ABSTRACT
Secretory leukocyte protease inhibitor (SLPI), a pleiotropic protein expressed by healthy intestinal epithelial cells, functions as an inhibitor of NF-κB and neutrophil proteases and exerts antimicrobial activity. We previously showed SLPI suppresses intestinal epithelial chemokine production in response to microbial contact. Increased SLPI expression was recently detected in various types of carcinoma. In addition, accumulating evidence indicates SLPI expression is favorable for tumor cells. In view of these findings and the abundance of SLPI in the colonic epithelium, we hypothesized SLPI promotes colorectal cancer (CRC) growth and metastasis. Here, we aimed to establish whether SLPI expression in CRC is related to clinical outcome. Using a cohort of 507 patients with CRC who underwent resection of liver metastases, we show that high SLPI protein expression in both liver metastases and primary CRC is associated with significantly shorter overall survival after resection of liver metastases. The prognostic value of SLPI in CRC patients with liver metastases implies a role for SLPI in the formation of metastasis of human CRC. Based on the immune regulatory functions of SLPI, we anticipate that expression of SLPI provides tumors with a mechanism to evade infiltration by immune cells.
Introduction
The pleiotropic protein secretory leukocyte protease inhibitor (SLPI) is constitutively expressed and secreted by human epithelial cells.Citation1,Citation2 SLPI exerts diverse functions, including the ability to act as a potent NF-κB inhibitorCitation3 and inhibit proteases such as neutrophil elastaseCitation4 and also exhibits broad antimicrobial properties.Citation5–7 We previously showed that repetitive microbial contact-induced expression of SLPI in intestinal epithelial cells and that SLPI suppressed chemokine production in response to microbial signals by inhibiting NF-κB activation.Citation8 Thus, SLPI prevents excessive inflammation during intestinal homeostasis.
Tumors frequently modulate the expression of immunomodulatory proteins to evade anti-tumor immune responses. Most investigations of immune invasion in CRC have focused on the interactions between tumor cells and T cells or natural killer (NK) cells.Citation9,Citation10 However, innate immune proteins can also regulate the anti-tumor immune response.Citation11 Increased SLPI protein expression is observed in several types of cancer, including colorectal cancer (CRC), Citation12 gastric cancer, Citation13 non-small cell lung cancerCitation14 and ovarian cancer.Citation15 While the roles of SLPI in tumor formation and progression have not been fully elucidated, multiple human and mouse studies indicate a role for SLPI in the formation of metastases. In particular, in a mouse model of polyclonal breast cancer, clones expressing SLPI entered the vasculature and formed metastases more efficiently than clones that did not express SLPI.Citation16 In addition, SLPI promoted spontaneous lung metastasis in an orthotopic mouse model of breast cancer .Citation17 Moreover, high tumor SLPI mRNA expression was associated with shorter overall survival in patients with triple-negative breast cancerCitation17 and expression of SLPI was associated with poorer five-year overall survival in gastric cancer.Citation13 However, some studies have not reported a tumor-promoting role for SLPI. For example, overexpression of SLPI in lung carcinoma cells reduced the number of liver metastases in a mouse model.Citation18 This protective effect was associated with suppressed production of TNF-α and E-selectin in the liver, suggesting that formation of liver metastases in this model requires a proinflammatory environment.Citation18 Thus, the precise role of SLPI in cancer has not yet been fully elucidated.
The role of SLPI in human CRC has not been investigated. However, SLPI was one of four secreted proteins upregulated in the conditioned medium of a highly metastatic human colorectal cancer cell line compared to the poorly metastatic parental cell line.Citation17 In addition, overexpression of SLPI enhanced tumor growth in murine colon cancer cells.Citation19 In view of the immunoregulatory functions of SLPI in the intestine and its potential tumor-promoting role, we hypothesized that SLPI promotes tumor growth and metastasis in CRC. In this study, we aimed to establish whether expression of SLPI in human CRC metastases is associated with patient survival.
The liver is the most common site of metastasis in CRC; 25–30% of patients with CRC develop colorectal cancer liver metastases (CRCLM).Citation20–22 Approximately 25% of patients with CRCLM are eligible for surgical resection of the affected part of the liver, which is currently the only treatment with curative intent for CRC patients with liver metastases.Citation23,Citation24 However, survival outcomes after resection of CRCLM are highly variable, even among patients with similar clinical and pathology-based risk scores.Citation25 A better understanding of tumor biology may help to predict survival for patients with CRCLM. Using a series of 507 patients with CRC who underwent resection of liver metastases, we show that SLPI protein expression in CRCLM and the matched primary tumors is associated with shorter overall survival.
Materials and methods
Patient cohort and tissue microarray (TMA) generation
Histologically confirmed, formaldehyde-fixed paraffin-embedded (FFPE) CRCLM tissue samples and, when available, samples of the corresponding primary tumor were collected from 507 patients who underwent resection of CRCLM between 1990 and 2010 in seven Dutch hospitals (the DeCoDe PET group), as described previously.Citation26 A previous power calculation indicated a sample size of 361 patients was required for a similar analysis.Citation26 We assumed similar proportions of patients would exhibit low and high SLPI expression (50:50); therefore, we assumed that the sample size of this cohort (n = 507) would be sufficient. Patients with more than one primary tumor were excluded from this study. Tissue microarrays (TMAs) were generated from the original FFPE tissue blocks, according to previously described protocols.Citation27 From every paraffin block, three tissue core biopsies of 0.6 millimeter in diameter were punched from morphologically representative areas and transferred into recipient TMA paraffin blocks. Four-micrometer TMA sections were cut and subsequently mounted onto glass slides. Collection, storage and use of the tissue samples and clinical data were conducted in compliance with the Dutch code of conduct for responsible use of human tissue for medical research.Citation28
SLPI immunohistochemistry
TMA sections were deparaffinized in xylene and rehydrated in alcohol, incubated in 3% H2O2 in PBS for 20 min to quench endogenous peroxidase activity, and antigen retrieval was performed by microwave treatment in citrate buffer (10 mM, pH 6.0). Sections were blocked for one hour at room temperature in Tris buffer (10 mM, pH 8.0) containing 5 mM EDTA (pH 8.0), 0.15 M NaCl, 0.25% gelatin, 0.05% Tween-20 and 10% normal human serum (AB serum; Sanquin, Amsterdam, The Netherlands) plus 10% normal horse serum (Biowest, Nuaillé, France) or 10% normal rabbit serum (Jackson ImmunoResearch, West Grove, PA, USA) matching the species in which the secondary antibody was raised. Subsequently, the sections were stained with either a monoclonal anti-human-SLPI antibody (4 μg/mL, mouse IgG1, HM2037, clone 31; HycultBiotech, Uden, The Netherlands) or polyclonal anti-human-SLPI antibody (1 μg/mL, goat IgG, BAF1274; R&D Systems/Bio-Techne, Minneapolis, MN, USA) in PBS overnight at 4°C. Immunoreactive sites were detected by incubation with a biotinylated horse-anti-mouse antibody (1:500, Vector Laboratories, Burlingame, CA, USA) or biotinylated rabbit-anti-goat antibody (1:500, Vector Laboratories) for 1 hour at room temperature. Biotinylated antibodies were detected using a complex of avidin and biotin (Vectastain ABC Elite Kit, Vector Laboratories) and 3,3ʹ-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, Zwijndrecht, The Netherlands). Sections were counterstained with hematoxylin (Vector Laboratories) and subsequently dehydrated and immersed in xylene.
Scoring of SLPI expression
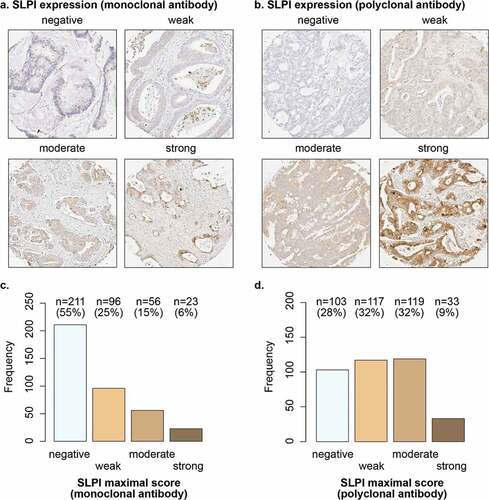
Images of stained sections were digitally captured using an Aperio AT2 scanner (Leica Microsystems B.V., Amsterdam, The Netherlands) equipped with a 20×/0.75 objective (UPlanSAPO; Olympus, Leiderdorp, The Netherlands). The intensity of SLPI protein expression in the cytoplasm of neoplastic epithelial cells was manually scored in a semi-quantitative manner as ‘negative’, ‘weak’, ‘moderate’ or ‘strong’ using the online platform Slide Score (www.slidescore.com). The scoring strategy was designed in consultation with a pathologist and based on the range of SLPI staining intensity observed in tumor cells; all sections were scored by the same investigator. In order to assess the reproducibility of the scoring, a second pathologist independently scored > 20% of the cores stained with the monoclonal antibody and > 20% of the cores stained with the polyclonal antibody, based on images of the scoring categories shown in ) and . Both observers were blinded to the clinicopathological information at time of assessment. The linear weighted kappa values were for 0.62 for the monoclonal antibody and 0.63 for the polyclonal antibody, indicating fair to good interobserver agreement. The scoring method was agreed on by both observers and discrepancies were discussed by the observers.
Figure 1. SLPI is expressed in a subset of CRCLM. Examples of TMA cores of CRCLM stained for SLPI using the monoclonal antibody (a) or polyclonal antibody (b); SLPI staining intensity in tumor cells was scored. The frequencies and percentages of CRCLM scored as ‘negative’, ‘weak’, ‘moderate’ or ‘strong’ after staining with the monoclonal SLPI antibody (c) or polyclonal SLPI antibody (d) are shown; only the maximal score for each patient was included
Statistical analysis
For each tissue type (CRCLM or primary tumor) and anti-SLPI antibody, the maximum score from the one to three TMA cores stained for each patient was used for analysis. Patients for whom none of the three cores were evaluable were excluded from the respective analyses (Supplementary Figure 1). Overall survival was defined as the time after resection of CRCLM until death in months, with a maximum follow-up period of 60 months. Patients who died within 2 months of CRCLM resection were excluded from the analysis, to avoid bias related to death due to surgical complications (Supplementary Figure 1). Patients with missing survival status or follow-up data were also excluded from the analysis (Supplementary Figure 1).
The prognostic value of SLPI protein expression in the liver metastases and primary tumors was evaluated separately by 500-fold cross-validation.Citation26 In short, in each of the 500 cycles, the study population was randomly divided into equally sized training and validation sets. The optimal cutoff for dichotomizing the training set was calculated in every cycle using receiver operating characteristic (ROC) curve analysis for 3-year overall survival. This cutoff was subsequently applied to the validation set to calculate a cross-validated hazard rate ratio (HRR) for 3-year overall survival in univariable Cox regression analysis. In addition, a corrected HRR was calculated in each validation cycle by multivariable Cox regression analysis, which included the following established clinical prognostic factors: number of CRCLM > 1, primary tumor-to-CRCLM interval < 12 months, lymph node positivity at time of CRC diagnosis, and maximal CRCLM diameter > 5.0 cm.Citation29 In both the univariable and multivariable analysis, the average HRR (HRRav) of the 500 HRRs was used and the P-value was calculated based on the percentage of HRR < 1 over the 500 cycles. The relation between SLPI expression and overall survival was visualized by Kaplan Meier curves both before and after dichotomization. Dichotomization for each antibody was performed using the cutoff that was most frequently selected during the automated 500-fold cross-validation procedure. Patients were classified as ‘SLPI-low’ or ‘SLPI-high’ based on the cross-validated cutoffs for each antibody. The log-rank test was used to determine whether overall survival varied significantly between the two groups. In addition, the time points at which 50% of the patients had died (median overall survival) and 95% confidence intervals were calculated for both groups. The clinicopathological features of the SLPI-high and SLPI-low groups were compared using Pearson’s Chi-squared test for categorical variables and the Kruskal–Wallis rank sum test for non-normally distributed continuous variables. The relationship between SLPI detected using the monoclonal antibody and SLPI detected using the polyclonal antibody was examined using Fisher’s exact test (two-sided). The relationship between SLPI expression in liver metastases and the corresponding primary tumors was also tested using Fisher’s exact test (two-sided). All statistical analyses and visualization were performed using R version 3.5.1.Citation30 The ‘survival’,Citation31,Citation32 ‘survminer’,Citation33 ‘pROC’Citation34 and ‘survivalROC’Citation35 packages were employed for survival analysis and cross-validation. The data in this study is reported in compliance with the REMARK recommendations for reporting tumor marker prognostic studies.Citation36
Results
SLPI is expressed in a subset of CRCLM
We assessed SLPI protein expression in CRCLM tissue samples from a Dutch cohort of 507 patients to establish the prognostic value of SLPI in CRCLM. The characteristics of this study population have been described previously.Citation26 To robustly assess the prognostic value of SLPI, we detected SLPI protein expression by immunohistochemistry using two different antibodies: a monoclonal antibody raised against human SLPI purified from sputum and a polyclonal antibody raised against Escherichia coli-derived recombinant human SLPI. CRCLM tissue samples stained with the SLPI monoclonal antibody from 386 patients were available for analysis and CRCLM samples stained with the SLPI polyclonal antibody from 372 patients were available for analysis (Supplementary Figure 1a).
In whole tissue sections of primary CRC samples from 10 patients, SLPI expression was homogeneous within each section, but varied between tumors from different patients (data not shown). SLPI expression was mainly observed in the cytoplasm on the luminal side of the tumor cells ()). We detected expression of SLPI in CRCLM in 45% of patients using the monoclonal antibody and in CRCLM in 72% of patients using the polyclonal antibody (). Overall, SLPI protein expression was detected in the CRCLM samples of a substantial subgroup of patients.
Expression of SLPI in CRCLM is associated with shorter overall survival
In order to assess the prognostic value of SLPI expression in CRCLM, we determined the optimal cutoffs for dichotomization of the cohort using 500-fold cross-validation.
For CRCLM stained with the SLPI monoclonal antibody, the optimal cutoff in all 500 cross-validation cycles was negative vs. weak/moderate/strong SLPI expression (data not shown). The patients were classified as ‘SLPI-low’ or ‘SLPI-high’ based on this cross-validated cutoff. Using this cutoff, high SLPI expression in CRCLM was associated with significantly shorter overall survival compared to low SLPI expression, with an average hazard rate ratio (HRRav) of 1.43 (P = .02; )). Furthermore, patients with high SLPI expression in CRCLM had significantly shorter overall survival after CRCLM resection compared to patients with low SLPI expression (log-rank test: P = .04; ). The median overall survival time for patients with high SLPI expression was 44 months (95% confidence interval: 38–60 months) compared to over 60 months (lower limit of the 95% confidence interval: 52 months) for patients with low SLPI expression in CRCLM ()).
Figure 2. SLPI expression in CRCLM is associated with shorter overall survival. Kaplan-Meier overall survival curves after resection of liver metastases (in months) for the CRCLM study population. Overall survival was stratified by SLPI expression after staining with the monoclonal antibody (a + b) or polyclonal antibody (c + d). Curves without a cutoff (a + c) and with the cutoff calculated from the 500-fold cross-validation procedure (b + d) are shown. P-values were calculated using the log-rank test. The dotted lines represent the time point at which 50% of the group had died (median overall survival time). OS = overall survival
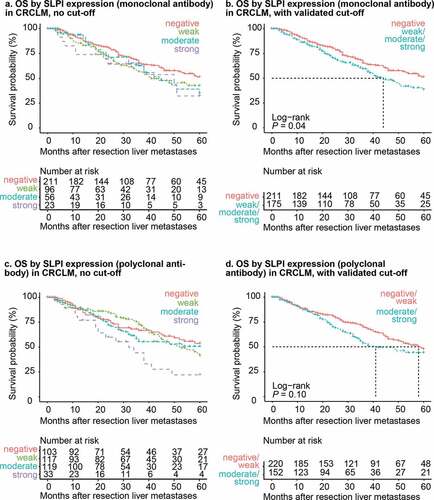
Using the polyclonal antibody, the optimal dichotomization cutoff was negative/weak (low) vs. moderate/strong (high) SLPI expression in all 500 cross-validation cycles (data not shown). Patients with high SLPI expression tended to have shorter overall survival compared to patients with low SLPI expression, though the HRRav of 1.33 was not statistically significant (P = .07; Supplementary Figure 2c). In the survival analysis, overall survival was not significantly different between the SLPI-high and SLPI-low groups for CRCLM stained using the polyclonal antibody (log-rank test: P = .10, ). Median overall survival time was 41 months for the SLPI-high group (lower limit of the 95% confidence interval: 34 months, upper limit more than 60 months) and 58 months (lower limit of the 95% confidence interval: 48 months, upper limit more than 60 months) for the SLPI-low group ()).
Patient age, gender, the location of the primary tumor, the grade of differentiation of the primary tumor, the size of the primary tumor, presence of lymph node metastases, presence of extrahepatic metastases, the interval between the primary tumor diagnosis and detection of liver metastases, the size of the liver metastases and the number of liver metastases were not significantly different between patients with high or low SLPI expression in CRCLM tissues stained with either the SLPI monoclonal antibody or the polyclonal antibody (Supplementary Figure 3a + b).
We also compared the SLPI expression scores for CRCLM from 357 patients stained with both the monoclonal and polyclonal antibody (Supplementary Figure 4a). There was a significant association between detection of high SLPI expression with the monoclonal antibody and detection of high SLPI expression with the polyclonal antibody (two-sided Fisher’s exact test: P < .01). For 74% of patients, both the monoclonal and polyclonal antibody resulted in the same classification, either ‘SLPI-low’ or ‘SLPI-high’ (Supplementary Figure 4a). SLPI expression was more frequently scored as ‘weak’ in CRCLM stained with the polyclonal antibody when no staining was detected using the monoclonal antibody than vice-versa, indicating the monoclonal antibody has a higher threshold of detection for SLPI. In conclusion, patients with high SLPI expression in CRCLM, as detected using the monoclonal antibody, have significantly shorter overall survival compared to patients with low SLPI expression in CRCLM.
SLPI expression in CRCLM has prognostic value independently of established clinical risk factors
Next, we determined whether expression of SLPI in CRCLM has prognostic value independently of established clinical risk factors. The following factors have been demonstrated to be associated with shorter overall survival after resection of liver metastases in patients with CRCLM: more than one CRCLM, a primary tumor-to-CRCLM interval less than 12 months, lymph node positivity at time of CRC diagnosis, a maximal CRCLM diameter > 5.0 cm and a serum carcinoembryonic antigen (CEA) level > 200 ng/mL .Citation29 The prognostic value of these parameters was previously extensively assessed in the current cohort .Citation26 The presence of more than one liver tumor was significantly associated with shorter overall survival. A primary tumor-to-CRCLM interval less than 12 months, lymph node positivity at the time of CRC diagnosis and a maximal CRCLM diameter > 5.0 cm were also associated with shorter overall survival (HRR > 1), though these trends were not statistically significant .Citation26 Serum CEA > 200 ng/mL was not associated with overall survival in this cohort.Citation26 Therefore, we only included more than one CRCLM, a primary tumor-to-CRCLM interval less than 12 months, lymph node positivity at the time of CRC diagnosis, and a maximal CRCLM diameter > 5.0 cm in the multivariable Cox regression model.
Importantly, the prognostic value of SLPI expression detected using the monoclonal antibody was not confounded by these established clinical risk factors; high SLPI expression had a HRRav of 1.63 for overall survival in the multivariable model (P = .02; Supplementary Figure 2b). Detection of high SLPI expression using the polyclonal antibody was also associated with shorter overall survival compared to low SLPI expression in the multivariable model, though this trend was not statistically significant (HRRav 1.37; P = .10; Supplementary Figure 2d). Thus, detection of SLPI expression in CRCLM using the monoclonal antibody was associated with significantly shorter overall survival after surgical resection of liver metastases, independently of other known clinical risk factors.
SLPI is expressed in a subset of primary tumors from patients with CRCLM
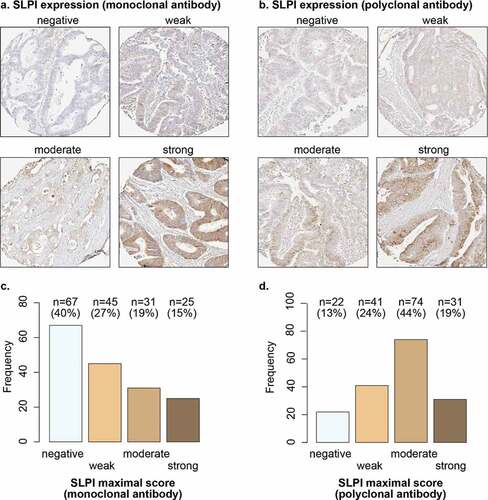
It is currently unknown whether SLPI expression alters during the progression of tumors to metastasis. Therefore, we compared SLPI expression in CRCLM samples and primary CRC tissues; 168 paired CRCLM and the matched primary tumors from the same patients were available for this analysis (Supplementary Figure 1b). We observed similar patterns of SLPI expression in the primary tumor samples and CRCLM samples, with SLPI mainly expressed in the cytoplasm on the luminal side of the tumor cells (). SLPI expression was detected in the primary tumors of 60% of patients using the monoclonal antibody and 87% of patients using the polyclonal antibody ().
Figure 3. SLPI is expressed in a subset of primary tumors from patients with CRCLM. Examples of TMA cores of primary CRC tumors stained for SLPI using the monoclonal antibody (a) or polyclonal antibody (b); SLPI staining intensity in tumor cells was scored. Frequencies and percentages of primary tumors scored as ‘negative’, ‘weak’, ‘moderate’ or ‘strong’ after staining with the SLPI monoclonal antibody (c) or SLPI polyclonal antibody (d) are shown; only the maximal score for each patient was included
Using the monoclonal antibody to detect SLPI expression in primary CRC, the optimal cutoff for dichotomizing the patients was negative (low) vs. weak/moderate/strong (high) in 263 of the 500 cycles (data not shown). Based on this cutoff, the monoclonal antibody detected high SLPI expression in both the primary tumor and CRCLM in 29% of patients and detected low SLPI expression in both the primary tumor and CRCLM in 26% of patients (Supplementary figure 5a). Expression of SLPI in the primary tumor was significantly associated with expression of SLPI in the corresponding liver metastases (two-sided Fisher’s exact test: P < .01). Using the monoclonal antibody, a higher proportion of primary tumors exhibited high SLPI expression than the matched CRCLM (Supplementary figure 5a).
Using the polyclonal antibody to detect SLPI expression in primary CRC, the optimal cutoff for dichotomization was negative/weak (low) vs. moderate/strong (high) in 480 out of 500 cycles (data not shown). In 28% of patients, both the primary tumor and CRCLM expressed low levels of SLPI (Supplementary figure 5b). We detected high SLPI expression in both the primary tumor and CRCLM in 37% of patients. As observed for the monoclonal antibody, high SLPI expression in primary CRC was significantly associated with high SLPI expression in CRCLM using the polyclonal antibody (two-sided Fisher’s exact test: P = .02). Moreover, using the polyclonal antibody, more primary tumors exhibited high SLPI expression than the matched CRCLM (Supplementary figure 5b).
Overall, these results indicate SLPI expression in primary tumor samples is related to SLPI expression in CRCLM samples, which leads to the question of whether expression of SLPI in primary CRC is associated with the prognosis of patients with CRCLM.
SLPI expression in primary CRC is associated with shorter overall survival in patients with CRCLM
In order to establish whether primary tumor SLPI expression has prognostic value in patients with CRCLM undergoing surgical resection of their metastases, we examined the association between SLPI expression in primary CRC and overall survival after CRCLM resection.
Detection of high SLPI expression in the primary tumor using the monoclonal antibody was associated with significantly shorter overall survival after CRCLM resection compared to low SLPI expression, with a HRRav of 1.80 (P = .02, Supplementary figure 6a). Furthermore, in the survival analysis, patients with high SLPI expression in the primary tumor had significantly shorter overall survival after CRCLM resection compared to patients with low SLPI expression in the primary tumor (log-rank test: P = .03; ). The median overall survival time of patients with high SLPI expression in the primary tumor was 46 months after CRCLM resection (lower limit of 95% confidence interval: 32 months; upper limit, > 60 months), compared to > 60 months (lower limit of 95% confidence interval: 52 months) for patients with low SLPI expression ()).
Figure 4. SLPI expression in primary CRC is associated with shorter overall survival in patients with CRCLM. Kaplan-Meier overall survival curves after resection of liver metastases (in months) for the CRCLM study population. Overall survival was stratified by SLPI expression after staining with the monoclonal antibody (a + b) or polyclonal antibody (c + d). Curves without a cutoff (a + c) and with the cutoff calculated from the 500-fold cross-validation procedure (b + d) are shown. P-values were calculated using the log-rank test. The dotted lines represent the time point at which 50% of the group had died (median overall survival time). OS = overall survival
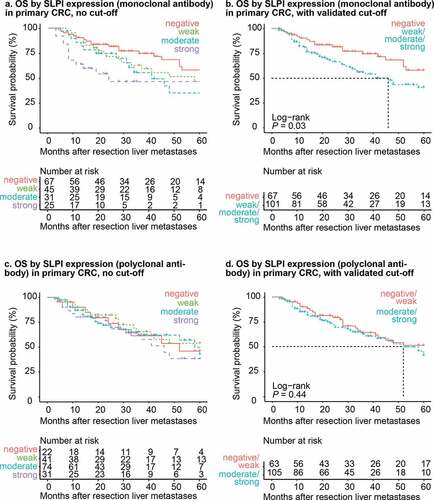
Detection of high SLPI expression in primary tumors using the polyclonal antibody was also associated with poorer overall survival, with an HRRav of 1.25; however, this effect was not statistically significant (P = .24; Supplementary figure 6 c). In addition, in the survival analysis there was no significant difference in overall survival between patients with low and high SLPI expression in the primary tumor detected using the polyclonal antibody (log-rank test: P = .44; ). Moreover, the median overall survival time for patients with high SLPI expression was 52 months (lower limit of the 95% confidence interval: 41 months, upper limit > 60 months), compared to > 60 months (lower limit of the 95% confidence interval, > 60 months) in patients with low SLPI expression based on the polyclonal antibody ()).
Expression of SLPI detected in primary CRC using either the monoclonal or polyclonal antibody was not related to patient age, the grade of differentiation of the primary tumor, primary tumor size, presence of lymph node metastases, presence of extrahepatic metastases, the interval between primary tumor diagnosis and detection of liver metastases, or the size or number of liver metastases (Supplementary Figure 3c + d). However, for primary tumors stained using the monoclonal antibody, the SLPI-high group more frequently had a primary tumor on the left side of the colon compared to the SLPI-low group (Pearson’s Chi-squared test: P = .04; Supplementary Figure 3c). However, we did not observe this association in analysis of primary tumors stained using the polyclonal antibody (Supplementary Figure 3d). In addition, in analysis of primary tumors stained with the polyclonal antibody, more patients in the SLPI-high group were female than in the SLPI-low group (Pearson’s Chi-squared test: P = .03; Supplementary Figure 3d), though we did not observe a similar association using the monoclonal antibody.
We were able to compare the SLPI scores for primary tumors stained using the monoclonal and polyclonal antibody for 157 patients (Supplementary Figure 4b). There was a significant association between detection of SLPI with the monoclonal antibody and the polyclonal antibody (two-sided Fisher’s exact test, P < .01). Moreover, the monoclonal and polyclonal antibodies resulted in the same classification as either ‘SLPI-low’ or ‘SLPI-high’ in 72% of patients, (Supplementary Figure 4b). As observed for the CRCLM, we observed relatively lower SLPI staining scores using the monoclonal antibody than the polyclonal antibody in primary tumors.
In conclusion, detection of high SLPI expression in the primary tumors using the monoclonal antibody was associated with shorter overall survival after surgical resection of liver metastases in patients with CRCLM.
SLPI expression in primary CRC has prognostic value in patients with CRCLM independently of established clinical risk factors
Next, we investigated whether SLPI expression in primary CRC had independent prognostic value in patients with CRCLM by adjusting for previously established clinical risk factors. Using the multivariable model described earlier, detection of high SLPI expression in the primary tumor using the monoclonal antibody was associated with significantly shorter overall survival after resection of liver metastases compared to low SLPI expression (HRRav 1.86, P = .04; Supplementary figure 6b). For primary tumors stained with the polyclonal antibody, high SLPI expression was not significantly associated with shorter overall survival in the multivariable model (HRRav 1.26; P = .28; Supplementary figure 6d).
In conclusion, detection of high SLPI expression in primary tumor samples with the monoclonal antibody was significantly associated with shorter overall survival, independently of established clinical prognostic factors.
Discussion
SLPI is a small protein produced in large quantities by healthy epithelial cells throughout the body. The many functions of SLPI include modulation of the immune response via suppression of chemokine production.Citation8 Recent studies indicated that SLPI drives metastasis in mammary carcinoma,Citation16,Citation17 but the role of SLPI in CRC tumor formation and progression is poorly characterized. In this analysis of a large cohort of patients with CRC who underwent resection of liver metastases, we demonstrate high expression of SLPI in both the liver metastases and primary tumors is associated with shorter overall survival. The prognostic value of SLPI was independent of established clinical risk factors that were previously associated with poorer overall survival in this cohort.Citation26 Therefore, our findings indicate that assessment of SLPI expression could help to predict the prognosis of patients with CRC after resection of liver metastases. Subsequent mechanistic analyses are required to investigate whether SLPI plays a causal role in CRC and may reveal previously unknown mechanisms involved in metastasis.
In the healthy intestine, SLPI prevents tissue damage by inhibiting neutrophil proteases and exerting antimicrobial activity and suppresses infiltration of immune cells to maintain intestinal homeostasis.Citation8,Citation37 Therefore, in CRC, SLPI may predominantly act on the tumor microenvironment, rather than on tumor cell proliferation itself. Most proteins known to mediate immune evasion in CRC, including programmed death ligand 1 (PD-L1) and CEA, act by suppressing T cell or NK cell activation in the tumor niche.Citation9,Citation10 Although the exact functional role of SLPI in CRC remains to be determined, it is likely that the pleiotropic functions of SLPI promote immune evasion via multiple processes. Firstly, as it directly suppresses the production of chemokines by intestinal epithelial cells under homeostasis, SLPI may also directly suppress chemokine gradients and thus prevent recruitment of immune cells to the tumor niche. This effect may be clinically relevant, as the absence of tumor-infiltrating lymphocytes in both primary CRCCitation38 and CRCLMCitation39 is associated with shorter overall survival. Secondly, soluble SLPI can exert generalized immune suppression by acting as an NF-κB inhibitor.Citation40,Citation41 Lastly, the function of SLPI as a protease inhibitor may provide tumor cells with the capacity to defend their niche by inhibiting the protease activity of infiltrating immune cells .Citation4 Moreover, multiple functions of SLPI may promote tumor metastasis. In particular, SLPI enhanced the formation of vessel-like structures and increased the metastatic potential of tumor cells in a mouse model of polyclonal mammary carcinoma.Citation16 The anticoagulative activity of SLPI partly explained the ability of SLPI to drive metastasis in this model.Citation16 Given its diverse activities, SLPI is likely to exert a number of functions in various processes related to tumor growth and metastasis. Future studies are required to elucidate the precise role of SLPI in processes related to the progression and metastasis of CRC, including immune modulation.
Other proteins with significant prognostic value in this cohort are aurora kinase A (AURKA), epidermal growth factor receptor (EGFR), prostaglandin-endoperoxide synthase 2 (PTGS2, also known as cyclooxygenase-2), glucose transporter 1 (SLC2A1) and vascular endothelial growth factor A (VEGFA) .Citation26,Citation42,Citation43 This study demonstrates SLPI expression has similar prognostic value in patients with CRCLM as these proteins. However, SLPI is likely to have a different biological function in CRC compared to these other prognostic proteins. In short, both AURKA and EGFR promote sustained proliferation of tumor cells, SLC2A1 expression is related to anaerobic glycolysis, VEGFA promotes angiogenesis and contributes to induction of regulatory T cellsCitation44 and PTGS2 is involved in both the proliferation and invasion of tumor cells. In addition, PTGS2-derived prostaglandin E2 acts on the tumor niche as it promotes activation of myeloid-derived suppressor cells, and thereby inhibits cytotoxic T cell and NK cell activation.Citation45 We hypothesize that SLPI prevents the recruitment of immune cells to the tumor, in contrast to VEGFA and PTGS2 which suppress the activation of T cells or NK cells in the tumor niche.
SLPI protein expression in both the primary CRC tissues and liver metastases varied substantially between patients. However, SLPI was invariably localized to the cytoplasm, mainly on the luminal side of the tumor cells, which is similar to the expression pattern observed in healthy intestinal epithelial cells .Citation37 The factors that trigger SLPI expression in some tumors, but not in others, remain unclear. We observed SLPI was strongly expressed in a subgroup of primary CRC tumors, and in these cases, high SLPI expression in the primary tumor was positively associated with high SLPI expression in the matched CRCLM, which indicates that SLPI may already be upregulated in non-metastatic tumor cells. Interestingly, SLPI was expressed at relatively higher levels in primary tumors than in the corresponding liver metastases, indicating that SLPI may be downregulated during the progression to metastasis, possibly due to the influence of the local environment in the liver. High SLPI expression in primary CRC was also associated with shorter overall survival after resection of the liver metastases, suggesting that assessment of SLPI expression in primary CRC samples holds prognostic value for patients for whom resected liver metastases are not available.
We used two antibodies to detect SLPI expression, one monoclonal and one polyclonal. We observed the same result using both antibodies: high SLPI expression in CRCLM and high SLPI expression in primary tumors were associated with shorter overall survival after resection of liver metastases in patients with CRCLM. Overall, the monoclonal antibody led to lower SLPI expression scores than the polyclonal antibody, which could be explained by the fact that monoclonal antibodies only recognize one epitope, in contrast to polyclonal antibodies. Indeed, we found that the monoclonal antibody was better at discriminating patients with a poorer prognosis, as the association between SLPI expression and overall survival was only significant for the monoclonal antibody. However, the similar results obtained using the monoclonal and polyclonal antibody strengthen our argument that SLPI protein expression is related to prognosis in CRCLM patients.
In conclusion, high SLPI expression in both CRCLM and primary CRC are associated with a poorer prognosis after resection of liver metastases. Further elucidation of the involvement of SLPI in various tumor-promoting processes may help to identify new targets for cancer therapy. In view of the role of SLPI in intestinal homeostasis, we suggest that SLPI influences the anti-tumor immune response in CRC.
Disclosures
The authors have no conflicts of interest.
Supplemental Material
Download ()Acknowledgments
We thank the DeCoDe PET group for collecting the patient tissue samples, Jan Hudeček for developing and making Slide Score available to us, Menno de Vries for managing the clinical database, Veerle M.H. Coupé for helping to write the script for the 500-fold cross-validation procedure and advice on statistical analyses, and Dimitris Rizopoulos for advice on the statistical analyses.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Maruyama M, Hay JG, Yoshimura K, Chu CS, Crystal RG. Modulation of secretory leukoprotease inhibitor gene expression in human bronchial epithelial cells by phorbol ester. J Clin Invest. 1994 Jul;94(1):368–10. doi:10.1172/JCI117331.
- Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 1994 Dec;11(6):733–741. doi:10.1165/ajrcmb.11.6.7946401.
- Taggart CC, Cryan SA, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O’Neill SJ, McElvaney NG. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med. 2005 Dec 19;202(12):1659–1668. doi:10.1084/jem.20050768.
- Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696.
- Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996 Nov;64(11):4520–4524. doi:10.1128/IAI.64.11.4520-4524.1996.
- Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997 Sep;176(3):740–747. doi:10.1086/514098.
- McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995 Jul;96(1):456–464. doi:10.1172/JCI118056.
- Menckeberg CL, Hol J, Simons-Oosterhuis Y, Raatgeep HR, de Ruiter LF, Lindenbergh-Kortleve DJ, Korteland-van Male AM, El Aidy S, Pieter P E van Lierop PPE, Kleerebezem M, et al. Human buccal epithelium acquires microbial hyporesponsiveness at birth, a role for secretory leukocyte protease inhibitor. Gut. 2014;64(6):(Jul):23.
- Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014 Apr 14;20(14):3738–3750. doi:10.3748/wjg.v20.i14.3738.
- de Vries NL, Swets M, Vahrmeijer AL, Hokland M, Kuppen PJ. The immunogenicity of colorectal cancer in relation to tumor development and treatment. Int J Mol Sci. 2016 Jun 29;17(7):1030. doi:10.3390/ijms17071030.
- Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474. doi:10.1146/annurev-immunol-032414-112043.
- Liu G, Yang J, Zhao Y, Wang Z, Xing B, Wang L, Shi D. Expression of secretory leukocyte protease inhibitor detected by immunohistochemistry correlating with prognosis and metastasis in colorectal cancer. World J Surg Oncol. 2014 Dec 2;12:369–7819-12-369. doi:10.1186/1477-7819-12-369.
- Cheng WL, Wang CS, Huang YH, Liang Y, Lin PY, Hsueh C, Wu Y-C, Chen W-J, Yu C-J, Lin S-R et al. Overexpression of a secretory leukocyte protease inhibitor in human gastric cancer. Int J Cancer. 2008 Oct 15;123(8):1787–1796. doi:10.1002/ijc.23746.
- Ameshima S, Ishizaki T, Demura Y, Imamura Y, Miyamori I, Mitsuhashi H. Increased secretory leukoprotease inhibitor in patients with nonsmall cell lung carcinoma. Cancer. 2000 Oct 1;89(7):1448–1456.
- Shigemasa K, Tanimoto H, Underwood LJ, Parmley TH, Arihiro K, Ohama K, O’Brien TJ. Expression of the protease inhibitor antileukoprotease and the serine protease stratum corneum chymotryptic enzyme (SCCE) is coordinated in ovarian tumors. Int J Gynecol Cancer. 2001 Nov-Dec;11(6):454–461. doi:10.1046/j.1525-1438.2001.01062.x.
- Wagenblast E, Soto M, Gutierrez-Angel S, Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY, Dickopf S et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015 Apr 16;520(7547):358–362. doi:10.1038/nature14403.
- Kozin SV, Maimon N, Wang R, Gupta N, Munn L, Jain RK, Garkavtsev I. Secretory leukocyte protease inhibitor (SLPI) as a potential target for inhibiting metastasis of triple-negative breast cancers. Oncotarget. 2017 Nov 26;8(65):108292–108302. doi:10.18632/oncotarget.22660.
- Wang N, Thuraisingam T, Fallavollita L, Ding A, Radzioch D, Brodt P. The secretory leukocyte protease inhibitor is a type 1 insulin-like growth factor receptor-regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res. 2006 Mar 15;66(6):3062–3070. doi:10.1158/0008-5472.CAN-05-2638.
- Amiano NO, Costa MJ, Reiteri RM, Payes C, Guerrieri D, Tateosian NL, Sánchez ML, Maffia PC, Diament M, Karas R, et al. Anti-tumor effect of SLPI on mammary but not colon tumor growth. J Cell Physiol. 2013 Feb;228(2):469–475. doi:10.1002/jcp.24153.
- Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014 Nov 4;14(1):810–2407-14-810. doi:10.1186/1471-2407-14-810.
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006 Aug;244(2):254–259. doi:10.1097/01.sla.0000217629.94941.cf.
- Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006 Apr;93(4):465–474.
- Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018 Jan 15;18(1):780173925x. doi:10.1186/s12885-017-3925-x.
- Ito K, Govindarajan A, Ito H, Fong Y. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010 Mar-Apr;16(2):103–110. doi:10.1097/PPO.0b013e3181d7e8e5.
- Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: the evolution of determining prognosis. World J Gastrointest Oncol. 2013 Dec 15;5(12):207–221. doi:10.4251/wjgo.v5.i12.207.
- Goos JA, Coupe VM, Diosdado B, Delis-Van Diemen PM, Karga C, Belien JA, Carvalho B, van den Tol MP, Verheul HMW, Geldof AA et al. Aurora kinase A (AURKA) expression in colorectal cancer liver metastasis is associated with poor prognosis. Br J Cancer. 2013 Oct 29;109(9):2445–2452. doi:10.1038/bjc.2013.608.
- Simon R, Mirlacher M, Sauter G. Tissue microarrays. BioTechniques. 2004 Jan;36(1):98–105. doi:10.2144/04361RV01.
- Federation of Dutch Medical Scientific Societies (FDMSS). Human tissue and medical research: code of conduct for responsible use. 2011; https://www.federa.org/code-goed-gebruik-van-lichaamsmateriaal-2011.
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999 Sep;230(3):309–318. discussion 318-21. doi:10.1097/00000658-199909000-00004.
- R Core Team, R Foundation for statistical computing, vienna, austria. r: a language and environment for statistical computing. 2018; https://www.R-project.org, version 3.5.1
- Therneau TA Package for survival analysis in S. 2015. https://CRAN.R-project.org/package=survival, version 2.38
- Therneau TM, Grambsch PM. Modeling survival data: extending the Cox 3. New York: Springer; 2000. ISBN 0-387-98784.
- Kassambara A, Kosinski M, Biecek P survminer: drawing survival curves using ‘ggplot2ʹ. 2019. https://CRAN.R-project.org/package=survminer, version 0.4.6
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011 Mar 17;12:77–2105-12-77. doi:10.1186/1471-2105-12-77.
- Heagerty PJ, Saha-Chaudhuri P survivalROC: time-dependent ROC curve estimation from censored survival data. 2013. https://CRAN.R-project.org/package=survivalROC, version 1.0.3
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005 Aug 22;93(4):387–391. doi:10.1038/sj.bjc.6602678.
- Si-Tahar M, Merlin D, Sitaraman S, Madara JL. Constitutive and regulated secretion of secretory leukocyte proteinase inhibitor by human intestinal epithelial cells. Gastroenterol. 2000 Jun;118(6):1061–1071. doi:10.1016/S0016-5085(00)70359-3.
- Pagѐs F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009 Dec 10;27(35):5944–5951. doi:10.1200/JCO.2008.19.6147.
- Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, Jarnagin W, Fong Y, Blumgart L, D’Angelica M, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009 Sep;16(9):2524–2530. doi:10.1245/s10434-009-0585-3.
- Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007 Mar;8(3):294–303. doi:10.1038/ni1434.
- Ding A, Thieblemont N, Zhu J, Jin F, Zhang J, Wright S. Secretory leukocyte protease inhibitor interferes with uptake of lipopolysaccharide by macrophages. Infect Immun. 1999 Sep;67(9):4485–4489. doi:10.1128/IAI.67.9.4485-4489.1999.
- Goos JA, de Cuba EM, Coupé VM, Diosdado B, Delis-Van Diemen PM, Karga C, Beliën JAM, Oordt CWM, Geldof AA, Meijer GA, et al. Glucose transporter 1 (SLC2A1) and vascular endothelial growth factor A (VEGFA) predict survival after resection of colorectal cancer liver metastasis. Ann Surg. 2016 Jan;263(1):138–145. doi:10.1097/SLA.0000000000001109.
- Goos JA, Hiemstra AC, Coupé VM, Diosdado B, Kooijman W, Delis-Van Diemen PM, Karga C, Beliën JAM, Menke-van der Houven van Oordt CW, Geldof AA et al. Epidermal growth factor receptor (EGFR) and prostaglandin-endoperoxide synthase 2 (PTGS2) are prognostic biomarkers for patients with resected colorectal cancer liver metastases. Br J Cancer. 2014 Aug 12;111(4):749–755. doi:10.1038/bjc.2014.354.
- Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013 Jan 15;73(2):539–549. doi:10.1158/0008-5472.CAN-12-2325.
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007 May 1;67(9):4507–4513. doi:10.1158/0008-5472.CAN-06-4174.
