacy.
Figure 3. Influence of hydroxychloroquine plus azithromycin on the efficacy of anticancer chemo-immunotherapy. Orthotopic NSCLC were established by intravenous (i.v.) injection of 5 × 105 TC1 cells stably expressing luciferase (TC1 Luc+) and tumor incidence in the lung was detected by bioluminescence. Mice (n = minimum of 8 mice per group) were treated with crizotinib (CRIZ) plus cisplatin (CDDP) or equivalent volumes of PBS, and combined or not with monoclonal antibody to PD-1 (αPD-1) or isotype antibody (ISO), either alone or in combination with hydroxychloroquine (HCQ, i.p.) plus azithromycin (AZI) in drinking water as illustrated in the scheme (a). Bodyweight of mice was measured every 2 days, and tumor size was monitored by luciferase activity every 3 to 4 days. Bodyweight (ration to 1st measurement after randomization) is reported as mean ± SEM (b). Statistical analysis was performed by means of two-way ANOVA test, ***P < .001 as compared to the PBS group (n = 8–11 mice per group). Representative time-lapse images of 3 mice from different groups are reported in (c and d).
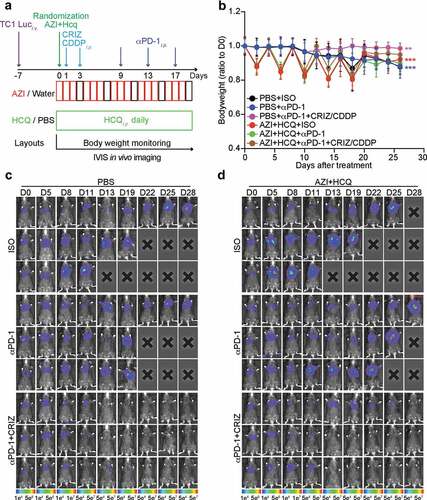
Figure 4. Influence of hydroxychloroquine plus azithromycin on the tumor development control by anticancer chemo-immunotherapy. Mice bearing orthotopic NSCLC were treated with crizotinib (CRIZ) plus cisplatin (CDDP) or equivalent volumes of PBS, and combined or not with monoclonal antibody to PD-1 (αPD-1) or isotype antibody (ISO), either alone or in combination with hydroxychloroquine (HCQ, i.p.) plus azithromycin (AZI) in drinking water. Tumor size was monitored by luciferase activity every 3 to 4 days and quantified as total flux of photons. Individual tumor growth curves are reported in (a, b) Average tumor growth curves (mean ± SEM) of different groups are reported in (c, d) Statistical analysis was performed by means of two-way ANOVA test. Significance are expressed as **P < .01 and ***P < .001 as compared to the PBS group; or ##P < .01 as compared between indicated groups (n = 8–11 mice per group).
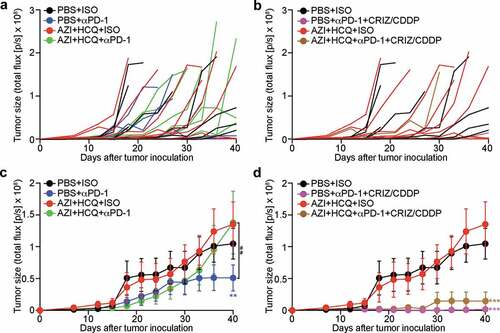
Figure 5. Influence of hydroxychloroquine plus azithromycin on the tumor-free and overall survival of animals treated by anticancer chemo-immunotherapy. Mice bearing orthotopic NSCLC were treated with crizotinib (CRIZ) plus cisplatin (CDDP) or equivalent volumes of PBS, and combined or not with monoclonal antibody to PD-1 (αPD-1) or isotype antibody (ISO), either alone or in combination with hydroxychloroquine (HCQ, i.p.) plus azithromycin (AZI) in drinking water. Tumor incidence was monitored by luciferase activity every 3 to 4 days and mice that lost the diagnostic pulmonary bioluminescence signal were regarded as tumor-free. Tumor-free (total influx of image photons < 16,000) survival rates (a, b) and overall survival rates are reported as Kaplan-Meier curves. Statistical analysis was performed by means of the Likelihood ratio test. Significances are expressed as *P < .05 and **P < .01 as compared to the PBS group (n = 8–11 mice per group).
Discussion
COVID-19 is perceived as a major menace to human health, causing the lockdown of entire countries over several months. Here, we evaluated a possible, but highly controversial treatment option, the combination of HCQ+AZI, for its possible interference with oncological treatments in preclinical models.
HCQ is a lysosomotropic agent, meaning that its physicochemical characteristics (lipophilic, cell membrane permeable weak base) cause it to enrich in acidic cellular compartments, such as lysosomes, endosomes, autophagolysosomes, leading to their alkalization and to a nonspecific interference with viral replication.Citation3 HCQ also causes the inhibition of autophagy,Citation13-Citation15 a cytoprotective mechanism that is essential for cellular stress management,Citation22 explaining why HCQ sensitizes cells to cytotoxic agents.Citation23,Citation24 However, HCQ may also mediate autophagy-independent cell killing through lysosomal membrane permeabilization,Citation13 causing the release of cytotoxic enzymes including cathepsinsCitation25 and secondary mitochondrial membrane permeabilization,Citation25 unleashing the apoptotic cascade.Citation26 In vivo, even high doses of HCQ (50 mg/kg per day) are unlikely to fully inhibit autophagy. Indeed, genetically-induced autophagy inhibition (by inducible knockout or knockdown of the essential autophagy genes Atg5 or Atg7) causes a dramatic acceleration of aging that provokes the degeneration of all internal organs and kills young adult mice in 2–3 months.Citation27,Citation28 No such side effects are known for HCQ treatment, meaning that autophagy inhibition cannot be complete, in spite of the prolonged half-life of the molecule.Citation29 Autophagy induction in cancer cells is required for the induction of immunogenic cell death (ICD) by OXACitation30 or the combination of CDDP+crizotinib,Citation20 meaning that cancer cells in which Atg5 expression has been suppressed do not respond to these combination regimens in vivo.Citation20,Citation30,Citation31 Hence, the fact that HCQ fails to interfere with the efficacy of ICD-based therapeutic regimens might be explained by its incapacity to fully block autophagic flux.
Tumor immunosurveillance and protective immune responses against viruses both require functional type 1 IFN receptor signaling.Citation32,Citation33 However, HCQ was associated with impaired ability of plasmacytoid dendritic cells from systemic lupus erythematosus patients to produce IFN-α and TNF-α upon stimulation with TLR-9 and TLR-7 agonists.Citation34 This might explain why, to some extent, HCQ reduced the efficacy of anti-PD1 Abs as a standalone therapy against our lung cancer tumor. However, given that a sustained type 1 IFN response causes secondary resistance to immune checkpoint blockade,Citation35 HCQ may actually curtail this feed -back loop, as suggested in our combinatorial regimen.
AZI is a macrolide antibiotic. Combinations of broad-spectrum antibiotics (that however did not include macrolides) capable of causing temporary sterilization of the gut interfere with the anticancer effects of OXACitation36 and PD-1 blockade.Citation37,Citation38 There are also clinical reports suggesting that treatment with macrolides or other broad-spectrum antibiotics during the month preceding immunotherapy negatively affect therapeutic responses.Citation39-Citation41 However, in the specific setting that we investigated here, AZI did not interfere with the efficacy of OXA or a combination therapy including PD-1 blockade.
In sum, the preclinical data contained in this work suggest that HCQ+AZI do not interfere with anticancer treatments. Additional work is required to determine whether HCQ+AZI have therapeutic utility against COVID-19 in oncological patients (as suggested for instance in Ref.Citation5 or whether this treatment, which is reputed to cause an increase in the Q-T interval causing potentially lethal arrhythmiaCitation42 should be discontinued).
Materials and methods
Cell lines and reagents
Murine colon adenocarcinoma cell line MC38 was purchased from the American Type Culture Collection (Virginia, MA, USA). Luciferase-expressing murine non-small lung cancer (NSCLC) TC1 Luc+ cell line was kindly shared by Dr T.C. Wu.Citation43 All cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, at 37°C in a humidified incubator supplied with 5% CO2. All cell culture media and supplements were from Gibco-Invitrogen (Carlsbad, CA, USA) and all plastic material was purchased from Corning Costar (Corning, NY, USA). Hydroxychloroquine sulfate (PHR1782), oxaliplatin (Y0000271), cisplatin (C2210000), and crizotinib (PZ0191) were purchased from Sigma-Aldrich (St Louis, MI, USA). Azithromycin was obtained from the local pharmacy (250 mg tablet, Pfizer France, Paris, France). Beetle luciferin (E1601) was purchased from Promega (Madison, WI, USA). Monoclonal antibody to LC3B (#3868) and p62 (#39749) werwas purchased from Cell Signaling Technology (Danvers, MA, USA) and HRP-conjugated monoclonal antibody to β-Actin (ab49900) came from Abcam (Cambridge, UK). Monoclonal antibody to mouse PD-1 (Clone: 29 F.1A12 Catalog#: BE0273) and isotype control antibody (Clone: LTF-2 Catalog#: BE0090) were purchased from BioXcell (Lebanon, NH, USA).
Protein extraction and immunoblot
Naïve female C57Bl/6 mice (6-week old) were treated with hydroxychloroquine and azithromycin for two day2 days as described below. Four hours after the 2nsecond treatment, mice were sacrificed and livers and hearts were snap-frozen in liquid nitrogen. For purification of tissue proteins, 30 mg tissue was immersed in 1 mL of RIPA lysis buffer (#89901, Invitrogen, Carlsbad, CA, USA) in Precellys lysing tubes (#CK28_2 mL, Bertin Technologies SAS, France). The tissues were then dissociated using the Precellys 24 homogenizer (Bertin Technologies SAS) at 6,500 rpm for 60 seconds, and centrifuged at 14,000 g for 15 mins to collect the supernatant that contains soluble proteins. Protein concentration was quantified with the DC Protein Assay (Bio-Rad, Hercules, CA, USA). The protein solution was mixed with 4X loading buffer, and denatured at 100°C for 1 h hour before being used for western blotting.
Forty micrograms of protein were resolved on SDS polyacrylamide gel electrophoresis (PAGE) gels (Invitrogen) and transferred to PVDF membranes (Merck Millipore), which were blocked in TBS containing 0.01% Tween-20 and 5% nonfat dry milk for 1 h before the incubation with primary antibodies overnight at 4°C on a rocking shaker. Membranes were then washed five times with TBST for 10 min each, and incubated with HRP-conjugated secondary antibody for 2 h at room temperature. At last, the membranes were washed five times with TBST for 10 min each and subjected to chemiluminescence-based detection with the Amersham ECL Prime detection reagent kit and the ImageQuant LAS 4000 software-assisted imager (GE Healthcare, Piscataway, NJ, USA). Band quantification was performed using the ImageQuant TL software (GE Healthcare).
In vivo experimentation
All animal experiments have been approved by the ethics committee of Gustave Roussy Cancer Campus (project number: 24771–2020032413235413) and were performed in accordance with governmental and institutional guidelines and regulations. All mice were maintained in a temperature-controlled specific pathogen-free environment with 12-h light/dark cycles, with food and water ad libitum. Six to eight-week-old female C57Bl/6 mice were purchased from Charles River (France) and were adapted for at least 1 week before being used for experiments. All animal experiments were performed once with a number of animals per group sufficient for statistical analysis by means of the freely available software TumorGrowth (https://github.com/kroemerlab/TumGrowth).Citation44
Hydroxychloroquine was intraperitonially administrated at a dose of 50 mg/Kg in 200 uL PBS daily for 3 weeks. Azithromycin was dissolved in autoclaved drinking water at a concentration of 1 mg/Kg or 3 mg/Kg, and the solution was changed daily throughout the treatment period. For the toxicity test, azithromycin was supplied continuously for 5 days; while for the treatment of tumor-bearing mice, it was used in a 3-day on/1-day off mode (3 days with azithromycin in drinking water plus 1 day without). Oxaliplatin (10 mg/Kg), crizotinib (40 mg/Kg), and cisplatin (0.25 mg/Kg) wawere administrated intraperitoneally at day 0 (when mice were randomized) and day 2. Monoclonal antibody to PD-1 or an isotype control antibody was administrated intraperitoneally at day 8, 12, and 16 after the 1sfirst chemotherapy, at a dose of 10 mg/Kg.
Tumor establishment and monitoring
For the establishment of MC38 colon adenocarcinoma, mice were subcutaneously (s.c.) injected with 5 × 105 cells/mouse in 100 mL cold PBS in a low-flow isoflurane anesthesia vaporizer. When the tumor surface (calculated as longest dimension x perpendicular dimension x π/4) reached around 20–30 mm2, tumor-bearing mice were randomized into treatment groups as described above. Tumor surface was then monitored every 3 days and animals bearing neoplastic lesions that exceeded 250 mm2 were euthanized.
To establish the orthotopic NSCLC model, 5 × 105 TC1 Luc+ cells (in 100 µL PBS) were intravenously (i.v.) injected to 6-week old female C57BL/6 mice. Tumor incidence and development were visualized by in vivo photonic imaging of tumor cells’ luciferase activity. Mice were i.p. injected with 150 mg/Kg beetle luciferin and 8 mins later, photons were acquired on aa Xenogen IVIS 50 bioluminescence imager (Caliper Life Sciences Inc., Hopkinton, MA, USA). In vivo imaging was conducted every 3–4 days with an exposure time starting at 4 min, and gradually reduced to 3 min, 2 min, 1 min when photon saturation occurred. When tumors could be detected at 4 min exposure, the tumor bearintumor-bearing mice were randomized into treatment groups as described above. Mice showing photon saturation in less than 1 min of exposure were euthanized.
Disclosure of Potential Conflicts of Interest
G.K. and O.K. are cofounders of Samsara Therapeutics.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
References
- Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–8. doi:10.15698/cst2020.04.216.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020. doi:10.1093/cid/ciaa237.
- Carmona-Gutierrez D, Bauer MA, Zimmermann A, Kainz K, Hofer SJ, Kroemer G, Madeo F. Digesting the crisis: autophagy and coronaviruses. Microb Cell. 2020;7:119–128. doi:10.15698/mic2020.05.715.
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi:10.1016/j.tmaid.2020.101663.
- Rogado J, Obispo B, Pangua C, Serrano-Montero G, Martín Marino A, Pérez-Pérez M, López-Alfonso A, Gullón P, Lara MÁ. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020. doi:10.1007/s12094-020-02381-z.
- Park SJ, Yu KM, Kim YI, Kim S-M, Kim E-H, Kim S-G, Kim EJ, Casel MAB, Rollon R, Jang S-G, et al. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio. 2020;11. doi:10.1128/mBio.01114-20.
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi:10.1136/bmj.m1849.
- Mahevas M, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, Fois E, Lepeule R, Szwebel T-A, Lescure F-X, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi:10.1136/bmj.m1844.
- van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D, Valenza F, de Braud F, De Petris L, Bergh J, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26:665–671. doi:10.1038/s41591-020-0874-8.
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi:10.1172/JCI35180.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi:10.1016/j.ccell.2015.10.012.
- Zitvogel L, Rusakiewicz S, Routy B, Ayyoub M, Kroemer G. Immunological off-target effects of imatinib. Nat Rev Clin Oncol. 2016;13:431–446. doi:10.1038/nrclinonc.2016.41.
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini J-L, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi:10.1128/MCB.25.3.1025-1040.2005.
- Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–666. doi:10.1158/1078-0432.CCR-10-2634.
- Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi:10.1038/nrclinonc.2011.71.
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi:10.1038/onc.2009.356.
- Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi:10.1038/onc.2010.500.
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350:972–978. doi:10.1126/science.aad0779.
- Levesque S, Le Naour J, Pietrocola F, Paillet J, Kremer M, Castoldi F, Baracco EE, Wang Y, Vacchelli E, Stoll G, et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology. 2019;8:e1657375. doi:10.1080/2162402X.2019.1657375.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10:1486. doi:10.1038/s41467-019-09415-3.
- Liu P, Zhao L, Kepp O, Kroemer G. Crizotinib - a tyrosine kinase inhibitor that stimulates immunogenic cell death. Oncoimmunology. 2019;8:1596652. doi:10.1080/2162402X.2019.1596652.
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi:10.1016/j.molcel.2010.09.023.
- Rosich L, Xargay-Torrent S, Lopez-Guerra M, Campo E, Colomer D, Roue G. Counteracting autophagy overcomes resistance to everolimus in mantle cell lymphoma. Clin Cancer Res. 2012;18:5278–5289. doi:10.1158/1078-0432.CCR-12-0351.
- Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V, Agostinis P, Bouche G. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 2017;11:781. doi:10.3332/ecancer.2017.781.
- Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini J-L, Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi:10.1038/sj.onc.1206622.
- Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. doi:10.1038/s41580-019-0173-8.
- Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014;4:914–927. doi:10.1158/2159-8290.CD-14-0363.
- Cassidy LD, Young ARJ, Young CNJ, Soilleux EJ, Fielder E, Weigand BM, Lagnado A, Brais R, Ktistakis NT, Wiggins KA, et al. Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat Commun. 2020;11:307. doi:10.1038/s41467-019-14187-x.
- Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27:771–779. doi:10.1111/j.1365-2125.1989.tb03439.x.
- Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini J-L, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21:79–91. doi:10.1038/cdd.2013.75.
- Wang Y, Xie W, Humeau J, Chen G, Liu P, Pol J, Zhang Z, Kepp O, Kroemer G. Autophagy induction by thiostrepton improves the efficacy of immunogenic chemotherapy. J Immunother Cancer. 2020;8:e000462. doi:10.1136/jitc-2019-000462.
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–414. doi:10.1038/nri3845.
- Kepp O, Senovilla L, Galluzzi L, Panaretakis T, Schlemmer F, Madeo F, Zitvogel L, Kroemer G. Viral subversion of immunogenic cell death. Cell Cycle. 2009;8:860–869. doi:10.4161/cc.8.6.7939.
- Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R155. doi:10.1186/ar3895.
- Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, Ferrere G, Becharef S, Vétizou M, Daillère R, et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 2019;29:846–861. doi:10.1038/s41422-019-0224-x.
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi:10.1126/science.1240527.
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi:10.1126/science.aan3706.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi:10.1126/science.aao3290.
- Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science. 2018;359:1366–1370. doi:10.1126/science.aar6918.
- Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, Belanger K, Miller W, Jamal R, Letarte N, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019;8:e1568812. doi:10.1080/2162402X.2019.1568812.
- Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020. doi:10.1016/j.eururo.2020.04.044.
- Christine MA, Chugh S, Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, Cingolani E,Cheng S, et al. Experience with hydroxychloroquine and azithromycin in the COVID-19 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9:e017144. doi: 10.1161/JAHA.120.017144.
- Chang CL, Tsai YC, He L, Wu T-C, Hung C-F. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer Res. 2007;67:10047–10057. doi:10.1158/0008-5472.CAN-07-0523.
- Enot DP, Vacchelli E, Jacquelot N, Zitvogel L, Kroemer G. TumGrowth: an open-access web tool for the statistical analysis of tumor growth curves. Oncoimmunology. 2018;7:e1462431. doi:10.1080/2162402X.2018.1462431.
