ABSTRACT
We had conducted phase 1/2 studies of cancer vaccination therapy using neo-tumor antigens in patients with refractory/persistent cervical cancer (CC) and ovarian cancer (OC) to assess the feasibility and efficacy. Enrollees must be refractory/persistent disease for usual treatments with Human Leukocyte Antigen-A*0201 or A*2402. The targets were epitope peptides obtained from driver genes in surviving pathways as follows: for CC A*0201, peptides from Up Regulating Lung Cancer 10 gene (URLC10) and Hypoxia-inducible gene 2 (HIG-2) and for OC A*0201, HIG2, VEGFR (vascular epithelial growth factor receptor) 1 and 2 were used. For CC A*2402, Forkhead Box M1 (FOXM1), Maternal Embryonic Leucine zipper Kinase (MELK), and Holliday Junction Recognition Protein (HJURP) were used. For OC A*2402, cocktails of peptides from FOXM1, MELK, HJURP, VEGFR1, and VEGFR2 were used. Subcutaneous administration was performed with adjuvant weekly. The toxicity profiles and tumor-response were analyzed in eight-week interval. Sixty-six patients were accrued, and 64 were evaluable for adverse events (AEs), and 35 for response. AEs of G2/3 dermatologic reaction (DR) of injection site had been identified in 15.6% and no other severe AEs were detected. Response rate in OC and CC were 22.9% and 20%, respectively. Median overall survival showed longer in performance status (PS) 0 (versus PS1/2), in CRP negative (versus positive) and in DR positive (versus negative) such as 8.7 m versus 1.2 m (p < .001), 8.8 m versus 3.0 m (p < .05) and 10.2 m versus 1.2 m (p < .001), respectively. In conclusion, our vaccination therapy was feasible and effective in this cohort of patients.
Introduction
Immunotherapy has been gaining importance in the treatment of cancer, particularly advanced-stage cancer. Currently, immunotherapy is recognized as a promising fourth standard therapy for patients with refractory disease despite good performance status (PS). The main immunotherapy agents are immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4),Citation1 programmed cell death protein-1 (PD-1),Citation2 and programmed death-ligand 1 (PD-L1).Citation3 However, in patients with platinum-refractory ovarian cancer, the response rates to ICIs are not satisfactory, and failed to 8% to 20%.Citation4,Citation5 Furthermore, in a phase 3 study of nivolumab versus chemotherapy in patients with platinum-resistant recurrent OC, nivolumab did not improve overall survival (OS) compared with chemotherapy (gemcitabine or pegylated liposomal doxorubicin) (ESMO 2020).Citation6
To overcome this limitation, multiple peptide cocktail vaccines started being explored. Peptide vaccines contain human leukocyte antigen (HLA)-restricted tumor-specific antigens (epitope peptides) combined with an incomplete Freund’s adjuvant such as the Montanide ISA 51 VG. The epitope peptides were first identified in a genome-wide association study (GWAS).Citation7 Since then, peptide vaccines working through the activation of the intrinsic pathway of CTL-mediated apoptosis have been reported in gastric cancer,Citation8–10 lung cancer,Citation7 pancreatic cancer,Citation11,Citation12 esophageal cancer,Citation13 colorectal cancer,Citation12 and head and neck cancer.Citation14
From the immunotherapy and precision medicine points of view, we selected new tumor-specific antigens that are key drivers of tumor escape mechanisms in refractory/chemoresistant tumors. We have previously conducted phase 1 and phase 2 trials to study the safety and efficacy of peptide vaccines containing these new tumor-specific antigens in patients with refractory, treatment-resistant OC and cervical cancer (CC). We have previously reported the results of the phase 1 trial, including the doses with good safety and efficacy profiles.Citation15 Here, we report the results of the phase 2 trial.
Patients and methods
Study design
This study was a prospective, exploratory, non-randomized, single-arm phase 2 trial. All patients received treatment with the test vaccines.
Ethical matters and trial registration
Protocols and informed consent forms were approved by the institutional review board (IRB; Iwate Medical University IRB#1 approved by the US Federal Wide Assurance for the Protection of Human Subjects). After being informed of the details of the clinical trial using approved documents, all patients provided written informed consent. Patients were assigned a number and their anonymity was preserved. Their personal data were kept confidential in a correspondence table that remained in possession of our department’s data protection administrator.
The clinical trials have been registered with UMIN at https://www.umin.ac.jp/ctr/(UMIN IDs: 000003860, 000003862, 000003902, and 000003903).
Study setting
The study was conducted at the Department of Obstetrics and Gynecology of the outpatients’ clinic of Iwate Medical University School of Medicine, Morioka, Iwate, Japan.
Study period
The accrual period was scheduled to last 3 years, and the observation period was scheduled to last 5 years. Patient accrual was initiated in June 3, 2010 (as approved by the IRB) and ended in March 31, 2016 for patients with CC and in July 10, 2017 for patients with OC. The last treatment intervention was performed in July 10, 2017. Patients were followed until death. The last observation was made on April 30, 2018.
Sample size
The phase 1 trial included 6 patients per group, and dose increments followed the modified Fibonacci method. The phase 2 trial included 30 patients per group. According to the literature, the response rate of patients with platinum-resistant OC or CC to immunotherapy is 20%, at best. In this study, the threshold response rate was set at 20% and the expected response rate was set at 40%. Expected prolongation of time to progression and expected prolongation of OS were each set at 9 months. Expected progression-free time (PFS) was set at 15 months, and OS was set at 24 months. Based on these parameters, an α-level of 0.05 in a one-tailed test, and a power of 80%, the sample size was calculated to be 22 patients. However, we included a total of 30 patients per group.
Patient eligibility and classification criteria
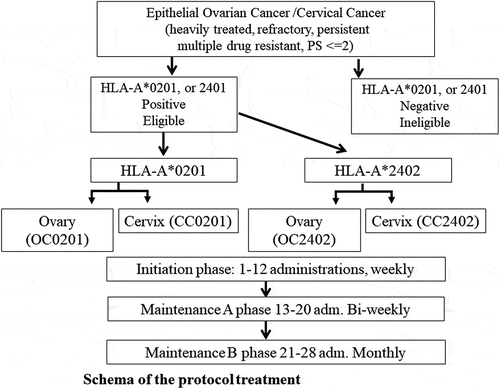
Subjects were patients who had received all possible treatments and had thus been recommended best supportive care (BSC) alone. Patients had histologically confirmed advanced and/or recurrent CC or OC, for which they received more than three lines of chemotherapy after standard treatment. Their Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) was 0–2. Patients were classified into four groups according to the combination of disease and HLA-A* subtype positivity: patients with CC and HLA-A*0201 positivity (CC02 group); patients with CC and HLA-A*2402 positivity (CC24 group); patients with OC and HLA-A*0201 positivity (OC02 group); and patients with OC and HLA-A*2402 positivity (OC24 group).
Inclusion criteria
The inclusion criteria were as follows: age 20–80 years old; an absolute neutrophil count (ANC) of 2,000–10,000/mm3 to exclude complications of inflammatory disease (inflammatory disease is known to induce regulatory T cells); and a 28-day interval between any previous treatment and patient accrual.
Exclusion criteria
The exclusion criteria were as follows: HLA-A*0201 or HLA-A*2402 negativity; double cancer, with the exception of synchronous or anachronous nonmalignant melanoma skin cancer; life-threatening disease, including another active cancer or brain metastasis; use of systemic steroids or other immunogenic agents such as Chinese herbal medicines or biological response modifiers such as some fungi, alternative therapies, and substitutional foods; and concurrent combination treatment (surgery, chemotherapy, and radiotherapy), with the exception of treatment for ascites or pleural effusion.
Study endpoints
The primary endpoint was safety and the secondary endpoints were clinical response, immunological evaluation using the enzyme-linked immunospot (ELISpot) assay, and patient outcomes. OS was defined as the period between patient accrual and the last known day of survival. In patients who did not receive further chemotherapy or radiotherapy, we also determined whether peptides vaccines could prolong OS and investigated the predictive and prognostic factors for longer OS.
Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0, and tumor response was assessed using the Immune-related Response Evaluation Criteria in Solid Tumors (ir-RECIST).Citation16 Dose-limiting toxicities (DLTs) were considered grade 4 (G4) hematologic toxicities; non-hematologic toxicities, except nausea, vomiting, and dermatologic reactions, were considered grade 3 (G3) or more. The immunological response was assessed using peripheral blood mononuclear cells (PBMCs) in the ELISpot assay.
Tumor response was assessed by spiral computed tomography (CT) sequences with enhancement according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and ir-RECIST. Best response, as evaluated by RECIST or ir-RECIST, was illustrated on waterfall and spider plots. Response rate was defined as the sum of complete response (CR) and partial response (PR) among all treated patients. Disease control rate was defined as the sum of CR, PR, and stable disease among all treated patients.
All evaluations were performed at every 9th administration.
Methods
Depending on the results of the HLA-A* locus, two, three, or five multiple tumor-associated HLA-A*0201- and HLA-A*2402-restricted epitope peptides were used as vaccines in combination with Montanide ISA 51 VG (SEPPIC Co. Ltd., Paris, France). Vaccines were administered subcutaneously. In the phase 1 trial, dose increments followed the modified Fibonacci method; therefore, in the phase 2 trial, the first six patients in each group were those of the phase 1 trial.
Peptide vaccines were as follows: for the CC02 group, we used two peptide vaccines containing epitope peptides from up-regulating factor of lung cancer 10 (epURLC10)-A*02-10-211 (LLLASIAAGL)Citation17–20 and hypoxia-inducible gene 2 (epHIG2)-A*0201-9-4 (VLNLYLLGV) ;Citation21–24 for the CC24 group, we used three peptide vaccines containing epitope peptides from forkhead box M1 (epFOXM1)-A*24-9-262 (IYTWIEDHF),Citation25–28 maternal embryonic leucine zipper kinase (epMELK)-A*24-9-87-7 N (EYCPGGNLF),Citation29–34 and Holliday junction recognition protein (epHJURP)-A*2402-9-408 (KWLISPVKI) ;Citation35–38 for the OC02 group, we used peptide vaccines containing epHIG2 and epitope peptides from vascular endothelial growth factor receptor 1 (epVEGFR1)-A*0201-9-770 (TLFWLLLTL) and vascular endothelial growth factor receptor 2 (epVEGFR2)-A*0201-9-773 (VIAMFFWLL); for the OC24 group, we used peptide vaccines containing epFOXM1, epMELK, epHJURP, epVEGFR1-A*2402-9-1084, and epVEGFR2-A*2402-9-169 ().Citation15 All epitope peptides had good manufacturing product (GMP) quality; 1 mg of each epitope peptide was mixed aseptically with 1 ml of Montanide ISA 51 VG.
Table 1. Epitope peptides and their expression profile in clinical samples
Patients negative for HLA-A*0201 or HLA-A*2402 received BSC at our institution or were referred to another institution or hospital. The OS of patients receiving BSC was used as a control (if possible).
Vaccination schedule
The vaccination schedule included 12 consecutive subcutaneous injections administered weekly followed by 8 injections administered bi-weekly (initiation phase or phase A). The schedule was continued with monthly injections beyond disease progression (phase B). After 1 year, the frequency of vaccine administration was monthly or every 3 or 4 months as per patient’s choice ().
Figure 1. Schema of the process of protocol treatment: Vaccination intervals and number of administrations (adm.): After classification of patient groups, peptides vaccination was performed in three phases. First, twelve weekly administrations were initiation phase. Next eight administrations were biweekly adm up to 20 as maintenance A phase. Further 8 adm were monthly adm (maintenance B). After A and B maintenance, further adm was performed every 3–4 month as patients’ needs. Maximum number of administrations was 48 times
ELISpot assay
To monitor antigen-specific immune responses, we performed the ELISpot assay using the human interferon (IFN)-γ ELISpotPLUS kit (Mabtec, Nacka Strand, Sweden).Citation13 Briefly, 96-well plates with nitrocellulose membranes (Millipore, Molsheim, France) were pre-coated with primary anti-IFN-γ antibody (1-DIK) overnight at 4 ºC. The plates were then pre-reacted with RPMI medium containing 10% fetal bovine serum (FBS; Invitrogen). For HLA-A-positive groups, pulsed TISI cells (stimulators; 2 × 104) were incubated with each epitope peptide (10 μg/ml), the human immunodeficiency virus (HIV)-specific peptide (ILKEPVHGC, 10 μg/ml), or the HIV-specific peptide (RYLRDQQLL, 10 μg/ml) and responder cells (from 2 × 104 to 2.5 × 103/well) in a total of 200 μl/well at different stimulator/responder cell ratios for 24 h in triplicate. Stimulation with phorbol 12-myristate 13-acetate (PMA, 25 ng/ml; Sigma-Aldrich, St. Louis, MO) plus ionomycin (500 pM; Sigma Aldrich) was used as a positive control for T-cell activity. For HLA-A-negative groups, responder cells (2 × 104/well) were incubated with each epitope peptide (10 μg/ml) or HIV-specific peptide (10 μg/ml) in a total of 200 μl/well without antigen-presenting cells for 24 h in triplicate, using PMA+ ionomycin as a positive control. Cells were treated with biotinylated secondary anti-IFN-γ antibody (7-B6-1) for 2 h. Then, they were incubated with horseradish peroxidase and stained with 3,3ʹ,5,5ʹ-tetramethylbenzidine (TMB; Mabtech). Immunospots were quantified with the ImmunoSpot S4 auto-analyzing system (Cellular Technology Limited). Antigen-specific T-cell response was quantified according to our original evaluation tree algorithm. In brief, peptide-specific spots were the average of triplicates calculated by subtracting the HIV peptide-pulsed well from the immunized peptide-pulsed well. Then, antigen-specific T-cell response was classified into four grades (-, +, ++, and +++) depending on the number of peptide-specific spots and invariability of peptide-specific spots at different stimulator/responder cell ratios. When the algorithm indicated +, ++, or +++ at either the 5th or 10th vaccine administration, we judged it as positive.
Data collection and statistical analysis
Predictive and prognostic factors were analyzed using the uni- and multi-non-parametric Cox hazard method, and Kaplan–Meier curves were analyzed using the log-rank method. Stat View J 5.0 (SAS, NC, US) and R 3.3.1 software were used for Kaplan–Meier curve and OS univariate and multivariate analyses by the Cox proportional hazard model.
Hazard risk for OS was analyzed by univariate and multivariate non-parametric proportional hazard tests in various subgroups: age (<50 years versus ≥50 years), ECOG-PS (0 versus 1 and 2), tumor size (<2.0 cm, 2.0–10.0 cm, and >10.0 cm), baseline ANC (<5000/mm3 versus ≥5000/mm3), baseline peripheral lymphocyte count (<2000/mm3 versus ≥2000/mm3), neutrocyte-to-lymphocyte ratio (NLR),Citation39 platelet-to-lymphocyte ratio (PLR),Citation40 baseline C-reactive protein (CRP) value (0 versus]0–2.0[or ≥2.0), HLA*A subtype (A*0201 versus A*2402), disease (OC versus CC), histological subtype in CC (squamous cell carcinoma versus adenocarcinoma), histological subtype (high-grade serous carcinoma versus others), and dermatologic reactions (positive [1+, 2+, 3+] versus negative). Data were collected and accumulated by the data manager in a file with accessible anonymization. Data were monitored by a third-party academic research organization (ARO), Captivation Network (http://ccpvc.org/; Kou Obara, MD, PhD).
Results
Patient accrual
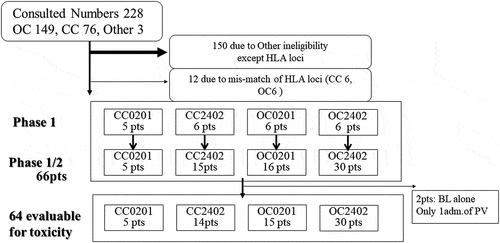
From October 1, 2010 to March 31, 2016, 230 patients had an appointment (149 OC, 76 CC, 1 primary peritoneal cancer, and 2 other cancers). In the CC02 group, the accrual was five patients; these patients had no treatment-related adverse events and the phase 1 trial has been completed.Citation15 In the CC24, OC02, and OC24 groups, the accrual was 15, 16, and 30, respectively (). Observation was continued up to April 30, 2018; four patients were still alive with disease.
Figure 2. Diagram of patients’ accrual: eligible patients and evaluable patient’s numbers
Patient characteristics
Of the 66 patients included in the study, two received only one vaccine administration because they could not visit our hospital due to disease progression (deterioration of PS). Therefore, adverse events were analyzed in 64 patients. Finally, tumor response was analyzed by ir-RECIST in 35 patients with OC and 15 patients with CC using multidetector CT. OS was analyzed in all 66 patients.
Patient characteristics are shown in . Most had previously received multiple treatments, and the median number of previous chemotherapy regimens was 5 and 3 in patients with OC and CC, respectively. The frequency of PS 0 was 31/46 (67.4%) among patients with OC and 16/20 (80%) among patients with CC. In patients with OC, the most common tumor subtype was high-grade serous carcinoma (32/46 [69.6%]), followed by clear-cell carcinoma (7/46 [15.2%]). In patients with CC, the most common tumor subtype was squamous cell carcinoma (11/20 [55%]), followed by adenocarcinoma (including a special subtype of adenosquamous carcinoma and gastric-type adenocarcinoma; 9/20 [45%]).
Table 2. Patients’ characteristics
Toxicity profile
No hematologic adverse events other than lymphocytopenia were observed (). Peripheral lymphocyte counts decreased in 43/64 (67.2%) patients, including 10 patients with hematologic toxicities over G3 (15.6%). No anemia was observed.
Table 3. Hematologic and non-hematologic toxicity profiles concerning peptide vaccine (NCI-CTCAE v.3.0)
Table 4a. CC 0201 and 2402: ELISPOT positivity in all case (Baseline and total most strong intensity during protocol treatment)
Table 4b. OC 0201: ELISPOT positivity in all case (Baseline and total most strong intensity during protocol treatment)
Table 4c. OC 2402: ELISPOT positivity in all case (Baseline and total most strong intensity during protocol treatment)
As for non-hematologic toxicities, some dermatologic reactions such as pain, itching, redness, swelling, induration, and ulceration, were detected. Five out of 64 (7.8%) patients had G3 ulceration and needed treatment by a dermatologist. Fifty-one out of 64 (79.7%) patients had adverse events over G1.
Response to treatment
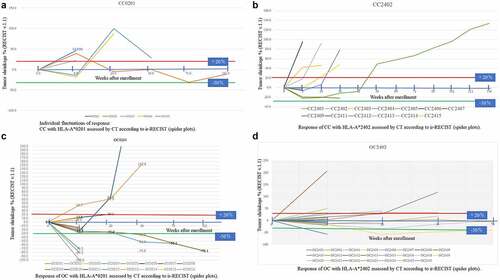
All patients with measurable disease were evaluated by CT at every 9th administration. Tumor response was assessed using the RECIST version 1.1 and ir-RECIST, and only a few patients with small lesions or peritoneal disease were evaluated by positron emission tomography–CT. Tumor response is illustrated by spider plots (). There were fluctuations in tumor size (first an increase, followed by a gradual decrease in size), indicating an immune response to the tumor.
Figure 3. Individual fluctuations of response assessed by CT according to ir-RECIST (spider plots).: 3-a: CC with HLA-A*0201, 3-b: CC with HLA-A*2402, 3-c: OC with HLA-A*0201, 3-d: OC HLA-A*2402. Red line showed 20% increase and green line was 30% decrease
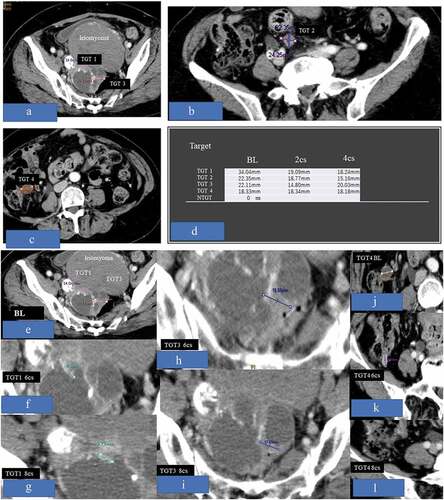
shows a representative case of the OC02 group (case OC0209). At baseline, four targets were detected without non-target lesion. However, TGT-2, which was initially thought to be a metastatic tumor of the mesenterium, was finally proven to be the small intestine. TGT-1 and TGT-4 had disappeared, and TGT-3 alone decreased and remained small (response rate: 76.4%).
Figure 4. Figures 4-a, b, c, d, e, f, g, h, i, and l demonstrated representative case of OC0209. At baseline, 4 targets were detected without non-target lesion, but the TGT-2 which was thought to be metastatic tumor of mesenterium at first was not the target and proved to be small intestine. The TGT-1 and TGT-4 had disappeared, and TGT-3 alone decreased and remained small (response rate = 76.4%)
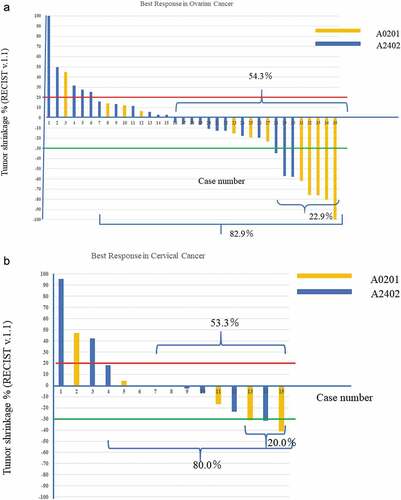
The best response is illustrated by waterfall plots (). Among the 35 patients with OC, 1 had CR, 7 had PR, 21 had stable disease (SD), and 6 had progressive disease (PD). The response rate was 22.9% ([1 CR + 7 PR]/35), and disease control rate was 82.9% (29/35). Among the 15 patients with CC, 0 had CR, 3 had PR, 9 had SD, and 3 had PD. The response rate was 20% (3 PR/15) and disease control rate was 80.0% (12/15) (). The red line indicates a 20% increase of the sum of target lesions and the green line indicates a 30% decrease. SD is shown in the area between the red and green lines. PD is shown above the red line, and PR is shown below the green line. Each patient was followed for 28 days or more.
Figure 5. Best response rate of each patients (water fall plots). Red line showed 20% increase and green line was 30% decrease. 5-a: OC group: yellow bar was HLA-A*0201 and blue bar was HLA-A*2402. 5-b: CC group. Response was complete response (CR) and partial response (PS). Disease control rate (DCR) was CR+PR+SD (stable disease). Responding period was not considered. It seemed that CR+PR cases demonstrated HLA-A*0201
OS
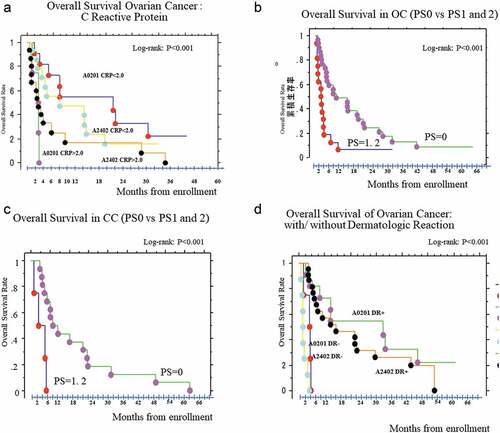
There were no significant differences in OS by age, residual tumor burden, baseline ANC (an indicator of preserved bone marrow function), peripheral lymphocyte count (an indicator of the general cellular immune status), serum globulin status (an indicator of liquid immune function [data not shown]), and disease type or subtype. Baseline CRP, baseline ECOG-PS, and treatment-related dermatologic reactions affected the OS ().
Figure 6. Overall Survival rate: Kaplan-Meier Curve (Logrank method). 6-a: Stratification by C-reactive protein level at baseline (BL). 6-b: Stratification by ECOG-Performance status (PS) at BL in OC
Sub-analyses
There were no significant associations among patient age, residual tumor burden, HLA-A* subtype, histological subtype, and baseline ANC, peripheral lymphocyte count, NLR, and PLR. PS, negative baseline CRP, and treatment-related dermatologic reactions were strongly associated with prolonged median OS (mOS) (). mOS was longer in patients with PS0 than in those with PS1 or PS2 (8.79 months versus 1.32 months, respectively; log-rank test; p < .0001, hazard ratio [HR]: 0.03, p = 1.5 × 10−Citation5, 95% confidence interval [CI]: 0.005–0.13). Patients with OC with lower CRP level had longer mOS than those with higher CRP level (19.0 months versus 3.0 months, respectively; log-rank; p = .0016, HR: 0.33, 95% CI: 0.16–0.68). Patients with CC without or with dermatologic reactions (over G1) had an mOS of 3.3 months versus 21.2 months, respectively (log-rank test; p = .0065, HR: 6.4, 95% CI: 1.39–29.24); in patients with OC, it was 1.4 months versus 17.7 months (log-rank test; p < .0001, HR: 14.5, 95% CI: 4.84–43.48).
Figure 7. From the cox proportional multi variate HR analysis, ECOG performance status (PS) and CRP value at baseline (BL) were the predictive value of this therapy, and the dermatologic reactions (DR) expression was the prognostic factor. Performance Status (PS) and C-reactive protein level (CRP) at baseline, and positivity of dermatologic reactions (DR) during courses were significantly related overall survival time and HR was 0.03, 6.04, and 0.23, respectively
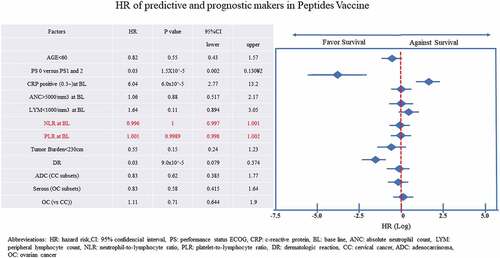
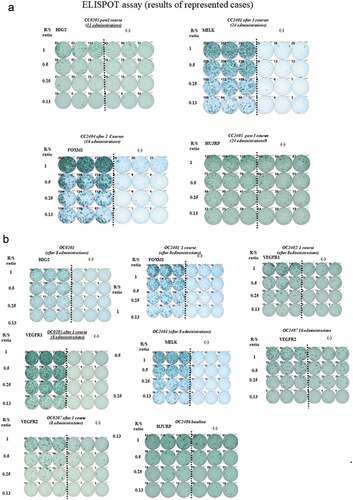
Figure 8. In 54 patients were assessed out of 65 patients. 27 patients had been already positive at baseline. After administrations of vaccination, one patient did not express during protocol treatment (CC0201). In these patients of cohorts, the positivity of ELISPOT assay for any peptides were 50% of accrual patients. After adm of vaccine therapy, one patient remained negative, but she had dermatologic reactions
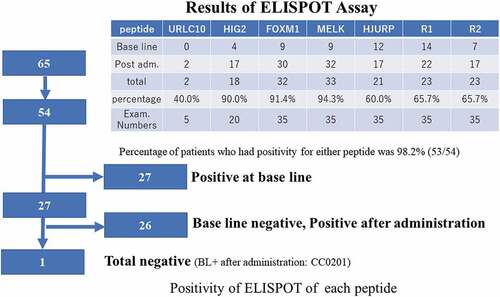
Figure 9. Peripheral blood lymphocytes (PBLs) obtained from each case after each administration of vaccination were cultured in recombinant interleukin 2 (rIL-2) for 14 days with 2 times of each peptide stimulation. The cultured lymphocytes were subjected to the ELISPOT assay after deletion of CD4-poitive cells by magnetic beads. TISI cell were incubated with responder cells in the presence of each peptide or HIV peptide as irrelevant control, and the spot counts were quantified. The cultured lymphocytes were analyzed with HLA-A*2402 or 0201 specific pentamer in the combination with CD8 and CD3 mAbs with flow cytometry. The value of pentamer (+)/CD8 (+) among CD3(+) cells was shown. R/S, responder/stimulator
PS, baseline CRP level, and dermatologic reactions were significantly associated with OS (HR: 0.03, 6.04, and 0.23, respectively).
Age at baseline, ANC, and absolute lymphocyte count, which might relate to the preservation of bone marrow function, were not associated with longer mOS. Baseline NLR and PLR also showed no significant association with OS.
As for the histological subtype, among patients with CC, those with adenocarcinoma had an HR of 0.83, which was better than that of patients with squamous cell carcinoma, yet not significantly so. In patients with OC, high-grade serous carcinoma showed better response than other subtypes.
As for tumor burden in the initiation phase, patients with smaller tumors tended to have better OS, yet not significantly so ().
Immunological evaluation
Twenty-two out of 23 patients were included in the immunological analysis (a sample was not taken from one patient). The ELISpot assay revealed that only 1/22 of patients showed no immunological response, despite dermatologic reactions (, , ). Some patients were not tested, and some had peripheral blood mononuclear cell deterioration. At baseline, 5/22 (23%) of patients were already positive for the antigens (epitope peptides). As for VEGFRs, 4/12 (33%) of patients were positive for epVEGFR1 but not for epVEGFR2. After vaccination, the positive rate for epitope peptides was 50% for epURLC10, 82% for epHIG2, 100% for epFOXM1, 100% for epMELK, 17% for epHJURP, 73% for epVEGFR1, and 64% for epVEGFR2. Sequential analysis of activated CTL revealed two cases of decreased-activated CTL count for epHIG2. One patient received general anesthesia for surgery (lymph-venous anastomosis in lower limb). There were no other noteworthy events.
Discussion
In advanced gynecological cancer, only complete tumor resection is curative. Even in chemo-sensitive tumors such as high-grade serous carcinoma of the ovary and squamous cell carcinoma of the cervix, complete cure by chemotherapy, radiotherapy, or computer-controlled radiotherapy is impossible in advanced stages. However, theoretically, the human cellular immune system should be able to eliminate tumors completely. We conducted cancer vaccination trials in patients with chemoresistant-advanced and persistent OC or CC using tumor-specific neo-antigens, such as epitope peptides obtained from essential genes of driver pathways of surviving tumor cells, which are usually expressed on the tumor cell membrane by HLA-A. These peptides are restricted by HLA-A*0201 or HLA-A*2402, and are disease-specific (i.e., they are not expressed in normal cells, except in the testes). The presence of HLA-A* subtypes such as A*2402 and A:0201 were 60% and 20% in Japanese population. That is, 80% of Japanese people express HLA-A*0201 or HLA-A*2402. In men, the use of peptides restricted by HLA-A*0201 or HLA-A*2402 could compromise the testicular function. However, they were good candidates for gynecological cancer. It is very important to note that the genes selected are essential to proliferation, metastasis, mitosis, angiogenesis, and stemness, and are involved in salvage pathways after several treatments, as reported in our previous paper.Citation15
Hematologic toxicities
Hasegawa et al. performed a study in a small number of patients with CC and reported a lack of efficacy and a high frequency of anemia.Citation41 On the contrary, our previous phase 1 trial and a phase 2 trial involving 20 patients with CC and 46 patients with OC revealed no cases of anemia related to the peptide vaccines.
Lymphocytopenia, which was not detected in small phase 1 studies, was detected in this phase 2 trial. The peripheral lymphocyte count decreased during treatment, and was decreased further with each successive vaccine administration in some patients. However, it did not affect OS (). We speculate that during the initial immune response of vaccination, peripheral T cells are mobilized to sensitized lymph nodes or the thymus; then, they are educated to detect epitope peptides by dendritic cells and grow into activated CTLs. Next, they move into systemic circulation and travel to tumors by homing to targets (epitope peptides expressed on the tumor cell membrane by HLA-A*), where activated CTLs attack tumor cells selectively. Targets also support new angiogenic endothelium cells. In some patients, the peripheral lymphocyte count recovered. Neither baseline NLR not PLR predicted response or OS. However, both NLR not PLR increased at 2 or 4 weeks before deterioration of PS. The role of peripheral lymphocytes should be investigated in future studies.
Furthermore, heavily treated patients usually have bone marrow suppression, including loss of monocytic stem cells. In such patients, rapid mobilization of peripheral T cells was facilitated after vaccination. However, there was not enough supply or there was a delay in the supply from the bone marrow (damage of the monocytic pathway), so peripheral T cells might be depleted during the early vaccine administrations. From these findings, immuno-preservation ability might be assessed by counting peripheral lymphocytes. Patients with fewer peripheral lymphocytes showed shorter OS than those in the non-depleted group (4.93 months versus 14.5 months, respectively; HR: 0.77, 95% CI: 0.269–0.80, p = .0057, log-rank test; p = .007) (Supplemental Figure 1).
Non-hematologic toxicities
Dermatologic reactions comprised redness, pain, swelling, induration, and skin changes such as scaling and ulceration at injection sites. Dermatologic reactions were assessed by NCI-CTC version 3.0. Dermatologic reactions were a prognostic factor of prolongation of OS of peptide vaccine therapy (, ).
As for fluid retention (pleural effusion and ascites), they were difficult to discriminate from disease progression or adverse events. Because both factors (disease progression and adverse events) were confounding, it would be important to compare the speed of retention of fluid in patients with BSC and the progression of fluid retention in patients treated with peptide vaccines. We identified some patients in the OC group, but not in the CC group, in whom retention was possibly accelerated (three of G2, and two of G3, out of five patients in total). This might be caused by VEGFR1 and VEGFR2 blockade using our epVEGFR1 and epVEGEFR2 peptides. VEGFs are expressed by tumor cells because their microenvironment is usually hypoxic. We speculate that the positive feedback of hypoxia might decrease the expression of VEGFRs, which might facilitate VEGF secretion from tumor cells. Unfortunately, this could not be confirmed, because we did not evaluate serum VEGF-A levels.
Response to treatment
Generally, it is important to save cost and medical resources, for which it is necessary to identify the cohort of responders to treatment. In this study, predictive and prognostic factors were ECOG-PS (less than PS1) and CRP level (less than 0.02 ng/ml) before treatment and dermatologic reactions at the injection site, respectively. Other factors such as age, tumor burden, adenocarcinoma in patients with CC, and high-grade serous carcinoma in patients OC tended to prolong survival, yet not significantly so (). High levels of ANC and absolute lymphocyte counts were also not significantly associated with OS. Adenocarcinoma had poorer prognosis than squamous cell carcinoma.Citation42 However, in this study, adenocarcinoma showed etter response than expected. Longer survivors showed fluctuating results (), and at the end of the study period, three patients were still alive with disease. The response rate was 20.0% in patients with CC and 22.9% in patients with OC. The disease control rate was 80% in patients with CC and 82.9% in patients with OC (). The response rates were similar to those of ICIs.
Immunological examination
Immunological examination showed that nearly half of patients in this cohort already had activated CTLs (, ). It is well known that irradiated patients recruit many onco-antigens in circulation, so that the PDL-1 inhibitor, pembrolizumab, is effective after irradiation,Citation43 especially hypofractionated radiation therapy. However, we selected genes that are not expressed in normal organs other than the testes.Citation15 Therefore, there were nearly no severe adverse events. On the other hand, ICIs are not tumor-specific, and several immune-related adverse events might occur.
In this protocol, the administration intervals had been set in a logarithmic interval to avoid the immunologic tolerance in maintenance phase. As a result, the immune tolerance had not been identified in this vaccination schedule.
Secondly, unknown depletion of CTL for epHIG2 had been detected the other patient. This case had showed other activated CTLs for epVEGFR1 and R2 had been detected. This suggests that the tumor cells (or microenvironment cells) expressing HIG2 (in other words, activated HIF-1 alpha pathway of epithelial mesenchymal transition [EMT]) would be eliminated by peptide vaccines with HIG2-sensitive CTLs and the VEGF pathway would be facilitated as a salvage pathway to survive hypoxia). Concerning the hypoxia, from the microenvironment point of view, especially in OC, the tendency of ascitic fluid retention which is usually caused by VEGF had been accelerated during these peptide vaccines. Concomitant administration with VEGF inhibitor, bevacizumab, might be decrease such fluid retention stated above and dual attacks for VEGF pathway would be anticipated in this peptide vaccine therapy for OC. Further preliminary study of concomitant use of ICIs and VEGF inhibitor would be necessary.
Thirdly, the inflammation had suppressed activation of CTLs. From the results of the expression of serum CRP, which is the surrogated marker of inflammation, high CRP patients showed shorter OS than CRP-negative patients. Inflammation usually accompanies with Treg expression and the Treg supposed to be suppressed-activated CTLs. Therefore, concurrent administration of such ICIs might overcome this resistance by regulatory T cells.
Predictive biomarkers were good performance status (PS 0 by ECOG) and low serum CRP (<0.2 mg/dl) before administration, and the prognostic biomarker was earlier expression of dermatologic reactions after peptide administration.
All peptides we used were derived from the actionable genes of treatment-resistant tumor cells and their micro-environmental endothelial cells, which were essential to survive the tumor under chemotherapy or radiotherapy. Thus, these peptides may be appropriate and absolute markings to notice the targets to activated CTLs and the CTLs can be performed pin-point attack to the cancer cells or tumor-induced microenvironmental vascular endothelial cells. The expression of these epitope peptides had not identified except testis, thus, for women, normal cells (normal tissue) did not receive attacks and the adverse events related to these vaccination treatments depressed at minimal extent.
Limitation of this treatment was that peptide vaccines would not be suitable for first line treatment or maintenance treatment after first line. Because, in the first-line therapy, the driver genes pathways are thought to be still main streams like usual MAPK cascade pathway (EGFR–RAS–RAF–MEK–ERK) pathway, PIK3CA–mTOR–AKT–S6 pathway, HGF–cMET–STAT3, and other salvage pathways (Fas ligand–FAS, Wnt–beta-catenin–Frizzled, Wnt–calcium) would not have induced just after surgery. Only when, after down-regulation of major driver gene pathway by chemotherapy or computer-controlled radiotherapy and inhibition of repairing pathway like BRCAness by PARP inhibitors, our peptide vaccines would be effective to eliminate the residual tumor cells. The secondary limitations were suppression by Treg with acquired inflammation or increase of VEGF secretion from tumor cells after using VEGFR1 and VEGFR2 antibodies. The former limitation would be overcome by using an anti-regulatory T-cell treatment and the latter would be overcome by using an anti-VEGF-A antibody.
Summary
Our multiple epitope peptide vaccines targeted genes in resistant/refractory OC and CC as precision medicine. Using the intrinsic immune system, the activated CTLs attack and destroy the tumor cells without severe adverse events. The limitations were attenuation by regulatory T cells with acquired inflammation or increased VEGF secretion from tumor cells. Further clinical study of peptide vaccines in combination with ICIs and/or an anti-VEGF antibody such as bevacizumab will be mandatory.
In conclusion, for patients with heavily treated, recurrent, and resistant OC and CC, peptide vaccines were safe and effective for disease control. Peptide vaccines might provide a promising therapeutic alternative to treat cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participant
IRB approval was obtained before initiation of this clinical study and every enrollees’ written consent was obtained after explanation of the protocols.
Consent for publication
All authors’ consent for publication was obtained.
Availability of data and material
Partly available.
Authors’ contributions
Takeuchi S., Shoji T, Kagabu M, Honda T, Nagasawa T were the investigator (physician). Nitta Y was data manager and clinical research coordinator. Yoshimura S examined the ELISPOT assay at Tokyo University. Nakamura Y had discovered and provided the several specific peptides for theses clinical study in GMP grade. Sugiyama T was supervisor of these clinical study.
Supplemental Material
Download ()Acknowledgments
We thank for co-operation of the institute of medical science the university of Tokyo in ELISPOT assay and delivery for all peptides. As for submission, we thank very much for Dr. Peter G Rose, Cleveland Clinic Main Campus, Gynecologic Oncology in the review of this manuscript in English.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O’Day SJ, Hoos A, Humphrey R, Berman DM, Long berg N, Korman AJ. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291(1):1–13.
- Koppolu V, Rekha Vasigala VK. Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma. J Cancer Res Ther. 2018;14(6):1167–1175.
- Coleman R, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–16. doi:10.1056/NEJMoa1503093.
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi:10.1200/JCO.2015.62.3397.
- Matulonis AU, Shapira-Frommer R, Santin A, Lisyanskaya SA, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: interim results from the phase 2 KEYNOTE-100 study. J Clin Oncol. 2018;36(15_suppl):5511. ASCO 2018 Procedings. doi:10.1200/JCO.2018.36.15_suppl.5511.
- Omatsu K, Hamanishi J, Katsumata N, Nishio S, Sawada K, Takeuchi S, Aoki D, Fujiwara K, Sugiyama T, Konishi I. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patinets with platinum-resistant (advanced or recurrent) ovarian cancer: open-label, randomized trial in Japan (NINJA trial). Ann Oncol. 2020:8070. ESMO2020 procedings. https://cslide.ctimeetingtech.com/esmo2020/attendee/confcal/session/list?q=Omatsu
- Suda T, Tsunoda T, Daigo Y, Nakamura Y, Tahara H. Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci. 2007;98(11):1803–1808. doi:10.1111/j.1349-7006.2007.00603.x.
- Suda T, Tsunoda T, Uchida N, Watanabe T, Hasegawa S, Satoh S, Ohgi S, Furukawa Y, Nakamura Y, Tahara H, et al. Identification of secernin 1 as a novel immunotherapy target for gastric cancer using the expression profiles of cDNA microarray. Cancer Sci. 2006;97(5):411–419. doi:10.1111/j.1349-7006.2006.00194.x.
- Masuzawa T, Fujiwara Y, Okada K, Nakamura A, Takiguchi S, Nakajima K, MIYATA H, YAMASAKI M, KUROKAWA Y, OSAWA R, et al. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol. 2012;41(4):1297–1304. doi:10.3892/ijo.2012.1573.
- Yamaue H, Tsunoda T, Tani M, Miyazawa M, Yamao K, Mizuno N, Okusaka T, Ueno H, Boku N, Fukutomi A, et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC study. Cancer Sci. 2015;106(7):883–890. doi:10.1111/cas.12674.
- Imai K, Hirata S, Irie A, Senju S, Ikuta Y, Yokomine K, Harao M, Inoue M, Tsunoda T, Nakatsuru S, et al. Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers. Clin Cancer Res. 2008;14(20):6487–6495. doi:10.1158/1078-0432.CCR-08-1086.
- Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med. 2013;11(1):291. doi:10.1186/1479-5876-11-291.
- Kono K, Mizukami Y, Daigo Y, Takano A, Masuda K, Yoshida K, Tsunoda T, Kawaguchi Y, Nakamura Y, Fujii H, et al. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci. 2009;100(8):1502–1509. doi:10.1111/j.1349-7006.2009.01200.x.
- Yoshitake Y, Nishimura Y, Nakamura Y, Shinohara M. A clinical trial of multiple peptides vaccination for advanced head and neck cancer patients induced immune responses and prolonged OS. Oncoimmunology. 2015;4(8):e1022307. doi:10.1080/2162402X.2015.1022307.
- Takeuchi S, Shoji T, Kagabu M, Honda T, Nagasawa T, Nitta Y, Sugiyama T, Yoshimura S, Nakamura Y. Anti-cancer immunotherapy epitope-peptides vaccination in patients with refractory/persistent disease of cervical cancer and ovarian cancer (phase 1 studies). Cancer Res J. 2019;7(3):106–116. doi:10.11648/j.crj.20190703.15.
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi:10.1158/1078-0432.CCR-09-1624.
- Ishikawa N, Takano A, Yasui W, Inai K, Nishimura H, Ito H, Miyagi Y, Nakayama H, Fujita M, Hosokawa M, et al. Cancer-testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas. Cancer Res. 2007;67(24):11601–11611. doi:10.1158/0008-5472.CAN-07-3243.
- Suzuki H, Fukuhara M, Yamaura T, Mutoh S, Okabe N, Yaginuma H, Hasegawa T, Yonechi A, Osugi J, Hoshino M, et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J Transl Med. 2013;11(1):97. doi:10.1186/1479-5876-11-97.
- Fujiwara Y, Okada K, Omori T, Sugimura K, Miyata H, Ohue M, Kobayashi S, Takahashi H, Nakano H, Mochizuki C, et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol. 2017;50(5):1655–1662. doi:10.3892/ijo.2017.3955.
- Iinuma H, Fukushima R, Inaba T, Tamura J, Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, et al. Phase I clinical study of multiple epitope peptide vaccine combined with chemoradiation therapy in esophageal cancer patients. J Transl Med. 2014;12(1):84. doi:10.1186/1479-5876-12-84.
- Togashi A, Katagiri T, Ashida S, Fujioka T, Maruyama O, Wakumoto Y, Sakamoto Y, Fujime M, Kawachi Y, Shuin T, et al. Hypoxia-inducible protein 2 (HIG2), a novel diagnostic marker for renal cell carcinoma and potential target for molecular therapy. Cancer Res. 2005;65(11):4817–4826. doi:10.1158/0008-5472.CAN-05-0120.
- Nishimura S, Tsuda H, Ito K, Takano M, Terai Y, Jobo T, Kigawa J, Sugiyama T, Yaegashi N, Aoki D, et al. Differential expression of hypoxia-inducible protein 2 among different histological types of epithelial OC and in clear cell adenocarcinomas. Int J Gynecol Cancer. 2010;20(2):220–226. doi:10.1111/IGC.0b013e3181ca1e16.
- Seo T, Konda R, Sugimura J, Iwasaki K, Nakamura Y, Fujioka T. Expression of hypoxia-inducible protein 2 in renal cell carcinoma: A promising candidate for molecular targeting therapy. Oncol Lett. 2010;1(4):697–701. doi:10.3892/ol_00000122.
- Nishimura S, Tsuda H, Nomura H, Kataoka F, Chiyoda T, Tanaka H, Tanaka K, Susumu N, Aoki D. Expression of hypoxia-inducible 2 (HIG2) protein in uterine cancer. Eur J Gynaecol Oncol. 2011;32(2):146–149.
- Yokomine K, Senju S, Nakatsura T, Irie A, Hayashida Y, Ikuta Y, Harao M, Imai K, Baba H, Iwase H, et al. The forkhead box M1 transcription factor as a candidate of target for anti-cancer immunotherapy. Int J Cancer. 2010;123(9):2153–2163.
- Yang H, Wen L, Wen M, Liu T, Zhao L, Wu B, Yun Y, Liu W, Wang H, Wang Y, et al. FoxM1 promotes epithelial-mesenchymal transition, invasion, and migration of tongue squamous cell carcinoma cells through a c-Met/AKT-dependent positive feedback loop. Anticancer Drugs. 2018;29(3):216–226. doi:10.1097/CAD.0000000000000585.
- Chen H, Zou Y, Yang H, Wang J, Pan H. Downregulation of FoxM1 inhibits proliferation, invasion, and angiogenesis of HeLa cells in vitro and in vivo. Int J Oncol. 2014;45(6):2355–2364. doi:10.3892/ijo.2014.2645.
- Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85(5):644–652. doi:10.1016/j.bcp.2012.10.013.
- Gu C, Banasavadi-Siddegowda YK, Joshi K, Nakamura Y, Kurt H, Gupta S, Nakano I. Tumor-specific activation of the C-JUN/MELK pathway regulates glioma stem cell growth in a p53-dependent manner. Stem Cell. 2013;31(5):870–881. doi:10.1002/stem.1322.
- Minata M, Gu C, Joshi K, Nakano-Okuno M, Hong C, Nguyen CH, Kornblum HI, Molla A, Nakano I. Multi-kinase inhibitor C1 triggers mitotic catastrophe of glioma stem cells mainly through MELK kinase inhibition. PLoS One. 2014;9(4):e92546. doi:10.1371/journal.pone.0092546.
- Kim SH, Joshi K, Ezhilarasan R, Myers TR, Siu J, Gu C, Nakano-Okuno M, Taylor D, Minata M, Sulman E, et al. EZH2 protects glioma stem cells from radiation-induced cell death in a MELK/FOXM1-dependent manner. Stem Cell Rep. 2015;4(2):226–238. doi:10.1016/j.stemcr.2014.12.006.
- Cheng J, Qin B, Liu B, Huang T, Li Y, Ma L. Maternal embryonic leucine zipper kinase inhibits epithelial-mesenchymal transition by regulating transforming growth factor-β signaling. Oncol Lett. 2017;13(6):4794–4798. doi:10.3892/ol.2017.6081.
- Janostiak R, Rauniyar N, Lam TT, Ou J, Zhu LJ, Green MR, Wajapeyee N. MELK promotes melanoma growth by stimulating the NF-κB pathway. Cell Rep. 2017;21(10):2829–2841. doi:10.1016/j.celrep.2017.11.033.
- Kohler RS, Kettelhack H, Knipprath-Mészaros AM, Fedier A, Schoetzau A, Jacob F, Heinzelmann-Schwarz V. MELK expression in OC correlates with poor outcome and its inhibition by OTSSP167 abrogates proliferation and viability of OC cells. Gynecol Oncol. 2017;145(1):159–166. doi:10.1016/j.ygyno.2017.02.016.
- Kato T, Sato N, Hayama S, Yamabuki T, Ito T, Miyamoto M, Kondo S, Nakamura Y, Daigo Y. Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 2007;67(18):8544–8553. doi:10.1158/0008-5472.CAN-07-1307.
- Bassett EA, DeNizio J, Barnhart-Dailey MC, Panchenko T, Sekulic N, Rogers DJ, Foltz DR, Black B. HJURP uses distinct CENP-A surfaces to recognize and to stabilize CENP-A/histone H4 for centromere assembly. Dev Cell. 2012;22(4):749–762. doi:10.1016/j.devcel.2012.02.001.
- Heo JI, Cho JH, Kim JR. HJURP regulates cellular senescence in human fibroblasts and endothelial cells via a p53-dependent pathway. J Gerontol A Biol Sci Med Sci. 2013;68(8):914–925. doi:10.1093/gerona/gls257.
- Stankovic A, Guo LY, Mata JF, Bodor DL, Cao XJ, Bailey AO, Shabanowitz J, Hunt DF, Garcia BA, Black BE, et al. A dual inhibitory mechanism sufficient to maintain cell-cycle-restricted CENP-A assembly. Mol Cell. 2017;65(2):231–246. doi:10.1016/j.molcel.2016.11.021.
- Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonopavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):124
- Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. 2018;37(1):4–11
- Hasegawa K, Ikeda Y, Kunugi Y, Kurosaki A, Imai Y, Kohyama S, Nagao S, Kozawa E, Yoshida K, Tsunoda T, et al. Phase I study of multiple epitope peptide vaccination in patients with recurrent or persistent CC. J Immunother. 2018;41(4):201–207. doi:10.1097/CJI.0000000000000214.
- Takeuchi S. Biology and treatment of cervical adenocarcinoma. Chin J Cancer Res. 2016;28(2):254–262. doi:10.21147/j.issn.1000-9604.2016.02.11.
- Derer A, Spiljar M, Baumler M, Hecht M, Fietkau R, Frey B, et al. Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells. Front Immunol. 2016;7:610. doi:10.3389/fimmu.2016.00610.
