ABSTRACT
After neoadjuvant chemoradiotherapy for esophageal adenocarcinoma, up to 29% of patients have a pathological complete response (pCR). What to do afterward is still under debate. The aim of this prospective study was to define which local markers of immune response might act as predictors of pCR and of recurrence after pCR. The peritumoral healthy mucosa of the surgical specimen was sampled at esophagectomy and analyzed by immunohistochemistry, flow cytometry and Real-Time PCR. One hundred and twenty-three patients received neoadjuvant therapy for esophageal adenocarcinoma and were included in the study. Significantly higher rate of natural killer (NK) cells (CD57+), intraepithelial CD8 + T lymphocytes and degranulating T- and NK-cells (CD107+) were observed in the healthy mucosa of patients with pCR. Moreover, pCR was characterized by a lower immune-check points gene expression level. T-cell activation markers mRNA levels were significantly lower in patients with pCR and recurrent disease, showing an excellent accuracy in the prediction of the postoperative recurrence. Costimulatory molecules mRNA relative levels tended to be lower in patients with pCR and recurrent disease, showing a good accuracy in the prediction of postoperative recurrence in patients with pCR. The immune profile identified in this study might further be tested in large prospective trials as marker of pCR after neoadjuvant therapy and as predictor of recurrence after pCR.
Introduction
Esophageal adenocarcinoma (EAC) is an increasingly common cancer with a poor prognosis. This mainly depends on the fact that most patients present with locally advanced or widespread metastatic disease. Combined modality treatment protocols such as neoadjuvant radiation and/or chemotherapy (CTRT) followed by surgery represent the current treatment option.Citation1,Citation2 Neoadjuvant chemoradiotherapy is commonly proposed to downstage the tumor and enhance the R0 resection rate.Citation3,Citation4 In fact, neoadjuvant therapy can effectively downstage cancer in patients with locally advanced esophageal disease, Citation5,Citation6 and prolonged survival has been observed in patients with a pathological complete response (pCR).Citation7,Citation8 In the CROSS trial, preoperative chemoradiotherapy improved survival among patients with potentially curable esophageal or esophagogastric-junction cancer with acceptable adverse-event rates.Citation9 In this study, pCR rate was 29% in the group of patients who underwent resection after chemoradiotherapy.Citation9
The immune system can specifically identify and eliminate tumor cells based on their expression of tumor-specific antigens or molecules induced by cellular stress.Citation10 In this process, known as tumor immune surveillance or immunoediting, the immune system identifies cancerous and/or precancerous cells and eliminates them before they can cause harm. In colorectal cancers, signs of loss of immune response within are associated with the pathological evidence of early metastatic invasion and with poor survival.Citation11 Thus, there is evidence to support that the immunological data might be a better predictor of patient survival than the histopathological methods currently used to stage colorectal cancer.Citation12 In esophageal cancer, the expression of the co-stimulatory molecule CD80 is significantly down-regulated and inversely correlated with TGF-β1 and IL-10 expression.Citation13,Citation14 Moreover, metastatic esophageal cancer cells are less sensitive to specific cytotoxic lymphocytes.Citation15 In addition, in these neoplasms, CD3+ and CD8+ tumor-infiltrating lymphocytes (TIL) show functional exhaustion and express high levels of PD-1.Citation16 In fact, PD-1 shows a 2- or 3-fold expression in esophageal adenocarcinoma in comparison to normal healthy tissue.Citation17 Furthermore, in esophageal cancer patients, the response to neoadjuvant chemoradiation, as measured according to tumor regression grade (TRG) system, is inversely correlated to the frequency of Tregs invading the tumor and the rarefaction of local Tregs also correlated with overall and cancer-specific survival.Citation17 These are the main reasons leading to immune escapeCitation18 of cancer cells in esophageal cancer and these might be the main potential markers of failure of neoadjuvant therapy.
While it is well established that in cervical esophageal cancer the complete response to CTRT should only be treated surgically in case of recurrence, what to do after complete response in thoracic and abdominal esophageal cancer is still debated.Citation19 Thus, evaluating the immune surveillance markers in the healthy mucosa close to the cancer site in patients with pCR after neoadjuvant CTRT compared to that in non-responders may have a prognostic/predictive role and therefore allow improved patient outcomes. Thus, the aims of this prospective study were to investigate the role of the immune microenvironment molecules in the healthy esophageal mucosa: 1. Before neoadjuvant therapy, as predictor of pCR; 2. After neoadjuvant therapy, as marker of pCR; 3. After pCR, as predictor of recurrence.
Materials and methods
Study design
This is a prospective study on the immune surveillance markers in the esophageal mucosa in patients with esophageal cancer who had undergone neoadjuvant CTRT. The peritumoral healthy mucosa of the surgical specimen was sampled at the end of esophagectomy and a part of the samples was preserved in formalin, another part was snap frozen and then stored at −80°C for RT-PCR, and a third part was used fresh for flow cytometry. The study was conducted according to Helsinki Declaration principles and patients gave their consent to participate in the study. This prospective study was performed as part of a research project on immunosurveillance mechanism in the esophageal and colorectal carcinogenesis (MICCE1) which was approved by the Ethical Committee of Veneto Oncology Institute (IOV-IRCCS).
External explorative cohort
To identify among immune microenvironment markers possible molecular predictors of pCR after neoadjuvant therapy, we explored GEO datasets and we identified as explorative external cohort the series GSE13898 from M.D. Anderson Cancer Center.Citation20 In this study, using DNA microarray, genome-wide gene expression profiling was performed on 75 biopsy samples from patients with untreated EAC. We selected the microarray results from the 28 surrounding normal fresh frozen tissues obtained before the neoadjuvant therapy (28 patients with PR and 6 patients with pCR). We selected the following genes as possible markers of immune microenvironment on the healthy mucosa surrounding the EAC: CD80, CD28, CD38, CTLA4, CD4, CD8alpha, CD8beta, CD107a (LAMP1), CD69, Tbet (TBX21), SERPINB3, TP53, HER2 (ERBB2), PD1 (PDCD1), PD-L1 (CD274), PD-L2 (PDCD1LG2), MLH1, MSH6, MSH3, PMS2, BRAF, IFNγ, FOXP3, CD25 (IL2RA), CD94 (KLRD1), CTLA4 and TNFβ (LT; TNFSF1).
Patients
All 206 consecutive patients presenting with esophageal adenocarcinoma at the Veneto Institute of Oncology in Padua (Italy) between April 2011 and May 2017 were evaluated for inclusion. Forty-seven patients who did not receive neoadjuvant therapy and 36 patients who did not participate to MICCE1 study were excluded. Finally, 123 patients who received neoadjuvant therapy and participated in the MICCE1 study were included in this study ().
Preoperative staging and neoadjuvant therapy
Based on preoperative staging, tumors staged T3N0 or any T N1 (locally advanced esophageal cancer) were considered suitable for neoadjuvant therapy. Patients were evaluated resectable when staged below T3N0 or, after the termination of neoadjuvant treatment, when there was no evidence of distant metastases (M1) or of local invasion of adjacent organs (T4) at restaging. TNM staging was evaluated in accordance with the 7th edition of the TNM classification.Citation21
The most common preoperative chemotherapy regimen consisted of 5-fluorouracil and a platinum agent (standard regimen was 100 mg/m2 DDP on day 1, and 5-FU 1000 mg/m2 per day in continuous infusion from day 1–5 for 3–4 cycles), but taxanes were also prescribed as part of the treatment regimen in some of the patients. Chemotherapy was usually administered concurrently with radiation therapy, but the exact sequence depended on the clinical protocol or on the physician’s preference. Standard radiotherapy was usually performed in 1.8 Gy daily fractions for a total dose of 41.4–45 Gy.
Surgical resection
Details concerning surgical techniques have been published elsewhere.Citation22 Briefly, esophagectomy was performed using an Ivor-Lewis procedure, through laparotomy and right thoracotomy, for tumors of the mid-lower esophagus and esophagogastric junction. At least 6–8 cm of healthy esophagus was resected above the proximal edge of the tumor to avoid neoplastic involvement of the resection margins. In this group of patients, en bloc lymph node dissection was performed. Patients were examined by members of the surgical team after 1, 3, 6, and 12 months and every 6–12 months thereafter. Fresh specimens were immediately placed in ice-cold HBSS, or frozen in liquid nitrogen, or fixed in formalin for subsequent analysis.
Histopathology
Histopathological examination of all resected specimens consisted in evaluation of tumor stage, residual tumor, grading, and number of lymph nodes involved. The specimens were fixed in 10% formaldehyde and set in paraffin. Nodal status (N0, N1) was evaluated in accordance with the 7th edition of the TNM classification, but for the purpose of this study the number of metastatic lymph nodes and their site is also analyzed.Citation21 In patients who previously underwent neoadjuvant therapy and if no neoplastic residue was found in the operative piece, the pathological response was defined complete, with stage ypT0N0 (pCR). In all cases of regression after secondary to neoadjuvant treatment, the score proposed by Mandard, Citation23 was applied (Suppl. Table S1).
Immunohistochemistry
Immunohistochemical (IHC) analyses were performed using standard procedures, and the resulting sections were evaluated by a single pathologist in a blinded fashion. Immunocomplexes were detected using the Dako Real Envision System Peroxidase and 3–3ʹ di-aminobenzidine tetrahydrochloride chromogen as a substrate (Dako) in formalin-fixed paraffin-embedded sections. IHC staining was performed using monoclonal antibodies for CD8 (clone C8/144B, 1:200; Dako), CD107 (LAMP1 marker of degranulating T cells and NK cells; clone H5G11, 1:50; Santa Cruz Biotechnologies) and CD57 (marker of NK cells, 1:300, clone NK-1, Cell Marque). Sections were independently evaluated by a gastrointestinal histopathologist (M.F.) who graded CD8, CD57 and CD107 expressions on a semi-quantitative scale (low and high prevalence of positive inflammatory cells). Furthermore, for all the immunohistochemical markers, the absolute number of positive cells was obtained by considering the mean number of positive cells observed in 5 High Power Field (HPF) (40×). High CD8 infiltration was defined as above 256.5 positive cells in 5 HPF (40x) while high CD107 and high CD57 were defined as above 156 and 3 positive cells in 5 HPF (40x), respectively. These thresholds were defined as the upper quartile values (Supplementary Table S2). The frequency of patients with high markers expression was compared with those of patients with no, low or moderate expression.
Flow cytometry
Esophageal mucosal samples were incubated in HBSS supplemented with 1 mM DTT and 0.5 mM EDTA with shaking at 37°C for 20 min. After washing, the tissues were treated with 1 U/ml dispase (Stemcell Technologies) in DMEM containing 5% FCS at 37°C for 30 min with gentle stirring. Single-cell suspensions were subjected to flow cytometry to determine the proportion of activated CD8 + T cells (positive for CD28 and CD38) and epithelial cells acting as antigen-presenting cells (Cytokeratin+HLA-ABC+ and Cytokeratin+CD80+). Flow cytometric analysis was performed using a FACSCalibur based on CellQuest software (Becton Dickinson, Franklin Lakes, NJ, United States). The antibodies used are summarized in Suppl. Table S3.
RNA extraction and real-time PCR of esophageal mucosa
Briefly, total RNA from frozen esophageal peritumoral mucosa was extracted using the SV Total RNA Isolation System (Promega). This method is based on a lysis-centrifugation process followed by a column filtration through a silica membrane. Briefly, 30 mg of tissue was homogenized in SV RNA Lysis Buffer using the Mixer Mill MM300 (Qiagen). RNA isolation was then performed as recommended by the manufacturer’s protocol. Complementary DNA (cDNA) synthesis was performed using the Applied Biosystems cDNA Synthesis kit according to the manufacturer’s directions. Specific mRNA transcripts were quantified with SYBR Green PCR Master Mix in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). We analyzed the mRNA expression of CD80, CD86 (costimulatory molecules), CD38 and CD69 (markers of lymphocytes activation), TLR4 and MyD88 (markers of innate immunity), TNFα, Tbet (Th1 marker) and IFNγ (Th1 cytokines), PD1, CTLA4 and PD-L1 (checkpoints molecules)., FoxP3 (Treg marker), CD25 (IL2 receptor) and CD8β (high-affinity CD8 receptor). The expression of the target molecule was normalized to the expression of the Actb housekeeping gene. The primers are summarized in Suppl. Table S4.
Statistical analysis
Continuous data were expressed as median and interquartile range (IQR), and categorical data as percentage. Patients who achieved complete response after neoadjuvant therapy were compared with those who did not achieve complete response using Mann-Whitney test (continuous data) and Fisher’s exact test (categorical data). Receiving operating curve (ROC) curve analysis was used to assess the accuracy and the threshold values of the possible predictors with their specificity and sensitivity. All tests were 2-sided and a p-value less than 0.05 was considered statistically significant. Statistical analysis was performed using R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).Citation24
Results
Patient characteristics
One hundred and twenty-three patients (111 males and 12 females; median age 61 y) received neoadjuvant therapy for esophageal adenocarcinoma and participated in this part of the MICCE1 project. Twenty patients had pCR to neoadjuvant CTRT, while the remaining 103 had partial response or no response (pPR). Patient characteristics according to response to neoadjuvant therapy are shown in . Chemotherapy drugs and radiotherapy doses in pCR and pPR groups are shown in Supplementary Table S5.
Table 1. Patient characteristics.
Immunological predictors of pCR after neoadjuvant therapy in normal esophageal mucosa
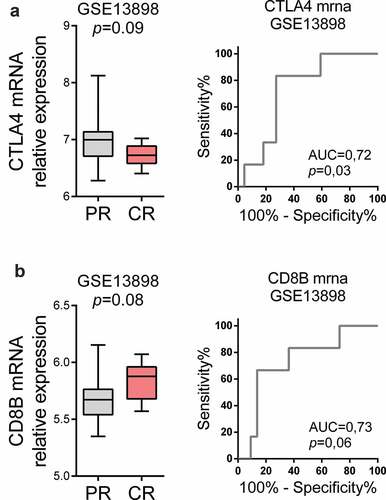
Our first aim was to identify possible predictors of pCR after neoadjuvant therapy for locally advanced EAC in an external series of patients from the M.D. Anderson Cancer Center (Geo series GSE13898). As shown in , in patients who experienced pCR after neoadjuvant therapy, CTLA4 mRNA expression tended to be lower and CD8B mRNA expression tended to be higher than in the peritumoral esophageal mucosa samples of patients who had pCR after neoadjuvant therapy. Moreover, ROC curves demonstrated the good accuracy of CTLA4 and CD8B mRNA in normal esophageal mucosa to predict pCR after neoadjuvant therapy.
Figure 2. Immunological markers in normal esophageal mucosa as predictors of pathological complete response (pCR) after neoadjuvant therapy. (a) CTLA4 and (b) CD8B mRNA expression in the peritumoral esophageal mucosa samples (before neoadjuvant therapy) of Geo dataset GSE13898 was compared in patients who experienced PR vs. patients with pCR afterward. ROC curves are shown to demonstrate the accuracy of CTLA4 and CD8B mRNA in normal esophageal mucosa to predict pCR. Mann-Whitney U test was performed. Data are represented as boxplots showing median and min to max values.
Immunological markers of pCR after neoadjuvant therapy in normal esophageal mucosa
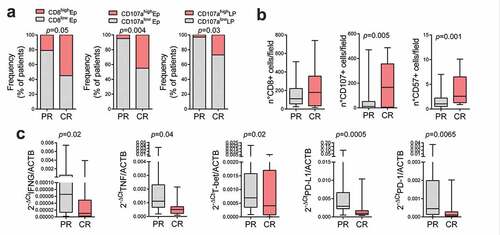
Our second aim was to identify possible immune microenvironment markers of pCR to neoadjuvant therapy in the healthy esophageal mucosa. Patients with high intraepithelial CD8 + T lymphocytes and degranulating cells (CD107+ marker of degranulating T cells and NK cells), quantified by immunohistochemistry, in the healthy esophageal mucosa were more frequent in the group with complete response compared to the group with partial response or progression (p = .05 and p = .004, respectively) ()). Moreover, patients with high degranulating cells (CD107+), in the lamina propria of the healthy esophageal mucosa were more frequent in the group with complete response compared to those with partial response or progression (p = .03) ()). Finally, patients with complete response showed a globally higher number of CD107+ cells (p = .005) but not CD8+ cells ()). On the contrary, healthy esophageal tissue infiltration by CD57 positive cells (NK cell marker) was significantly higher in patients with pCR (p = .001) ()).
Figure 3. Lymphocytes and natural killer (NK) infiltration and activation in the non-cancerous esophageal mucosa after neoadjuvant therapy. Immunological markers in non-cancerous esophageal mucosa specimen of patients with pathological complete response (CR) and patients with partial or no response (PR) after neoadjuvant therapy were compared. (a) Epithelium (Ep) and lamina propria (LP) CD8 and CD107a expression were graded on a semiquantitative scale in the non-cancerous esophageal mucosa specimen of patients with CR and PR, and the frequency of patients with high markers expression (high) was compared with those of patients with no, low or moderate expression (low). Fisher exact test was performed. (b) CD8+, CD107+ and CD57+ cells counts were compared in patients with PR vs patients with CR. (c) IFNG, TNF, T-bet, PD1 and PD-L1 mRNA expression were compared in patients with PR vs patients with CR. Mann-Whitney U test was performed. Data are represented as boxplots showing median and min to max values.
Moreover, at flow cytometry, CD8+/CD28+ and CD8+/CD38+ rates resulted similar in patients with complete response to neoadjuvant therapy and in those with partial response or progression. Epithelial cell acting as nonprofessional antigen-presenting cell rate was similar in the two groups. Flow cytometry analysis is shown in Supplementary Table S6 and supplementary Figure S1.
In the healthy esophageal mucosa, CD80, CD86, MyD88, CD69, TLR4, FoxP3, CD25, CD8β and CD38 mRNA relative levels were similar in patients with complete response to neoadjuvant therapy and in those with partial response or progression (Supplementary Table S7). On the contrary, IFNγ, TNFα, T-bet, PD1 and PD-L1 mRNA expression were statistically lower in patients with pCR than in patients with pPR (p = .02, p = .04, p = .02, p = .0065, and p = .0005, respectively) ()).
Immunological predictors of recurrence after pCR after neoadjuvant therapy in normal esophageal mucosa
Our third aim was to explore the predictive value of the immunosurveillance markers in patients who had a pCR to neoadjuvant therapy, analyzing their levels in patients who had recurred within the first 2 y. We identified 23 patients with tumor regression grade 1 (TRG1), thus, mucosal complete response. Three of them were excluded from this analysis because they had nodal metastasis and four of them recurred in the first 2 y.
No difference in terms of high intraepithelial or lamina propria CD8 + T lymphocytes and degranulating cells (CD107+) was observed in mucosal complete response patients who recurred or not. Similarly, activated CD8+ ve T cell rates as well as epithelial cell acting as antigen-presenting cell rate resulted similar in patients with mucosal complete response who recurred or not.
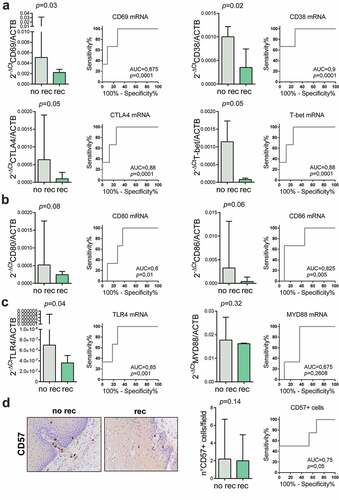
On the contrary, CD38, CD69, CTLA-4, T-bet and TRL4 mRNA relative levels were significantly lower in patients with mucosal complete response who recurred (p = .03, p = .02, p = .05, p = .05 and p = .04, respectively) (,)). ROC curve analysis showed a good accuracy in the prediction of the postoperative recurrence in patients with mucosal complete response (AUC = 0.90 [95%CI: 0.62–0.98], p = .0001; AUC = 0.87 [95%CI: 0.59–0.98], p = .0001; AUC = 0.88 [95%CI: 0.62–0.98], p = .0001; AUC = 0.88 [95%CI: 0.59–0.98], p = .0001; AUC = 0.85 [95%CI: 0.56–0.97], p = .001; respectively). Moreover, CD80, and CD86 mRNA relative levels tended to be lower in patients with mucosal complete response who recurred (p = .08 and p = .06, respectively) ()). ROC curve analysis showed a good accuracy in the prediction of the postoperative recurrence in patients with mucosal complete response (AUC = 0.80 [95%CI: 0.50–0.95], p = .015; AUC = 0.82 [95%CI: 0.53–0.96], p = .004; respectively). Accuracy, specificity and sensitivity of the immunological markers for recurrence after pCR are shown in . MyD88 mRNA relative levels were similar in patients with mucosal complete response who recurred or not ()).
Table 2. Accuracy, specificity and sensitivity of immunological markers for recurrence after pCR.
Figure 4. Immunological markers as predictors of recurrence after pathological complete response (pCR). Immunological markers in non-cancerous esophageal mucosa specimen of patients with pCR who experienced (rec) or not (no rec) disease recurrence were compared. (a) Lymphocytes markers activation (CD69, CD38), exhaustion (CTLA-4) and differentiation (T-bet) mRNA expression and ROC curves are shown to demonstrate the accuracy of the different lymphocytes markers mRNA to predict recurrence after pCR. (b) Costimulatory molecules CD80 and CD86 mRNA expression were compared and ROC curves are shown to demonstrate the accuracy of costimulatory molecules mRNA to predict recurrence after pCR. (c) Innate immunity markers TLR4 and MYD88 mRNA expression were compared and ROC curves are shown to demonstrate the accuracy of innate immunity markers mRNA to predict recurrence after pCR. (d) The number of natural killer cells (CD57+ cells) was compared and ROC curves are shown to demonstrate the accuracy of NK cells number to predict recurrence after pCR. Representative images of CD57 immunohistochemical staining are shown (original magnification 40X). Mann-Whitney U test was performed. Data are represented as median ± IQR.
Discussion
After neoadjuvant chemoradiotherapy plus surgery for esophageal cancer, up to 29% of patients have a pCR in the resection specimen.Citation9 What to do after complete response is still under debateCitation19 but patients seem to be willing to trade a substantial 5-year survival for a reduction in the risk of a possible esophagectomy.Citation25 Thus, it appears essential to have precise prognostic markers to satisfy the patients’ need of avoiding overtreatment. Several studies have focused on this topic. A recent study on circulating tumor cells observed that the positive rate of mesenchymal circulating tumor cells was almost double in the progressive and stable disease group compared to the complete and partial response group.Citation26 Similarly, a different study aimed to examine the value of early changes in quantitative diffusion-weighted imaging and 18 F-fluorodeoxyglucose positron emission tomography/computed tomography for discriminating pCR to chemoradiation in esophageal cancer.Citation27 Moreover, the immune response to the tumor has been investigated to predict the pCR to neoadjuvant therapy. In fact, a higher absolute lymphocyte count during neoadjuvant chemotherapy was observed to be associated with a higher rate of pCR for esophageal cancer patients undergoing trimodally therapy.Citation28
We analyzed the external explorative cohort GSE13898 and, among several immune markers, CTLA4 and CD8β mRNA expression resulted promising immunological predictors of pCR after neoadjuvant therapy in normal esophageal mucosa. CTLA4 is the inhibitor of CD80 activation and it competes with CD28 to decrease the duration of CD80-CD28 binding and thus the duration of the costimulatory effect on antigen-presenting mechanisms. The role of CD80 in esophageal carcinogenesis was observed and well demonstrated in our pervious study.Citation29 A high expression of CTLA4 in esophageal healthy tissue might in part compromise the antigen presentation and, thus, the lymphocyte activation that is necessary for the immune response elicited by radiotherapy.Citation30 On the other hand, CD8β is the marker of CD8 cytotoxic activation and low level of it was observed in patients with ulcerative colitis and who did not manage to eliminate low-grade dysplasia.Citation31 CD8β is the high-affinity receptor of MHC-I and the more is expressed the more efficient is the antigen presentation and the lymphocyte activation. Its low levels may reduce the immune response to EAC elicited by the antigen liberation induced by CTRT.
The current standard of care for restaging after finishing neoadjuvant CTRT is based on endoscopy with (random) bite-on-bite biopsies of the primary tumor site in the esophagus as well as an endoscopic ultrasonography (EUS) with fine-needle aspiration of suspected lymph nodes.Citation32 Thus, immune markers might be easily retrieved at staging or restaging endoscopy to support the ensuing decision-making process. The immune microenvironment in the healthy esophageal mucosa on the endoscopic biopsies might provide a picture of the local immune response to cancer and to CTRT, thus giving further information on cancer progression and on the recurrence risk when cancer tissue is no more available, such in case of pCR. In our series, we observed that high infiltration of intraepithelial CD8 + T lymphocytes and intraepithelial and lamina propria degranulating lymphocytes and natural killer cells (CD107+) were more frequent in patients with pCR compared to those with partial response or progression. However, patients with pCR showed a globally higher number of CD57+ and CD107+ cells but not CD8+ cells suggesting that NK activated cells play a direct role in the pCR and that their high infiltration in the healthy mucosa can be seen as an echo of the past battle.
Our results seem to indicate that pCR is associated with a lower level of immune checkpoints molecules that possibly allowed a more effective immune response to esophageal cancer after neoadjuvant therapy. On the other hand, CD8 and Th1 lymphocytes and their effectors seem not to be associated with pCR. In our opinion, CD8 and Th1 lymphocytes have been directly involved during the immune response to neoadjuvant therapy but after 8 weeks this response might have faded away or even have been consumed in the struggle for pCR, especially in the surrounding healthy mucosa. Similarly, other previous studies have observed that peritumoral CD8+ lymphocytes infiltration in esophageal cancer specimens is not associated with a good prognosis in esophageal adenocarcinomas.Citation33 On the other hand, in our previous study, we had observed that immunonutrition administration before surgery was significantly associated with increased degranulating CD8 and natural killer cells (CD107+) infiltrating the healthy esophageal mucosa.Citation34 Moreover, CD107 was observed to be involved in the TNM stages and histological differentiation of the esophageal squamous cell carcinoma.Citation35 Thus, in our opinion, the predictive value of CD57 and CD107 immunostaining in healthy samples of esophageal mucosa as a marker of pCR after neoadjuvant therapy deserves further investigation and might be possibly tested in large prospective trials.
Currently, despite the standard treatment for those patients that experience recurrence after pCR implies esophagectomy, there is increasing interest from both the patient and provider for active surveillance with so-called “salvage” esophagectomies for local recurrence as an alternative treatment paradigm.Citation36 Therefore, the identification of recurrence markers is of primary interest. In the present work, we observed that CD38, CD69, and Tbet mRNA relative levels were significantly lower in patients with mucosal complete response (TRG1) who recurred. These results suggest that to prevent recurrence after pCR after neoadjuvant therapy for esophageal adenocarcinoma an adequate lymphocyte activation is required. In a recent study, CD4 + T-cells infiltrating esophageal adenocarcinoma tissue displayed a decreased activation profile, with significantly lower CD45RO and CD69 expression compared with normal tissue.Citation37 These data and those obtained in our series seem to suggest that CD38 and CD69 expression in the healthy mucosa surrounding the cancer or in the site of the previous cancer might reveal the immune status of the esophageal mucosa microenvironment, which is directly related to the immune tumor response. Therefore, since the clinical decision-making in this specific patient population includes the accuracy of post-induction clinical restaging, Citation36 these markers might further be tested in a large prospective trial as predictors of recurrence after mucosal complete response. In fact, with improved diagnostic accuracy, nonsurgical treatment can become a solid option for patients identified as cCR after treatment administered in a neoadjuvant setting.Citation38
Finally, TLR4 mRNA relative levels were lower in patients with pCR who recurred, and the ROC curve analysis showed a good accuracy in the prediction of the postoperative recurrence. In recent studies, increased TLR4 expression in cancer tissue associates with advanced stage and poor prognosis in esophageal adenocarcinoma, Citation39 with the pathogenesis of esophageal adenocarcinoma.Citation40 In healthy esophageal mucosa after pCR TLR4 might play in the innate immune activation binding to HMGB1 (High Mobility Group Box 1) released by cancer cell necrosis due to neoadjuvant therapy. The lack of innate immune activation might predispose to esophageal cancer recurrence after pCR.
This study has some limitations. First, it is a single-center study, so the generalization of the findings is limited to similar settings. Second, the small sample size prevented the performance of a multivariable analysis that would have helped in the analysis of possible confounders. The exploratory nature and single Citation41center setting of the study make our conclusion only suggestive and not definitive. A prospective validation cohort study should be specifically set up and concluded before drawing any definitive conclusion on the possible use of these markers in pCR in esophageal adenocarcinoma. In fact, in the series currently available in GEO datasets at the query (Esophageal adenocarcinoma OR EAC) AND (predictor markers in normal tissues OR predictor markers in surrounding esophageal tissues OR predictor markers in surrounding esophageal tumors) there is no series describing the analysis of immune microenvironment in the healthy esophageal tissue after neoadjuvant therapy comparing patients with pCR and those with no or partial response while there are some series describing the microarray results obtained from healthy esophageal tissue before the neoadjuvant therapy.Citation20 The lack of a validation cohort is a clear weakness of the study but, on the other hand, it demonstrates the original point of view of it. Finally, we did not observe any significant difference according to the chemotherapy scheme, but this is possibly due to the small sample size of the subgroups that we obtained.
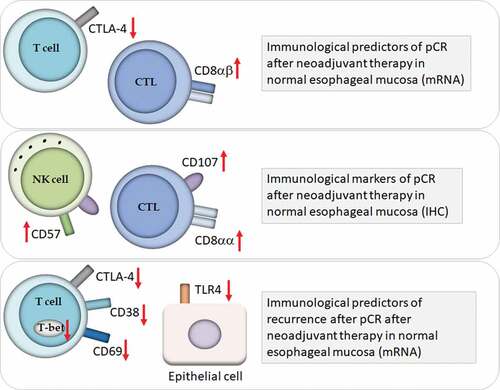
In conclusion, as shown in , CD8 and CD107 immunostaining in healthy samples of esophageal mucosa might be possibly tested as a marker of pCR after neoadjuvant therapy in large prospective trials. CD38 and CD69 mRNA (and possibly CD80 and CD86) expression in the healthy mucosa surrounding the cancer or in the site of the previous cancer deserves further investigation in large prospective trial as predictors of recurrence after mucosal complete response.
Figure 5. Immune surveillance in healthy esophageal mucosa: role in pathological complete response (pCR) after neoadjuvant therapy for esophageal adenocarcinoma. Markers of immune response in the healthy esophageal mucosa that might act as predictors of pCR and of recurrence after pCR. Abbreviations: Cytotoxic T Lymphocytes (CTL); Natural killer (NK) cells; immunohistochemistry (IHC).
Acknowledgments
The authors are extremely grateful to Professor Giuseppe Opocher, Scientific Director of the Veneto Institute of Oncology, for his constant support to this project. The authors are also grateful to Ms. Christina Drace, Scientific Direction Unit, Veneto Institute of Oncology, for her kind help in the final editing of the manuscript.
Disclosure statement
The authors declare no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8(3):226–10. doi:10.1016/S1470-2045(07)70039-6.
- Greer SE, Goodney PP, Sutton JE, Birkmeyer JD. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137(2):172–177. doi:10.1016/j.surg.2004.06.033.
- Mariette C, Triboulet JP. Is preoperative chemoradiation effective in treatment of oesophageal carcinoma? Lancet Oncol. 2005;6(9):635–637. doi:10.1016/S1470-2045(05)70295-36.
- Malaisrie SC, Untch B, Aranha GV, Mohideen N, Hantel A, Pickleman J. Neoadjuvant chemoradiotherapy for locally advanced esophageal cancer: experience at a single institution. Arch Surg. 2004;139(5):532–538; discussion 538–9. doi:10.1001/archsurg.139.5.532.
- Reynolds JV, Muldoon C, Hollywood D, Ravi N, Rowley S, O’Byrne K, Kennedy J, Murphy TJ. Long-term outcomes following neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg. 2007;245(5):707–716. doi:10.1097/01.sla.0000254367.15810.38.
- Zacherl J, Sendler A, Stein HJ, Ott K, Feith M, Jakesz R, Siewert JR, Fink U. Current status of neoadjuvant therapy for adenocarcinoma of the distal esophagus. World J Surg. 2003;27(9):1067–1074. doi:10.1007/s00268-003-7063-z.
- Brücher BL, Stein HJ, Zimmermann F, Werner M, Sarbia M, Busch R, Dittler HJ, Molls M, Fink U, Siewert JR. Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol. 2004;30(9):963–971. doi:10.1016/j.ejso.2004.06.008.
- Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, Wang H, Goldberg M. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–4337. doi:10.1200/JCO.2005.05.017.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al.; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi:10.1056/NEJMoa1112088.
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi:10.1172/JCI31405.
- Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi:10.1056/NEJMoa051424.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139.
- Yang WF, Yu JM, Zuo WS, Wang SZ. Expression of CD80, CD86, TGF-beta1 and IL-10 mRNA in the esophageal carcinoma. Zhonghua Zhong Liu Za Zhi. 2006;28:762–765.
- Yang W, Zhang Y, Yu J, Li S. The low expression of CD80 correlated with the vascular endothelial growth factor in esophageal cancer tissue. Eur J Surg Oncol. 2010 May;36(5):501–506. doi:10.1016/j.ejso.2010.01.007.
- Ichiki Y, Hanagiri T, Takenoyama M, Baba T, Nagata Y, Mizukami M, So T, Sugaya M, Yasuda M, Uramoro H, et al. Differences in sensitivity to tumor-specific CTLs between primary and metastatic esophageal cancer cell lines derived from the same patient. Surg Today. 2012;42(3):272–279. doi:10.1007/s00595-011-0083-7.
- Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J, Zhang X. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50(2–3):269–275. doi:10.1007/s12026-011-8227-9.
- Wagener-Ryczek S, Schoemmel M, Kraemer M, Bruns C, Schroeder W, Zander T, Gebauer F, Alakus H, Merkelbach-Bruse S, Buettner R, et al. Immune profile and immunosurveillance in treatment-naive and neoadjuvantly treated esophageal adenocarcinoma. Cancer Immunol Immunother. 2020 Jan 20;69(4):523–533. doi:10.1007/s00262-019-02475-w.
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017.
- Castoro C, Scarpa M, Cagol M, Alfieri R, Ruol A, Cavallin F, Michieletto S, Zanchettin G, Chiarion-Sileni V, Corti L, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg. 2013;17(8):1375–1381. doi:10.1007/s11605-013-2269-3.
- Kim SM, Park YY, Park ES, Cho JY, Izzo JG, Zhang D, Kim S-B, Lee JH, Bhutani MS, Swisher SG, et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One. 2010 Nov 30;5(11):e15074. doi:10.1371/journal.pone.0015074.
- Edge SB, Bryd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer-Verlag; 2010.
- Ruol A, Portale G, Castoro C, Merigliano S, Cavallin F, Battaglia G, Michieletto S, Ancona E. Management of esophageal cancer in patients aged over 80 years. Eur J Cardiothorac Surg. 2007;32(3):445–448. doi:10.1016/j.ejcts.2007.06.014.
- Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi:10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C.
- R Core Team. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2016.
- Noordman BJ, de Bekker-grob EW, Coene PPLO, van der Harst E, Lagarde SM, Shapiro J, Wijnhoven BPL, van Lanschot JJB. Patients’ preferences for treatment after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg. 2018;105(12):1630–1638. doi:10.1002/bjs.10897.
- Chen W, Li Y, Yuan D, Peng Y, Qin J. Practical value of identifying circulating tumor cells to evaluate esophageal squamous cell carcinoma staging and treatment efficacy. Thorac Cancer. 2018;9(8):956–966. doi:10.1111/1759-7714.12771.
- Fang P, Musall BC, Son JB, Moreno AC, Hobbs BP, Carter BW, Fellman BM, Mawlawi O, Ma J, Lin SH. Multimodal imaging of pathologic response to chemoradiation in esophageal cancer. Int J Radiat Oncol Biol Phys. 2018;102(4):996–1001. pii: S0360-3016(18)30328-6. doi:10.1016/j.ijrobp.2018.02.029.
- Fang P, Jiang W, Davuluri R, Xu C, Krishnan S, Mohan R, Koong AC, Hsu CC, Lin SH. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. 2018;128(3):584–590. pii: S0167-8140(18)30117-8. doi:10.1016/j.radonc.2018.02.025.
- Scarpa M, Fassan M, Kotsafti A, Realdon S, Dall’Olmo L, Morbin T, Cavallin F, Saadeh L, Cagol M, Alfieri R, et al. CD80 expression promotes immune surveillance in Barrett’s metaplasia. Oncoimmunology. 2019 Jul 23;8(10):e1636618. doi:10.1080/2162402X.2019.1636618.
- Ma JL, Jin L, Li YD, He CC, Guo XJ, Liu R, Yang YY, Han SX. The intensity of radiotherapy-elicited immune response is associated with esophageal cancer clearance. J Immunol Res. 2014;2014:794249. doi:10.1155/2014/794249.
- Kotsafti A, D’Incà R, Scarpa M, Fassan M, Angriman I, Mescoli C, Bortoli N, Brun P, Bardini R, Rugge M, et al. Weak cytotoxic T cells activation predicts low-grade dysplasia persistence in ulcerative colitis. Clin Transl Gastroenterol. 2019 Jul;10(7):e00061. doi:10.14309/ctg.0000000000000061.
- Borggreve AS, Mook S, Verheij M, Mul VEM, Bergman JJ, Bartels-Rutten A, Ter Beek LC, Beets-Tan RGH, Bennink RJ, van Berge Henegouwen MI, et al.; PRIDE study group. Preoperative image-guided identification of response to neoadjuvant chemoradiotherapy in esophageal cancer (PRIDE): a multicenter observational study. BMC Cancer. 2018 Oct 20;18(1):1006. doi:10.1186/s12885-018-4892-6.
- Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001 May 15;61(10):3932–3936.
- Scarpa M, Kotsafti A, Fassan M, Scarpa M, Cavallin F, Nardi T, Pinto E, Alfieri R, Cagol M, Agostini M, et al. Immunonutrition before esophagectomy: impact on immune surveillance mechanisms. Tumour Biol. 2017;39(10):1010428317728683. doi:10.1177/1010428317728683.
- Huang J, Li L, Liu J, Yu J, Wu X, Xu Y, Ma M, Wang W, Zhang R. Altered expression of lysosomal associated membrane protein 1 in esophageal squamous cell carcinoma. Pathol Res Pract. 2017;213(8):938–942. doi:10.1016/j.prp.2017.05.008.
- Semenkovich TR, Meyers BF. Surveillance versus esophagectomy in esophageal cancer patients with a clinical complete response after induction chemoradiation. Ann Transl Med. 2018;6(4):81. doi:10.21037/atm.2018.01.31.
- Kavanagh ME, Conroy MJ, Clarke NE, Gilmartin NT, O’Sullivan KE, Feighery R, MacCarthy F, O’Toole D, Ravi N, Reynolds JV, et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 2016;370(1):117–124. doi:10.1016/j.canlet.2015.10.019.
- Ohkura Y, Shindoh J, Ueno M, Iizuka T, Udagawa H. Comparison of outcome of esophagectomy versus nonsurgical treatment for resectable esophageal cancer with clinical complete response to neoadjuvant therapy. Ann Surg Oncol. 2018 Mar 21;(8):2428–2433.
- Huhta H, Helminen O, Lehenkari PP, Saarnio J, Karttunen TJ, Kauppila JH. Toll-like receptors 1, 2, 4 and 6 in esophageal epithelium, Barrett’s esophagus, dysplasia and adenocarcinoma. Oncotarget. 2016;7(17):23658–23667. doi:10.18632/oncotarget.8151.
- Verbeek RE, Siersema PD, Ten Kate FJ, Fluiter K, Souza RF, Vleggaar FP, Bus P, van Baal JW. Toll-like receptor 4 activation in Barrett’s esophagus results in a strong increase in COX-2 expression. J Gastroenterol. 2014;49(7):1121–1134. doi:10.1007/s00535-013-0862-6.
- Mariette C, Piessen G, Lamblin A, Mirabel X, Adenis A, Triboulet JP. Impact of preoperative radiochemotherapy on postoperative course and survival in patients with locally advanced squamous cell oesophageal carcinoma. Br J Surg. 2006;93(9):1077–1083. doi:10.1002/bjs.5358.