ABSTRACT
Our previous study has identified intratumoral CD103+CD8+ T cells as a favorable prognostic factor in gastric cancer. However, the significance of CD103+CD4+ T cells in gastric cancer hasn’t yet been elucidated. Here, we aimed to investigate the clinical significance and phenotype characteristics of intratumoral CD103+CD4+ T cells in gastric cancer. In our study, 469 formalin-fixed and paraffin-embedded samples and 24 fresh tissue specimens of patients with gastric cancer from Zhongshan Hospital were included. We manifested that intratumoral CD103+CD4+ T cells in gastric cancer predicted poor overall survival and inferior responsiveness to fluorouracil-based ACT. The density and phenotypic characteristics of CD103+CD4+ T cells in gastric cancer were detected by immunohistochemistry and flow cytometry, which showed that CD103+CD4+ T cells exhibited an immunosuppressive phenotype and higher retention capacity in tumor tissues. Furthermore, increased CD103+CD4+ T cells contributed to CD8+T cell dysfunction with decreased granzyme B (GZMB), interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and perforin (PRF-1) expression in gastric cancer. Overall, this study revealed that intratumoral CD103+CD4+T cell infiltration defined immunoevasive contexture and predicted poor prognosis and inferior responsiveness to fluorouracil-based ACT. Therefore, we recommended that CD103+CD4+ T cells might be a potential immunotherapeutic target for gastric cancer.
Introduction
Gastric cancer, the fifth most common malignancy worldwide, ranks the third among all cancers in terms of mortality.Citation1 Despite significant progress having been made in prevention, diagnose, and therapeutic options, the possibility of advanced metastasis and disease recurrence still makes the prognosis and therapeutic responsiveness of gastric cancer fairly unpredictable.Citation2 As first-line adjuvant chemotherapy (ACT), fluorouracil tends to be applied after radical gastrectomy for those advanced gastric cancer (AGC) patients.Citation3–5 However, drug resistance and poisonous side effect still remain a huge restriction to the efficacy of ACT.Citation6,Citation7 Thus, new stratification in gastric cancer to better predict its prognosis and therapy response should be drawn further attention.
Currently, the interplay between tumor and host immune system is considered to be associated with patient survival outcomes and chemotherapy resistance.Citation8–10 CD4+ T cells play an essential part in anti-tumor adaptive immunity, which not only help the activation of CD8+ T cells, but also contribute to the generation and maintenance of memory cytotoxic T lymphocyte (CTL) responses, and have direct anti-tumor functions.Citation11,Citation12 However, their effect on the prognosis of gastric cancer patients remains doubtful. Actually, CD4+ T cells are mainly helper T cells, which can be induced by different cytokines to differentiate into multiple subsets with diverse phenotypes, functions and gene expression, including Th1, Th2, Th17, regulatory T cells (Tregs) and so on.Citation13,Citation14 Consequently, to figure out the prognostic significance of CD4+ T cells and to identify biomarkers with therapeutic value, phenotypic and functional classification of CD4+ T cell subtypes is urgently needed.
CD103, or integrin αEβ7 (ITGAE), is a heterodimeric integral membrane protein composed of an alpha chain and a beta chain. It binds to β7 integrin to form E-cadherin binding integrin, thereby mediating lymphocyte adhesion, migration, and homing.Citation15 Our previous study revealed that CD103 expression on CD8+ T cells identified a subset with stronger anti-tumor effect and superior prognostic value.Citation16 Existing literature has indicated that CD103 was also expressed on CD4+ T cells.Citation17 Whereas, the clinical significance, phenotype and functional characteristics of intratumoral CD103+CD4+ T cells have not been reported in detail. Hence, our current study was aimed to explore the clinical significance and phenotypic characteristics of intratumoral CD103+CD4+ T cells in gastric cancer.
In our study, we found that intratumoral CD103+CD4+ T cell infiltration, rather than total CD4+ T cells had a predictive value for the survival of gastric cancer patients. Phenotypic analysis showed that CD103+CD4+ T cells exhibited tissue-resident and immunosuppressive characteristics. Furthermore, intratumoral CD103+CD4+ T cells were associated with impaired anti-tumor immunity by CD8+ T cells in gastric cancer. To summarize, our results demonstrated that CD103+CD4+ T cells contributed to the impairment of anti-tumor immunity and could be an effective prognostic and predictive biomarker for patients with gastric cancer.
Materials and methods
Study cohorts
496 patients with gastric cancer who underwent radical or palliative gastrectomy between 2007 and 2008 in Zhongshan Hospital were recruited. Due to clinical data incompleteness and point loss during immunohistochemistry (IHC), 27 patients were excluded. 469 patients were randomly divided into two independent cohorts: the discovery cohort (n = 235) and the validation cohort (n = 234). Demographic and clinical data were collected retrospectively. Cancer staging was identified with reference to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM Classification. Patients were treated with postoperative adjuvant chemotherapy (ACT) according to the NCCN guidelines and patients’ own will. In addition, a total of 24 fresh tumor tissue samples were obtained from gastric cancer patients during surgery at Zhongshan Hospital for flow cytometry analysis. None of the patients received radiation therapy. Written informed consent was provided from each patient, and the study was approved by the Institutional Review Board and Ethics Committee of Zhongshan Hospital, Fudan University.
Dual immunohistochemistry and immunofluorescence
The tissue microarrays (TMA) of the discovery cohort and the validation cohort were constructed and subjected to dual immunohistochemical staining. Briefly, the TMA slides were dewaxed in an oven and treated with xylene heated in a water bath and decreasing concentrations of alcohol. The slides were heated in sodium citrate buffer (0.01 M sodium citrate buffer, pH = 6) for 15 minutes to recover the antigen. The goat serum blocking solution was applied at 37 °C for 20 minutes, and then the slides were incubated with anti-CD103 antibody (Ra; Abcam, ab129202, diluted 1:300) at 37 °C for 2 hours. After primary antibody incubation, a two-step kit detection system with DAB (HRP, Mo/Ra; ZSGB-Bio, PV-9000) was used to achieve positive staining (brown). After completing this step, the slides were washed again, and the anti-CD4 primary antibody was added, overnight at 4 °C (Mo; ZSGB-Bio, TA500481, diluted 1:50). The next day, TMA slides were washed, incubated with AP-labeled secondary antibodies (AP, Mo; ZSGB-Bio, DS-0006) and stained with vector blue (VECTOR Blue AP Substrate Kit Detection System; Vector Lab, SK 5300). Finally, the sections were washed, dehydrated and fixed. For immunofluorescence staining, the sections were co-incubated with the primary antibody and left overnight at 4 °C. The samples were then incubated with FITC and TRITC-conjugated secondary antibodies at 37 °C for 2 hours. Finally, the slides were fixed with an anti-fading fixing solution containing DAPI. The Leica DMi8 micro system was used to capture the slides.
Assessment of the number of CD103+CD4+ T cell infiltration in IHC specimens
As mentioned above, after double staining with IHC, CD103 and CD4 were stained respectively brown and blue, while CD103+CD4+ T cells showed dark brown, which was easily distinguished from single positive cells of light brown or blue (). All specimens were observed using a Nikon Eclipse Ti-s microscope (Nikon, Tokyo, Japan), and each tumor area captured three of the most representative high-power fields at ×200 magnification (0.284 mm2 per field of view). Then, two pathologists (L. Chen and P. Zhang) who had no previous knowledge of the patient’s clinical data identified CD103+CD4+ cells (cells stained dark brown), CD103−CD4+ cells (cells stained blue) and CD103+CD4−cells (stained light brown). The total number of CD4+ T cells was counted as the sum of the numbers of CD103+CD4+ and CD103−CD4+ cells, and similarly, the total number of CD103+ cells was counted as the sum of CD103+CD4+ and CD103+CD4− cells. In case of disagreement, the pictures were reviewed and a consensus was reached between two observers. Eventually, average the 3 fields to the final score of each sample.
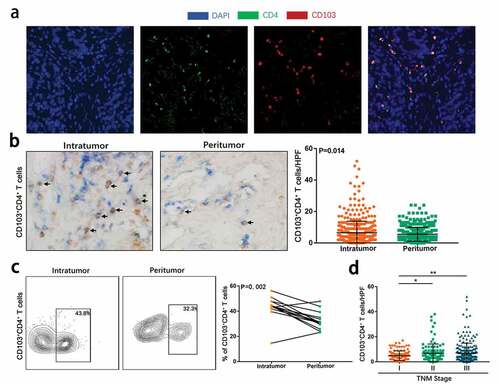
Figure 1. CD103+CD4+ T cells accumulate in gastric cancer and associates tumor progression. (a) Representative immunofluorescence staining with CD4(green), CD103 (red) and DAPI (blue) in gastric cancer tissues. (b) Representative dual immunohistochemical staining images(left) for CD4(blue) and CD103(brown) and quantitative analysis(right) of CD103+CD4+ T cells in intratumoral tissues (n = 469) and corresponding peritumoral tissues (n = 469). Significance was assessed by unpaired t test. (c) Representative flow cytometry (FCM) analysis images (left) and quantification (right) of CD103+CD4+ T cells in CD3+ cells in intratumoral tissues and corresponding peritumoral tissues of gastric cancer fresh samples (n = 14). Significance was assessed by Wilcoxon matched-pairs signed-ranks test. (d) Relationship between intratumoral CD103+CD4+ T cells infiltration and TNM stage was examined based on IHC staining. Significance was assessed by unpaired t test. Bar plots show mean ± SD
Flow cytometry
Freshly isolated gastric cancer tissues were disposed as previously reported.Citation18 After erythrocyte lysis, samples were co-incubated with human BD Fc blocker (BD Biosciences) and then stained with the indicated monoclonal antibodies (mAbs) in the dark at 4 °C for 30 minutes. The fixation/permeation solution kit or the transcription factor fixation/permeability buffer set (BD Biosciences) was applied according to the manufacturer’s instructions. The stained cells were washed and resuspended in cell staining buffer. Stained cells were then separated in a FACS Celesta flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar). The antibodies used in flow cytometry were listed in Table S1.
Statistical analysis
Using X-tile software, we determined the cutoff values for distinguishing between high-density and low-density groups by minimum P method for all markers. It turned out that the cutoff value of CD4+ T cells was 34/HPF, while the cutoff value of CD103+CD4+ T cells was 6/HPF. Kaplan-Meier method and log-rank test were used to compare survival outcomes between the two patient subgroups. One-sample Kolmogorov–Smirnov analysis was used to explore whether the data conformed to the normal distribution. Student t test was used to compare variables between normally distributed samples, while Mann Whitney U test or Wilcoxon matched-pairs signed-ranks test was applied if data wasn’t in line with normal distribution. The Spearman correlation test was used to assess the correlation between two variables. The data of all groups in the figure was expressed as mean ± SDs. A P-value of <0.05 was considered statistically significant. All analysis was performed using GraphPad Prism (version 6.00), MedCalc (version 12.7.0) or IBM SPSS Statistics (version 21) software.
Results
Accumulation of CD103+CD4+ T cells in gastric cancer and its correlation with clinicopathological characteristics
Initially, we intended to confirm the presence of CD103+CD4+T cell subpopulation in gastric cancer. Immunofluorescence staining on formalin-fixed paraffin-embedded gastric cancer tissues revealed the co-expression of CD103 and CD4 on tumor-infiltrating lymphocytes, suggesting that CD103+CD4+ T cells constituted a unique CD4+ T cell population (). We further quantified the CD103+CD4+ T cells in tumor and peritumoral tissues of gastric cancer by double staining IHC and flow cytometry. It was found that CD103+CD4+ T cells were more common and enriched in tumor tissues compared with peritumor tissues ( and c; P= .014 and P= .002). Additionally, IHC staining showed that compared with stage I tumors, stage II or III tumors had more CD103+CD4+ T cell infiltration ( and Table S2). Taken together, these findings suggested that CD103+CD4+ T cells accumulated in gastric cancer and were associated with tumor progression.
Intratumoral CD103+CD4+ T cells predict poor prognosis in gastric cancer patients
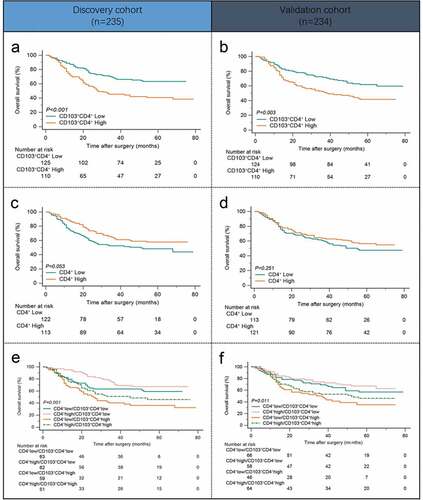
To clarify the clinical significance of intratumoral CD103+CD4+ T cells in gastric cancer, we compared the overall survival (OS) between patients with high and low CD103+CD4+ T cell infiltration. The results showed that high CD103+CD4+ T cell infiltration was associated with poor OS in both the Discovery cohort and Validation cohort ( and b; P< .001 and P= .003). Meanwhile, total CD4+ T cell infiltration was not significant prognostic factor for patients with gastric cancer ( and d; P= .053 and P= .251). However, it’s worth noting that when we used a combination of CD4+ T cells and CD103+CD4+ T cells for survival analysis, patients with CD103+CD4+ T cell high infiltration showed poorer overall survival than those with CD103+CD4+ T cell low infiltration in patients with similar CD4+ T cell infiltration level ( and f; P< .001 and P= .011). The above results revealed that CD103 expression identified a subpopulation of CD4+ T cells with prognostic value.
Figure 2. Intratumoral CD103+CD4+ T cells predict poor prognosis in gastric cancer patients. (a and b) Kaplan–Meier survival curves for overall survival of gastric cancer patients in Discovery cohort(a) and Validation cohort(b) on the basis of intratumoral CD103+CD4+ T cell infiltration. (c and d) Kaplan–Meier survival curves for overall survival of gastric cancer patients in Discovery cohort(c) and Validation cohort(d) on the basis of intratumoral total CD4+ T cell infiltration. (e and f) Kaplan-Meier survival curves for overall survival of gastric cancer patients in the Discovery cohort (e) and Validation cohort (f) further stratified on the basis of CD103+CD4+ T cells in the total CD4+ T cell strata
Intratumoral CD103+CD4+ T cells indicate inferior responsiveness to fluorouracil-based adjuvant chemotherapy in stage II/III patients
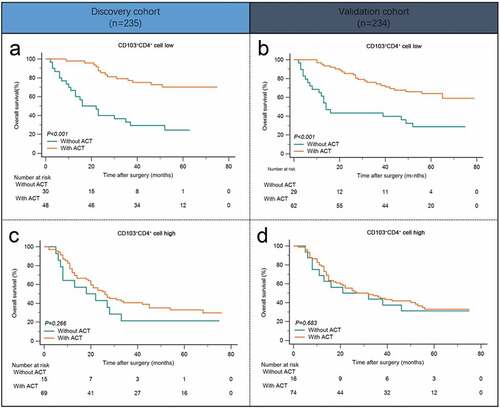
Previous studies have demonstrated the critical role of tumor infiltrating immune cells in chemotherapy response.Citation19–21 Consequently, we next evaluated the predictive value of intratumoral CD103+CD4+ T cells for efficacy of fluorouracil-based ACT. In patients with low CD103+CD4+ T cell infiltration, ACT could produce significant survival benefits in both Discovery cohort and Validation cohort ( and b; P< .001 and P< .001). However, in patients with high CD103+CD4+ T cell infiltration, OS was not improved even after ACT applied ( and d; P= .266 and P= .663). Conclusively, these data showed that high intratumoral CD103+CD4+ T cell infiltration indicated inferior responsiveness to fluorouracil-based adjuvant chemotherapy in stage II/III gastric cancer patients.
Figure 3. Intratumoral CD103+CD4+ T cells indicate inferior responsiveness to fluorouracil-based adjuvant chemotherapy in stage II/III patients. (a andb) Kaplan-Meier survival curves for overall survival of gastric cancer patients with low CD103+CD4+ T cell infiltration in Discovery cohort (a) and Validation cohort (b) on the basis of ACT therapy. (c and d) Kaplan-Meier survival curves for overall survival of gastric cancer patients with high CD103+CD4+ T cell infiltration in Discovery cohort (c) and Validation cohort (d) on the basis of ACT therapy
Intratumoral CD103+CD4+ T cells express high level of IL-10 and display features of tissue residency
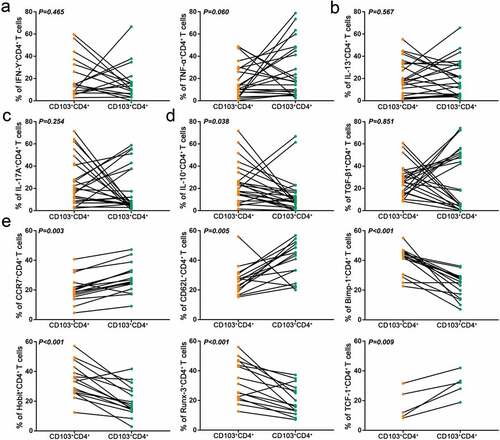
To explore the functional characteristics of intratumoral CD103+CD4+ T cells, flow cytometry was used to detect the expression of Th1 (IFN-γ, TNF-α)-, Th2 (IL-13)-, Th17 (IL-17A)- and Treg (IL-10, TGF-β1)-related cytokines in CD4+ T cells.Citation22–24 Results showed that CD103+CD4+ T cells showed higher expression of IL-10 compared with their CD103− counterparts, which indicated introtumoral CD103+CD4+ T cell subset possessed a suppressive secretory phenotype (–d). Existing researches have displayed that CD103+CD4+ T cells mainly resided in non-lymphoid tissues,Citation17 hence we attempted to investigate whether they retained this characteristic in gastric cancer. CCR7 and CD62L, which are related to lymph node homing and tissue egress of lymphocytes, were down-regulated in CD103+CD4+ T cells. Consistently, as transcription activation factors for CCR7 and CD62L, TCF-1 also showed significantly lower expression on the CD103+CD4+ T cells than CD103−CD4+ T cells. However, transcription factor related to lymphocyte tissue residency, including Blimp-1, Hobit, and Runx-3 were upregulated in CD103+CD4+ T cells, demonstrating that the tissue residency program is regulated by a unique set of transcription factors (). Taken together, these results indicated that CD103+CD4+ T cells in the tumor exhibited immunosuppressive and tissue-resident characteristics.
Figure 4. Intratumoral CD103+CD4+ T cells express high level of IL-10 and display features of tissue residency. (a) Flow cytometry analysis of Th1 type cytokines (IFN-γ, TNF-α) in CD103−CD4+ and CD103+CD4+ T cells from gastric cancer tissues (n = 17,24). (b) Flow cytometry analysis of Th2 type cytokine IL-13 in CD103−CD4+ and CD103+CD4+ T cells from gastric cancer tissues (n = 24). (c) Flow cytometry analysis of Th17 type cytokine IL-17A in CD103−CD4+ and CD103+CD4+ T cells from gastric cancer tissues (n = 24). (d) Flow cytometry analysis of Treg type cytokines (IL-10, TGF-β1) in CD103−CD4+ and CD103+CD4+ T cells from gastric cancer tissues (n = 24). (e) Flow cytometry analysis of tissue egression markers (CCR7 and CD62L) and transcriptional factors (Blimp-1, Hobit, Runx-3 and TCF-1) in CD103−CD4+ and CD103+CD4+ T cells from gastric cancer tissues(n = 16,16,16,16,16,5). Significance was assessed by paired t test
Intratumoral CD103+CD4+ T cells are associated with impaired anti-tumor immunity by CD8+ T cells
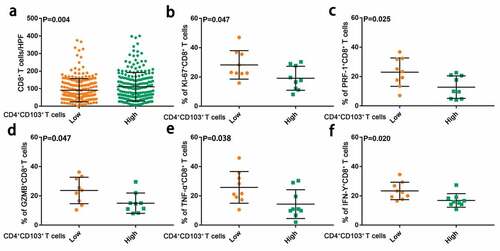
Based on the immunosuppressive characteristics of CD103+CD4+ T cells in gastric cancer, we went on to explore whether intratumoral CD103+CD4+ T cells were associated with anti-tumor immune response. Of interest, we observed an increased number of CD8+ T cells, which are regarded as the main effector cells in anti-tumor immune response, in patients with high CD103+CD4+ T cell infiltration (). Nevertheless, flow cytometry analysis showed that the frequency of Ki-67+CD8+ T cells in CD103+CD4+ T cell high group was significantly lower than that in CD103+CD4+ T cell low group. Moreover, CD8+ T cells in the CD103+CD4+ T cell high group exhibit lower expression of effector molecules related to cytolytic activity, such as granzyme B (GZMB), perforin (PRF-1), and produced less tumor necrosis factor-alpha (TNF-α) and interferon-gamma(IFN-γ) (–e). Altogether, these results indicated that CD103+CD4+ T cells associated with CD8+T cells dysfunction in gastric cancer.
Figure 5. Intratumoral CD103+CD4+ T cells are associated with impaired anti-tumor immunity by CD8+ T cells. (a) Numbers of CD8+ T cells in tissue microarray samples from patients with low and high CD103+CD4+ T cell infiltration. Significance was assessed by unpaired t test. (b) Ki67 expression of CD8+ T cells in gastric cancer tissue samples from patients with low(n = 9) and high(n = 9) CD103+CD4+ T cell infiltration. Significance was assessed by unpaired t test. (c and d) Cytolytic markers expression (PRF-1 and GZMB) of CD8+ T cells in gastric cancer tissue samples from patients with low(n = 9) and high(n = 9) CD103+CD4+ T cell infiltration. Significance was assessed by unpaired t test(c) and Mann Whitney U test(d). (e and f) Effector cytokines expression (TNF-α and IFN-γ) of CD8+ T cells in gastric cancer tissue samples from patients with low(n = 9) and high(n = 9) CD103+CD4+ T cell infiltration. Significance was assessed by Mann Whitney U test(e) and unpaired t test(f). Bar plots show mean ± SD
Discussion
In this study, we explored the clinical significance, phenotypic and functional characteristics of intratumoral CD103+CD4+ T cells in gastric cancer. We found that CD103+CD4+ T cell infiltration predicted poor overall survival and inferior response to adjuvant chemotherapy in patients with gastric cancer. Flow cytometry analysis showed that CD103+CD4+ T cells in gastric cancer exhibited immunosuppressive and tissue resident characteristics. In addition, we found that CD103+CD4+ T cells were associated with impaired anti-tumor immunity by CD8+ T cells. These findings demonstrated that intratumoral CD103+CD4+ T cells might be a useful prognostic factor and a potential target in gastric cancer.
Prognostic evaluation is critical to selecting the appropriate treatment for cancer patients. In recent years, the prognostic significance of tumor infiltrating immune cells has attracted more and more attention with their critical role in tumorigenesis and progression. Our previous studies have clarified the prognostic value of tumor-associated macrophages, tumor-infiltrating neutrophils, and IL-17A-producing cells in gastric cancer.Citation21,Citation25,Citation26 In this study, we identified and validated a subset of CD4+ T cells expressing CD103 conferred impaired anti-tumor immunity and poor clinical outcomes in gastric cancer. To our knowledge, this study is the first to identify intratumoral CD103+CD4+ T cell density as a useful prognostic factor in gastric cancer. Besides, it is worth noting that our study also revealed the predictive value of intratumoral CD103+CD4+ T cells for ACT. For patients with stage II/III gastric cancer, fluorouracil-based ACT is now generally used as a first-line adjuvant therapy.Citation4,Citation6,Citation27 However, not everyone can benefit from adjuvant chemotherapy, and the criteria for selecting proper candidates remains controversial.Citation28,Citation29 Therefore, it is of great significance to identify patients with gastric cancer who will benefit from chemotherapy to prevent excessive toxicity. Our findings indicated that ACT could only improve survival in patients with low CD103+CD4+ T cell infiltration, which will help to better select and manage patients receiving adjuvant chemotherapy.
By flow cytometry, we found that CD103+CD4+ T cells expressed higher level of anti-inflammatory cytokine IL-10, indicating that CD103+CD4+ T cells have an immunosuppressive phenotype. In addition, we also found that CD103+CD4+ T cells in gastric cancer showed a tissue-resident phenotype, which might contribute to persistent immunosuppressive effect. To further explored the association between CD103+CD4+ T cell infiltration and anti-tumor immunity, we analyzed the number and functional status of intratumoral CD8+ T cells in gastric cancer, which regarded as the main antitumor effector cells. As hypothesized, we observed that CD8+ T cells showed decreased proliferative capability and effector function in gastric cancer tissues with high CD103+CD4+ T cell infiltration. These data might give possible explanations for the poor prognosis and inferior ACT response of gastric cancer patients in CD103+CD4+ T cell low infiltration subgroup.
The main limitation of our study was the absence of in vivo validation of our findings, as there were no suitable mouse-derived cell lines for gastric cancer to establish immune-capable mouse models. Besides, minimum P method was used to determine the cutoff values, so these cutoff values might not be reproducible. Thirdly, manual pathological analysis for IHC images might be influenced by visual bias. In addition, we have not identified how the gastric cancer CD103+CD4+ T cell subsets formed and differentiated, which was needed to be clarified in our future studies.
Conclusion
Conclusively, we for the first time identified CD103+CD4+ T cells as an unfavorable prognostic factor and predictor for adjuvant chemotherapy. CD103+CD4+ T cells exhibit immunosuppressive and tissue resident features and might be potential therapeutic target in gastric cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Y. Gu, Y. Chen and K. Jin for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; Y. Cao, X. Liu, K. Lv, X. He, C. Lin, H. Liu, H. Li, H. He and J. Qin for technical and material support; R. Li, H. Zhang and W. Zhang for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Competing interests
The authors declare no conflict of interest.
Consent for publication
All authors provide their consent for publication of the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from each patient included and the protocol of all study cohorts were approved by the institutional review board and ethics committee of Zhongshan hospital, Fudan University.
Acknowledgements
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their contribution to the evaluation of IHC results and excellent pathological technology help.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Availability of data and materials
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Prof. Zhang upon reasonable request.
Additional information
Funding
References
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (London, England). 2016 May 10;388(10060):2654–8. doi:10.1016/s0140-6736(16)30354-3.
- Cheong JH, Yang HK, Kim H, Kim WH, Kim Y-W, Kook M-C, Park Y-K, Kim -H-H, Lee HS, Lee KH, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018 Mar 24;19:629–638. doi:10.1016/s1470-2045(18)30108-6.
- De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005 Apr 28;92:1644–1649. doi:10.1038/sj.bjc.6602573.
- Nishida T. Adjuvant therapy for gastric cancer after D2 gastrectomy. Lancet (London, England). 2012 Jan 10;379:291–292. doi:10.1016/s0140-6736(11)61928-4.
- Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010 Apr 23;11:439–449. doi:10.1016/s1470-2045(10)70070-x.
- Noh SH, Park SR, Yang H-K, Chung HC, Chung I-J, Kim S-W, Kim -H-H, Choi J-H, Kim H-K, Yu W, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014 Dec 03;15(12):1389–1396. doi:10.1016/s1470-2045(14)70473-5.
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J clin Oncol. 2011 Oct 20;29(33):4387–4393. doi:10.1200/jco.2011.36.5908.
- Tsujimoto H, Ono S, Ichikura T, Matsumoto Y, Yamamoto J, Hase K. Roles of inflammatory cytokines in the progression of gastric cancer: friends or foes? Gastric Cancer. 2010 Dec 04;13:212–221. doi:10.1007/s10120-010-0568-x.
- Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014 Aug 29;345(2):196–202. doi:10.1016/j.canlet.2013.08.016.
- Yolanda LV, Sergio PD, Hugo ES, Isabel AFR, Rafael B-Z, Aldo T-D, Gonzalo C-R. Gastric cancer progression associated with local humoral immune responses. BMC Cancer. 2015 Nov 22;15:924. doi:10.1186/s12885-015-1858-9.
- Luckheeram RV, Zhou R, Verma AD, Xia B. CD4 + T cells: differentiation and functions. Clin Dev Immunol. 2012 Apr 05;2012:925135. doi:10.1155/2012/925135.
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008 Aug 30;112:1557–1569. doi:10.1182/blood-2008-05-078154.
- Colantonio L, Recalde H, Sinigaglia F, D’Ambrosio D. Modulation of chemokine receptor expression and chemotactic responsiveness during differentiation of human naive T cells into Th1 or Th2 cells. Eur J Immunol. 2002 May 01;32(5):1264–1273. doi:10.1002/1521-4141(200205)32:5<1264::Aid-immu1264>3.0.Co;2-s.
- Read KA, Powell MD, Sreekumar BK, Oestreich KJ. In vitro differentiation of effector CD4(+) T helper cell subsets. Methods Mol Biol (Clifton, NJ). 2019 Feb 25;1960:75–84. doi:10.1007/978-1-4939-9167-9_6.
- Boutet M, Gauthier L, Leclerc M, Gros G, de Montpreville V, Théret N, Donnadieu E, Mami-Chouaib F. TGFbeta signaling intersects with CD103 integrin signaling to promote T-Lymphocyte Accumulation and antitumor activity in the lung tumor microenvironment. Cancer Res. 2016 Feb 28;76:1757–1769. doi:10.1158/0008-5472.Can-15-1545.
- Li R, Liu H, Cao Y, Wang J, Chen Y, Qi Y, Lv K, Liu X, Yu K, Lin C, et al. Identification and validation of an immunogenic subtype of gastric cancer with abundant intratumoural CD103(+)CD8(+) T cells conferring favourable prognosis. Br J Cancer. 2020 Mar 25. doi:10.1038/s41416-020-0813-y.
- Klicznik MM, Morawski PA, Hollbacher B, Varkhande SR, Motley SJ, Kuri-Cervantes L, Goodwin E, Rosenblum MD, Long SA, Brachtl G, et al. Human CD4 + CD103 + cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci Immunol. 2019 Jul 07;4(37):eaav8995. doi:10.1126/sciimmunol.aav8995.
- Fu Q, Xu L, Wang Y, Jiang Q, Liu Z, Zhang J, Zhou Q, Zeng H, Tong S, Wang T, et al. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol. 2019 Oct 09;75(5):752–763. doi:10.1016/j.eururo.2018.09.030.
- Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011 Aug 19;71(17):5670–5677. doi:10.1158/0008-5472.Can-11-0268.
- Konig L, Mairinger FD, Hoffmann O, Bittner A-K, Schmid KW, Kimmig R, Kasimir-Bauer S, Bankfalvi A. Dissimilar patterns of tumor-infiltrating immune cells at the invasive tumor front and tumor center are associated with response to neoadjuvant chemotherapy in primary breast cancer. BMC Cancer. 2019 Feb 06;19(1):120. doi:10.1186/s12885-019-5320-2.
- Wang JT, Li H, Zhang H, Chen YF, Cao YF, Li RC, Lin C, Wei YC, Xiang XN, Fang HJ, et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol. 2019 Nov 18;30(2):266–273. doi:10.1093/annonc/mdy505.
- Guery L, Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015 Nov 20;2015:314620. doi:10.1155/2015/314620.
- Lee GR. The balance of Th17 versus treg cells in autoimmunity. Int J Mol Sci. 2018 Mar 08;19. doi:10.3390/ijms19030730.
- Muller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002 May 10;205:35–50. doi:10.1078/0171-2985-00109.
- Liu X, Cao Y, Li R, Gu Y, Chen Y, Qi Y, Lv K, Wang J, Yu K, Lin C, et al. Poor clinical outcomes of intratumoral dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin-positive macrophages associated with immune evasion in gastric cancer. Eur J Cancer (Oxford, England: 1990). 2020 Feb 29;128:27–37. doi:10.1016/j.ejca.2020.01.002.
- Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018 Oct 21;267(2):311–318. doi:10.1097/sla.0000000000002058.
- Bang YJ, Kim YW, Yang HK, Chung HC, Park Y-K, Lee KH, Lee K-W, Kim YH, Noh S-I, Cho JY, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet (London, England). 2012 Jan 10;379:315–321. doi:10.1016/s0140-6736(11)61873-4.
- Jiang Y, Xie J, Han Z, Liu W, Xi S, Huang L, Huang W, Lin T, Zhao L, Hu Y, et al. Immunomarker support vector machine classifier for prediction of gastric cancer survival and adjuvant chemotherapeutic benefit. Clin Cancer Res. 2018 Jul 26;24(22):5574–5584. doi:10.1158/1078-0432.Ccr-18-0848.
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003 May 02;3:330–338. doi:10.1038/nrc1074.
