ABSTRACT
In head and neck squamous cell carcinoma (HNSCC), data from studies using checkpoint-inhibiting antibodies that target programmed death 1 (PD-1) or its ligand the programmed death ligand 1 (PD-L1) demonstrated outstanding clinical activity. Translational investigations also suggested some correlations between therapeutic response and PD-L1 expression in tumor tissue. We comprehensively summarize results that have evaluated PD-L1 expression in HNSCC. We discuss flaws and strength of current PD-1/PD-L1 detection, quantification methods and the evaluation of PD-L1 as a prognostic and theragnostic biomarker. Understanding tumor microenvironment may help understanding resistance to checkpoint inhibitors, designing clinical trials that can exploit drug combinations.
Introduction
Targeted therapies, including immunotherapy, are currently an area of active development in head and neck oncology;Citation1,Citation2 however, to date, adequate biomarkers for selection of candidates who will benefit from this therapy have not been definitively identified. Emerging evidence has revealed that head and neck squamous cell carcinoma (HNSCC) is associated with an enriched immune landscape with critical immunological implications.Citation1 Current HNSCC clinical trials are addressing the need to manage systemic and local immunoregulation. One of the most promising pathways for manipulation involves programmed death-ligand 1 (PD-L1, also referred to as B7-H1 or CD274). Binding of PD-L1 which is expressed on tumor cells, to its receptor, programmed cell death protein 1 (PD-1), results in direct protection of the tumor from cell death.Citation3 PD-L1 can also reduce activity of tumor-infiltrating effector CD4 and CD8 T cells that express PD-1.Citation4 Membrane-anchored PD-L1 is also constitutively expressed by nonmalignant cells of the myeloid lineage, including macrophages and dendritic cells.Citation4–7
Two PD-1/PD-L1 inhibitors (nivolumab and pembrolizumab) have recently been approved by both US and EU regulatory agencies for second-line treatment of recurrent or metastatic (R/M) HNSCC.Citation8,Citation9 For melanoma, urothelial and non-small cell lung cancer (NSCLC), PD-L1 expression has been associated with clinical response to anti-PD-1 antibodies in multiple clinical trials.Citation10–13 For HNSCC, PD-L1 status was not requested by the regulatory authorities for the use of nivolumab. When pembrolizumab was investigated, better outcomes were reported among patients with high PD-L1 expression. Therefore, scoring systems were subsequently developed, either focusing on PD-L1 expression on tumor cells (TPS; tumor proportion score) or combining tumor and immune cell expression (CPS; combined positive score).
Using the first scoring system, the European Medicines Agency restricted the indication of pembrolizumab in HNSCC for patients with recurrent or metastatic tumor with a ≥50% TPS and progressing on or after platinum-containing chemotherapy.
In July 2019, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending a change to the terms of the marketing authorization for the medicinal product pembrolizumab.
Results from the KEYNOTE-048 study showed that pembrolizumab was efficient for first-line treatment of R/M HNSCC in patients with a tumor and/or surrounding cells expressing PD-L1, which was particularly pronounced with CPS ≥20, and less so when CPS ≥1.Citation14 This suggests a degree of correlation between PD-L1 expression and the effect of PD-1 inhibitors. Nonetheless, a proportion of patients with PD-L1-negative tumors also shows some levels of benefit from PD-1 inhibitors.Citation9,Citation14–16
The expression and topographic distribution of PD-L1 and PD-1 on nonmalignant cells in the tumor microenvironment has been well described for Hodgkin lymphomaCitation17 and melanoma.Citation10 For HNSCC, which displays intense intra-tumoral and peritumoral inflammatory infiltration, the distribution of PD-L1 and PD-1 on nonmalignant cells is not yet unclear, and the clinical phenotype most likely to benefit from immunotherapy remains to be defined.Citation1
PD-1 inhibitors are associated with a high rate of primary progression, and an unexpected effect which has recently been defined as “hyperprogression”.Citation18–21 The biological mechanisms of hyperprogression are yet to be fully elucidated, but are expected to help clinicians identify predictors of response or primary resistance, and thus improve the use of PD-1/PD-L1 inhibitors.
In this review, we focus on studies of PD-L1 expression in HNSCC tumors and the microenvironment, highlighting the different types of scoring used.
Quantifying PD-L1 expression with antibodies
The choice of antibody
PD-L1 expression is currently quantified by immunohistochemistry. However, methods analyzing PD-L1 staining are heterogeneous given the availability of multiple staining antibodies and interpretation protocols.
Several commercial PD-L1 immunohistochemistry antibodies are available and their detection rates of PD-L1 vary.Citation22 The wide range of anti-PD-L1 labeling antibodies precludes homogenous and comparable results for PD-L1 expression.Citation23 Importantly, results from the study by Scott et al. reported at the ESMO 2018 Congress, demonstrated that different algorithms for PD-L1 evaluation can select different populations and should thus not be used interchangeably.Citation24
Preclinical studies reporting PD-L1 expression use different antibodies (). Most clinical studies use the IHC 22C3 pharmDx assay (Dako, Carpinteria, CA) with the 22C3 anti-PD-L1 antibody (Merck, Kenilworth, NJ) that were validated by the FDA for characterization of NSCLC ().Citation15,Citation25,Citation26 Two ongoing clinical trials (e.g. nct02369874, nct03051906) require confirmed PD-L1-positive or -negative status by the Ventana SP263 assay. A number of studies using different tumor types have compared available assays to determine if they are interchangeable,Citation22,Citation27–29 as summarized in . The two international Phase III studies KEYNOTE-040 (second-line)Citation14 and KEYNOTE-048 (first-line)Citation16 used the 22C3 Dako antibody for PD-L1 expression, providing an argument for this to be considered the routine assay for HNSCC patients candidate for pembrolizumab, as is the case for lung carcinoma patients. In practice, PD-L1 expression is currently only taken into consideration in Europe for pembrolizumab in HNSCC.
Table 1. Results about prognostic value of PD-L1 expression in HNSCC pre-clinical studies
Table 2. Results about prognostic value of PD-L1 expression in HNSCC clinical trials
Table 3. Studies comparing different PD-L1 IHC assays
Cells choice of for PD-L1 scoring
Given the physiological mechanism of PD-1 inhibitors, PD-L1 expression on the tumor cells could be considered the most important parameter to predict efficacy. In the initial cohort of the Phase 1 KEYNOTE-012 study, patients with R/M HNSCC had to have at least 1% PD-L1 expression to be eligible, regardless of the cell type expressing PD-L1 (tumor, inflammatory cells or stroma).Citation15 Eighty-one (78%) screened patients were found to be PD-L1-positive.
In the expansion cohort of this study, all patients with R/M HNSCC, irrespective of PD-L1 or human papillomavirus status (HPV), were eligible. Two scoring methods for assessing PD-L1 expression were assessed: scoring only tumor cell PD-L1 expression, and scoring PD-L1 expression in both tumor and mononuclear inflammatory cell.Citation25 When scoring took into account staining in both tumor and immune cells, PD-L1 expression was more predictable of anti-PD-1 efficacy (P = .021, one-sided) and was better correlated with response rate, progression-free survival (PFS), and overall survival (OS) (PFS: P = .008; OS: P = .008, one-sided). Restricting scoring to tumor cells did not show significance, suggesting that PD-L1 expression is more representative when scoring immune cells (notably macrophages and activated T lymphocytes) than tumor cells. In another study by Kim et al., tumor and immune cell PD-L1 expression was evaluated separately according to each cell component. Overall, scoring immune cell expression was shown to be an important prognostic parameter to predict clinical outcome in HNSCC patients.Citation30
To further clarify the above findings, the KEYNOTE-055 study defined CPS as the “percentage of tumor and mononuclear inflammatory cells within the tumor nests and adjacent supporting stroma expressing PD-L1 at any intensity”.Citation26 In clinical practice, CPS is defined by membranous PD-L1 staining on both tumor and inflammatory cells. It is important to highlight that the CPS denominator includes only the viable invasive tumor cells. The CPS score can thus exceed 100. Moreover, only PD-L1 positive lymphocytes and macrophages are included in the count, although other immune cell subtypes (e.g. dendritic cells) might also be present in the microenvironment.
In contrast to NSCLC clinical trials that used TPS, the recent cutoff references used for PD-L1 positivity in HNSCC are more and more referring to CPS (). However, this score carries some difficulties for routine clinical practice. Pathologists are basically trained to focus on tumor cell component. Therefore, it requires additional attention and time for them to analyze also immune cells, and to separate them from tumor cells on immunohistochemistry staining, in order to perform and report CPS score. In HNSCC, available data suggest that CPS is the most appropriate score to determine PD-L1 expression; however, practical implementation requires further validation and pathological recommendations.
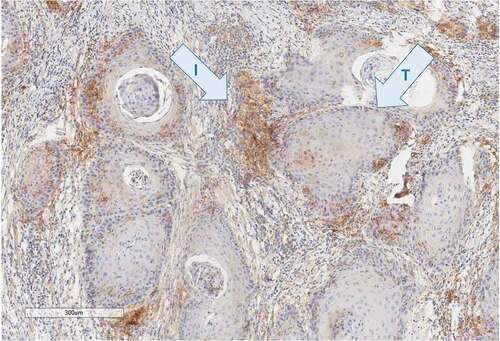
Figure 1. PD-L1 staining (E1L3N, Cell signaling technology) in head and neck carcinoma sample shows a weak staining of the tumor component (t) contrasting with a strong staining of the immune component (i) located in the stroma. Pathologists have scored this case 15% by tumor proportion score (TPS) and 40 by combined positive score (CPS)
PD-L1 expression cutoff values
Definition of a valid and widely accepted PD-L1 cutoff value remains a point of debate. In the case of NSCLC, different cutoff values and different scoring were used for different drugs: TPS>5% for nivolumab, CPS>1 for pembrolizumab, PD-L1 expression on at least 50% of tumor cells or at least 10% of tumor-infiltrating immune cells for atezolizumab and TPS>25% for durvalumab.Citation31–33 Likewise, for some drugs (e.g. pembrolizumab), different cancers have different cutoffs: CPS ≥10 for urothelial and esophageal carcinoma and CPS ≥1 for gastric and cervical cancer.
There is currently no absolute cutoff reference for PD-L1 positivity in clinical and preclinical studies in HNSCC ( and ). For most studies, binary classification of tumors was used; “positive or negative”. Most clinical trials used a 1% cutoff,Citation15,Citation25,Citation26 as this cutoff between a tumor which does not express PD-L1 at all and a positive case, is easy to apply in practice. The other cutoff values of 5%,Citation10,Citation12,Citation34,Citation35 20% (KEYNOTE-048),Citation16 or 50%Citation26 imply more complex determination and might be affected by interobserver bias (sensitivity of histopathological examination).
Other studies used a semiquantitative score to evaluate PD-L1 staining. A four-level score was used by Badoual et al.: 0 (<10% positive cells), + (10–20% positive cells), ++ (20–50% positive cells), +++ (>50% positive cells) based on reference staining slides using a high magnification (x400).Citation36 Cho et al. calculated the PD-L1 staining score according to staining intensity and distribution of positive tumor cells.Citation37
In all these studies, the scoring of PD-L1 expression was subjective with a labor-intensive procedure, representing a limitation for reproducible and comparative results.Citation38 Clinical trials in HNSCC need a standardized and ideally semi-quantitative score for PD-L1 expression, which includes tumor cells and immune-infiltrating cells. As automatic scoring improves, a more complex score may be used, delineating each type of positive cells (tumor, macrophages and lymphocytes). An alternative solution to determining PD-L1 expression scores may be a technique independent of histopathological examination, such as gene signatures using mRNA expression levels.Citation34,Citation39,Citation40 This could resolve the challenge of variable PD-L1 expression in different tissue sections. However, gene signatures may be highly dependent on the method used for tissue preservation (cryopreservation versus paraffin-embedded tissue) and the content of the sampling in terms of immune, normal and cancer cells. For those reasons, all methods need to be continuously evaluated in terms of evolving disease prognosis and therapeutic objectives.
Factors influencing PD-L1 expression in HNSCC
Variability in PD-L1 expression levels in HNSCC tumor tissues between studies remains important, ranging from 18% to 100% in preclinical studies, and 27% to 78% in clinical trials ( and ). This variability reflects the lack of uniformity in assays, different monoclonal antibodies employed for staining, fresh versus paraffin-embedded samples, scoring (or not) of PD-L1 expression on inflammatory cells and the different cutoffs.Citation37 Moreover, discrepancies arise due to true variability within the tumor tissue, between different clones, and between the primary site and any metastases. Furthermore, PD-L1 expression may also vary according to TNM stage, previous treatment, and time since disease diagnosis.
Primary tumor site and cervical lymph nodes
While most studies have analyzed surgical samples from the primary site at initial diagnosis, one study included 34 cases where PD-L1 expression was performed both on the primary tissue (oral and oropharyngeal SCC [OPSCC]) and the cervical lymph nodes. Among cervical lymph node samples, 71% were positive for PD-L1.Citation22 Of these 34 matched pairs, only 19 (56%) were concordant with PD-L1 expression at the primary site. Among discordant cases, there was a mix of increased PD-L1 expression in the lymph nodes (26%) and decreased PD-L1 expression (18%).
Moreover, with quantitative polymerase chain reaction (qPCR), Partlová et al. analyzed the mRNA expression levels of PD-L1 in the primary tumor tissue samples and in metastatic and control cervical lymph nodes. The only significant difference reported was higher mRNA expression in primary tumor versus negative lymph nodes, all other subsite comparison being non-significant.Citation40
Studies in other tumor types have reported discrepant expression of PD-L1 between primary and metastatic tissues, suggesting that assessing primary tumor tissue alone might be insufficient to accurately predict PD-L1 expression in advanced disease, an important parameter to bear in mind for treatment outcomes and prognosis in recurrent and metastatic HNSCC.Citation41–43
PD-L1 expression during disease evolution
Studies in melanoma and gastric cancer have also shown notable differences in PD-L1 expression between archival tissue from initial diagnosis and subsequent fresh tumor samples.Citation44 This suggests PD-L1 expression could be dynamic, increasing by the time disease progression occurs. Thus, analysis of PD-L1 in HNSCC biopsies from the initial diagnosis may not reflect expression at the time of immunotherapy initiation.
Influence of radiotherapy, chemotherapy and targeted therapy on PD-L1 expression
In preclinical studies using cell lines and mouse models, radiotherapy has been shown to increase tumor PD-L1 expression.Citation45,Citation46 This process depends on two main pathways: the production of IFNγ by CD8 + T cellsCitation45 and the DNA damage.Citation47 Radiotherapy causes cell death through DNA damage, which is reported to upregulate PD-L1 expression via ATM/ATR/Chk1 kinase activities and cGAS/STING-dependent pathway.Citation48–50
In a clinical study including soft tissue sarcoma, Patel et al. found that PD-L1 expression is significantly increased after radiotherapy both in tumor cell and TAM as compared to pre-radiotherapy samples.Citation51 The correlation between chemotherapy and PD-L1 expression was not investigated in clinical studies. However, since several chemotherapy compounds cause also cell death through DNA damage, it could be anticipated that those compounds could increase PD-L1 expression. So far, no pre-clinical and clinical studies report specifically the effects of targeted therapy, such as cetuximab, on PD-L1 expression.
The above results support immunotherapy-based strategies combined with chemotherapy and/or radiotherapy.
HPV status
The role of the PD-1/PD-L1 in genuine viral infection and adaptive immune resistance of HPV-positive OPSCC is well established.Citation5 Various studies have investigated a possible correlation between PD-L1 expression and HPV status in HNSCC; however, a consensus is yet to be reached. In the Kim et al. study, PD-L1 expression in HPV-negative and HPV-positive tumors was not significantly different (61% vs. 71%, respectively, P = .274).Citation52 Similarly, Badoual et al. reported that 52% of HNSCC tumors evaluated expressed a high level of PD-L1, but no correlation was observed between PD-L1 expression and HPV status (HPV-positive vs. negative, 63% vs. 40%, P = .08).Citation36 Ukpo et al. also reported no significant difference for the presence of PD-L1 expression in 138 OPSCC tumors according to HPV status (HPV-positive vs. negative, 49% vs. 34%, P = .08).Citation34
Conversely, three studies have reported a correlation between PD-L1 expression and HPV status. In a small cohort, Lyford-Pike et al. reported an increased PD-L1 expression in HPV-positive tumors compared with HPV-negative tumors (14/20 [70%] vs. 2/7 [29%]).Citation5 Hong et al. reported a similar correlation in a cohort of 99 patients (83.3% vs. 56.9%, P = .008).Citation53 Oguejiofor et al. also reported a significant – but inverse – correlation, with lower mean PD-L1 expression in HPV-positive tumors compared to negative cases (3.1 ± 1% vs. 6.1 ± 2%, P = .01).Citation35 Moreover, they stratified PD-L1 expression according to site of expression (stroma versus tumor), showing that HPV-positive tumors had lower stromal PD-L1 expression compared with negative tumors (P = .01). The authors hypothesize that this could be due to lower PD-L1 expression on CD68 cells in the stroma in HPV-positive tumors. Considering the importance of the increased incidence of HPV-positive patients and the potential of checkpoint inhibitors in therapy, larger prospective studies clarifying the whispered role of PD-L1 in HPV-positive tumors in patients with HNSCC are urgently warranted.
Other clinical characteristics
Studies evaluating correlations between PD-L1 expression and other clinical characteristics are to date relatively rare, with inconstant results. Zhang et al. reported that the expression of PD-L1 in nasopharyngeal carcinoma was significantly correlated with clinical TNM stage and lymphatic metastasis (P < .05), but not with age or sex.Citation54 Hong et al. found that patients with PD-L1-positive tonsillar carcinoma were more likely to be never-smokers and nondrinkers (P = .0001 and P = .0001, respectively), and have grade 3 disease, with a lower T stage and higher N stage (P = .0011, P = .0001, and P = .0001, respectively).Citation53 Conversely, Kim et al. did not identify any significant correlations between PD-L1 expression and age, sex, smoking history, location of tumor origin, and stage in OPSCC patients.Citation52
PD-L1 expression in the tumor microenvironment
The use of multiplexed immunofluorescence has allowed to report the expression and topographic distribution in PD-L1-positive malignant and nonmalignant cells throughout the tumor microenvironment.Citation2,Citation10,Citation17
Tumor-infiltrating lymphocytes
The incidence of tumor-infiltrating lymphocytes (TILs) has been closely studied according to the different tumoral patterns of PD-L1 expression. Two studies found that in the induced pattern, PD-L1 expression co-localized with invading CD3-positive lymphocytesCitation55 or CD8-positive lymphocytes.Citation5 However, Cho et al. showed by immunohistochemical analysis that the density of intratumoral CD8-positive lymphocytes was significantly inversely correlated with tumoral PD-L1 expression (P = .047). Moreover, neither intratumoral CD4-positive nor peritumoral TIL density correlated with the staining-intensity-distribution PD-L1 score.Citation37 Lyford-Pike et al. did not observe any difference in the density or quality of TILs when comparing the constitutive and induced patterns.Citation5 This correlation between the incidence of TILs and PD-L1 expression could help us to identify patients who may better respond to or develop resistance to PD-1 inhibitors.
Tumor-associated macrophages
Potential correlations between the PD-1/PD-L1 axis and tumor-associated macrophages (TAMs) have been studied in various cancer types including in OPSCC.Citation56,Citation57 TAMs express both PD-1 and PD-L1. Using an in vivo mouse model with colon cancer, Gordon et al. found that 50% of tumoral macrophages expressed surface PD-1. These PD-1-positive TAMs show less phagocytosis compared to their PD-1-negative counterparts.Citation58 This suggests that PD-1 inhibitors may increase macrophage phagocytosis and reduce tumor growth in a macrophage-dependent fashion, and that PD-1/PD-L1 therapies may also function through a direct effect on TAMs phagocytosis.
However, TAMs were also shown to express PD-L1. Some studies have reported that PD-L1 and PD-L2 are specific markers of M1 and M2 macrophages, respectively.Citation59,Citation60 Lyford-Pike et al. who studied the spatial distribution of PD-L1-positive TAMs, offered a potential explanation of macrophage functions.Citation5 For instance, PD-L1-positive TAMs are localized at the rim interface between the tumor periphery and the surrounding inflammatory stroma. As such, they may stand as a PD-L1 immuno-protective “barrier” surrounding the tumor nests and by such actively contributing to adaptive resistance in PD-L1-positive tumors. The authors also demonstrated that PD-L1 co-localized with CD68-positive TAMs in HPV-positive HNSCC, suggesting that TAMs mediate adaptive resistance through the PD-1/PD-L1 pathway to dampen tumor-specific T cell functions. This was supported by Oguejiofor et al., who showed that 7% of CD68-positive cells expressed PD-L1 in HPV-positive OPSCC compared with 16% in HPV-negative OPSCC.Citation35 The co-expression of PD-1 and PD-L1 on TAMs certainly deserves further analysis considering that these cells may be playing an important, or potentially ambivalent role, in the response to anti-PD-1 therapy.
PD-L1 tumoral expression patterns
HNSCC seems to present two distinct patterns of PD-L1 expression,Citation5,Citation22,Citation55 similar to what has been described previously in melanoma.Citation10 In the first pattern – termed “induced pattern”Citation22 – few PD-L1-expressing tumor cells are located at the periphery of the tumor nests adjacent to the tumor–stroma interface and concomitant expression of PD-L1 in the adjacent immunocytes ().Citation22 PD-L1 expression in these immune cells could be a result of tumor–immunocyte interactions, representing adaptive immune resistance.Citation5,Citation10,Citation22 Restricted staining was more common in HPV-positive OPSCC, in accordance with the proposed adaptive resistance hypothesis.Citation5 In the second pattern called “constitutive pattern”, described for HNSCCCitation5,Citation22 and melanoma,Citation10 PD-L1 is expressed uniformly by most tumor cells. This expression pattern appears to be an innate display rather than induced by changes in the microenvironment. Further research into potential correlations between these two distinct patterns and response to immunotherapy are currently ongoing.
Prognostic and predictive functions of PD-L1 expression
Relationships between PD-L1 expression and outcome
The prognostic value of PD-L1 expression in HNSCC is currently unclear. In the Hong et al. study analyzing 99 tonsillar cancer patients by immunochemistry, PD-L1 status was a significant positive prognostic factor for OS by univariate analysis (P = .019), although this was not maintained in a multivariate analysis.Citation53 For Solomon et al., high PD-L1 expression in intratumoral immune cells was significantly associated with improved OS in a cohort of 190 patients with HPV-positive OPSCC (HR = 0.37; 95%CI [0.15–0.93]; P = .023).Citation61 On the other hand, Kim et al. reported that PD-L1 expression did not affect OS in 133 OPSCC patients in univariate and multivariate analyses. Kaplan-Meier analysis showed no significant difference between PD-L1–positive and PD-L1–negative patients for PFS and OS (P = .519 and P = .625, respectively).Citation52
Using PD-L1 expression to predict benefit under therapy with PD-1 inhibitors
Progresses with immunotherapy have changed the therapeutic arsenal for patients with R/M HNSCC, improving both OS and clinical response. Unfortunately, the rate of responders remains low (~20%) () Citation9,Citation14–16,Citation25 highlighting an urgent need to identify predictive factors for patient subgroups likely to derive greater benefit.
Table 4. Response to the PD-1/PD-L1 inhibitors according to PD-L1 expression in HNSCC clinical trials
Most studies report that patients with tumors expressing PD-L1 are more likely to respond to PD-1/PD-L1 inhibition (). CPS (which includes both tumor and immune cells) appears to be a better predictive factor of response to pembrolizumab than when TPS alone is used.Citation25 When immune cells were included in the scoring system, a statistically significant increase in the probability of response to pembrolizumab for positive (≥1) vs. negative (<1) patients was observed (22% vs. 4%, P = .021). When only tumor cells were included, the difference between the two groups was not significant (19% vs. 16%, respectively; P = .348).
In a study reported by Bauml et al., 18% of patients with ≥1% PD-L1 expression responded to pembrolizumab compared with 12% with <1% expression, although the small size of the subgroup with CPS <1% (N = 26) should be kept in mind.Citation26 Moreover, several PD-L1–negative patients responded to pembrolizumab; 6- and 12-month PFS and OS rates were similar between PD-L1–negative and PD-L1–positive patients. As these data suggest that the therapeutic benefit of pembrolizumab is not limited to patients with PD-L1–positive tumors, CPS was not validated by Bauml et al. as an absolute predictive factor of response to anti-PD1 therapy.Citation26 The CheckMate-141 study suggested that patients with PD-L1 expression ≥1% experience a greater effect of nivolumab than those whose PD-L1 level <1%; however, interactions were neither significant nor corrected for multiple comparisons.Citation9
Across all the above-cited clinical trials, a number of patients defined as PD-L1 non-expressors also had a response to the PD-1/PD-L1 inhibitors (4–16%) (). Although in the low range, these response rates are not very different from those reported for PD-L1 expressors (17–21%). This should be factored into future decisions concerning guidelines on patient selection regarding PD-L1 status.
The investigation of additional biomarkers to aid in appropriate patient selection for PD-1 inhibitors continues to be an important area of research. In the case of NSCLC, the presence of EGFR mutations, IFN-γ expression signatures, and tumor mutational burden have been proposed as potential predictive markers for response to immunotherapy.Citation62 These factors also merit investigation, alone or associated with PD-L1 expression, as potential prognostic factors for response to immunotherapy in patients with HSNCC.
PD-L1 expression for detecting hyperprogressors to PD-1 inhibitors?
An emerging and heavily debated phenomenon is the identification of hyperprogressors among patients treated with immunotherapy. Initially described by Champiat et al., hyperprogressor patients were defined as showing RECIST progression at the first disease assessment with a ≥ two-fold increase in the tumor growth rate when treated with immunotherapy.Citation18 Of 131 evaluable patients with several types of malignancies, 12 patients (9%) were considered as having hyperprogression. In another study evaluating HNSCC patients, this reached 29% (10 cases among 34 patients).Citation19 Hyperprogressing disease was associated with increased age (66% vs. 55%, P = .007)Citation18 and regional recurrence (90% vs. 37%, P = .008).Citation19
Hyperprogressing disease was associated with decreased survival in both studies.Citation18,Citation19 In HNSCC patients, hyperprogression was associated with shorter PFS per RECIST (2.5 vs. 3.4 months, P= .003), and irRECIST (2.9 vs. 5.1 months, P = .02).Citation19 In the study reported by Champiat et al., there was a clear trend toward a worse outcome for hyperprogressors (median OS, 4.6 months; 95% CI, 2.0–NA) compared with non-hyperprogressors (median OS, 7.6 months; 95% CI, 5.9–16.0), although this was not significant (P = .19), likely due to the small sample size of hyperprogressors. However, the overall log-rank test was highly significant (P < .001) among all groups.Citation18 To our knowledge, the association between PD-L1 expression and hyperprogression has not yet been studied but may deserve a deeper evaluation.
Currently, reasons behind accelerated tumor growth are unknown. The flare progression occurring after few weeks of therapy with checkpoint inhibitors does not match classical concepts used for defining resistance to chemotherapy. Indeed, the inflammatory microenvironment has the potential for triggering mechanisms stimulating the release of growth factors or strongly unbalance checkpoint inhibition, stimulating the carcinogenic progression. Interestingly, Daste et al. described a patient previously treated for various HNSCCs (oral cavity, larynx, oropharynx) by surgery and radiotherapy, who presented two simultaneous tumors (HNSCC and a squamous lung cell carcinoma) showing rapid progression in the oral cavity tumor under immunotherapy while the lung tumor was stable for one year.Citation63 This case report supports that the response to PD-1 inhibitor is dependent on the phenotype of tumor cells and/or its microenvironment. Inhibition of the PD1/PD-L1 axis may induce collateral effects on other immunosuppressive cells, such as Treg cells, TAMs or myeloid cells, which are to date unknown and must be investigated.
Conclusion
The definition of a standard and universally shared laboratory method to determine PD-L1 tumor expression is an urgent challenge in head and neck oncology. Moreover, it is imperative for cutoff values, pertinent to clinical outcomes, to be better defined. Analyzing correlations between PD-L1 expression and clinical characteristics should help us to better understand which patient subgroups derive benefit from anti-PD-1 therapy. Translational research will contribute to characterizing other possible predictive markers, which will be valuable for optimal patient selection candidate for immunotherapy in the future.
Abbreviations
Declaration of interest statement
SF has received consultancy compensation and clinical trial research grants from Bayer, BMS, Merck Serono, MSD and Pfizer.
Acknowledgments
Sarah MacKenzie for manuscript editing.
Additional information
Funding
References
- Cavalieri S, Rivoltini L, Bergamini C, Locati LD, Licitra L, Bossi P. Immuno-oncology in head and neck squamous cell cancers: news from clinical trials, emerging predictive factors and unmet needs. Cancer Treat Rev. 2018;65:78–11. doi:10.1016/j.ctrv.2018.03.003.
- Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, Denize T, Mirghani H, Fabre E, Ménard M, et al. Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev. 2018;65:54–64. doi:10.1016/j.ctrv.2018.02.008.
- Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111(7):3635–3643. doi:10.1182/blood-2007-11-123141.
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi:10.1038/nm730.
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et al. Evidence for a role of the PD-1: PD-L1pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. doi:10.1158/0008-5472.CAN-12-2384.
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi:10.1038/70932.
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol Baltim Md 1950. 2002;169(10):5538–5545.
- Larkins E, Blumenthal GM, Yuan W, He K, Sridhara R, Subramaniam S, Zhao H, Liu C, Yu J, Goldberg KB, et al. FDA approval summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist. 2017;22(7):873–878. doi:10.1634/theoncologist.2016-0496.
- Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi:10.1056/NEJMoa1602252.
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi:10.1126/scitranslmed.3003689.
- Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematol. 2016;101:75–85. doi:10.1016/j.critrevonc.2016.03.007.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690.
- Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi:10.1016/S1470-2045(17)30616-2.
- Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, Soria A, Machiels J-P, Mach N, Mehra R, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet Lond Engl. 2019;393(10167):156–167. doi:10.1016/S0140-6736(18)31999-8.
- Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. doi:10.1016/S1470-2045(16)30066-3.
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland Å et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet Lond Engl. 2019 Oct 31;394(10212):1915–1928. doi:10.1016/S0140-6736(19)32591-7.
- Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, Hu X, Redd R, Freeman GJ, Neuberg D, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for reed-sternberg cells in Hodgkin lymphoma. Blood. 2017;130(22):2420–2430. doi:10.1182/blood-2017-03-770719.
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria J-C, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(8):1920–1928. doi:10.1158/1078-0432.CCR-16-1741.
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(7):1605–1611. doi:10.1093/annonc/mdx178.
- Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid progression of adult T-cell leukemia–lymphoma after PD-1 inhibitor therapy. N Engl J Med. 2018;378(20):1947–1948. doi:10.1056/NEJMc1803181.
- Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi:10.1001/jamaoncol.2018.3676.
- Scognamiglio T, Chen Y-T. Beyond the percentages of PD-L1-positive tumor cells: induced versus constitutive PD-L1 expression in primary and metastatic head and neck squamous cell carcinoma. Head Neck Pathol. 2018;12(2):221–229. doi:10.1007/s12105-017-0857-3.
- Economopoulou P, Kotsantis I, Psyrri A. Checkpoint inhibitors in head and neck cancer: rationale, clinical activity, and potential biomarkers. Curr Treat Options Oncol. 2016;17(8):40. doi:10.1007/s11864-016-0419-z.
- Scott M, Wildsmith S, Ratcliffe M, Al-Masri H, Scorer PW, Barker C, Rebelatto MC, Walker J. Comparison of Patient Populations Identified by Different PD-L1 Assays in Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 2018;29(8):mdy287-007.
- Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee S-H, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–3845. doi:10.1200/JCO.2016.68.1478.
- Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, Phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(14):1542–1549. doi:10.1200/JCO.2016.70.1524.
- Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, et al. PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2017;12(2):208–222. doi:10.1016/j.jtho.2016.11.2228.
- Karim LA, Wang P, Chahine J, Kallakury B. Harmonization of PD-L1 immunohistochemistry assays for lung cancer: a working progress. J Thorac Oncol. 2017;12(5):e45. doi:10.1016/j.jtho.2016.12.022.
- Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3(8):1051–1058. doi:10.1001/jamaoncol.2017.0013.
- Kim HR, Ha S-J, Hong MH, Heo SJ, Koh YW, Choi EC, Kim EK, Pyo KH, Jung I, Seo D, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6. doi:10.1038/srep36956.
- Brody R, Zhang Y, Ballas M, Siddiqui MK, Gupta P, Barker C, Midha A, Walker J. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. 2017;112:200–215. doi:10.1016/j.lungcan.2017.08.005.
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937.
- Ukpo OC, Thorstad WL, Lewis JS. B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;7(2):113–121. doi:10.1007/s12105-012-0406-z.
- Oguejiofor K, Galletta-Williams H, Dovedi SJ, Roberts DL, Stern PL, West CML. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 2017;8(9):14416–14427. doi:10.18632/oncotarget.14796.
- Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138. doi:10.1158/0008-5472.CAN-12-2606.
- Cho Y-A, Yoon H-J, Lee J-I, Hong S-P, Hong S-D. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47(12):1148–1153. doi:10.1016/j.oraloncology.2011.08.007.
- Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016;9:5023–5039. doi:10.2147/OTT.S105862.
- Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi:10.1038/ncomms3612.
- Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, Fučíková J, Truxová I, Tachezy R, Špíšek R, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4(1):e965570. doi:10.4161/21624011.2014.965570.
- Zhang X, Yin X, Zhang H, Sun G, Yang Y, Chen J, Zhu X, Zhao P, Zhao J, Liu J, et al. Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma. BMC Cancer. 2019;19. doi:10.1186/s12885-019-5578-4.
- Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47(1):52–63. doi:10.1016/j.humpath.2015.09.003.
- Manson QF, Schrijver WAME, Ter Hoeve ND, Moelans CB, van Diest PJ. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis. 2019;36(1):29–37. doi:10.1007/s10585-018-9950-6.
- Kakavand H, Wilmott JS, Menzies AM, Vilain R, Haydu LE, Yearley JH, Thompson JF, Kefford RF, Hersey P, Long GV, et al. PD-L1 expression and tumor-infiltrating lymphocytes define different subsets of MAPK inhibitor-treated melanoma patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(14):3140–3148. doi:10.1158/1078-0432.CCR-14-2023.
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi:10.1158/0008-5472.CAN-14-1258.
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francisca BJ, Velarde E, Deweese TL, Drake CG. Stereotactic radiation therapy augments antigen-specific PD-1-mediated anti-tumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3(4):345–355. doi:10.1158/2326-6066.CIR-14-0196.
- Sato H, Jeggo PA, Shibata A. Regulation of programmed death‐ligand 1 expression in response to DNA damage in cancer cells: implications for precision medicine. Cancer Sci. 2019;110(11):3415–3423. doi:10.1111/cas.14197.
- Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8. doi:10.1038/s41467-017-01883-9.
- Permata TBM, Hagiwara Y, Sato H, Yasuhara T, Oike T, Gondhowiardjo S, Held KD, Nakano T, Shibata A. Base excision repair regulates PD-L1 expression in cancer cells. Oncogene. 2019;38(23):4452–4466. doi:10.1038/s41388-019-0733-6.
- Iijima M, Okonogi N, Nakajima NI, Morokoshi Y, Kanda H, Yamada T, Kobayashi Y, Banno K, Wakatsuki M, Yamada S, et al. Significance of PD-L1 expression in carbon-ion radiotherapy for uterine cervical adeno/adenosquamous carcinoma. J Gynecol Oncol. 2020;31(2). doi:10.3802/jgo.2020.31.e19
- Patel KR, Martinez A, Stahl JM, Logan SJ, Perricone AJ, Ferris MJ, Buchwald ZS, Chowdhary M, Delman KA, Monson DK, et al. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology. 2018;7(7). doi:10.1080/2162402X.2018.1442168
- Kim HS, Lee JY, Lim SH, Park K, Sun J-M, Ko YH, Baek C-H, Son Y, Jeong HS, Ahn YC, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat Cancer Res Treat. 2015;48(2):527–536.
- Hong AM, Vilain RE, Romanes S, Yang J, Smith E, Jones D, Scolyer RA, Lee CS, Zhang M, Rose B. PD-L1 expression in tonsillar cancer is associated with human papillomavirus positivity and improved survival: implications for anti-PD1 clinical trials. Oncotarget. 2016;7(47):77010–77020. doi:10.18632/oncotarget.12776.
- Zhang F, Liu Z, Cui Y, Wang G, Cao P. [The clinical significance of the expression of costimulatory molecule PD-L1 in nasopharyngeal carcinoma]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi J Clin Otorhinolaryngol Head Neck Surg. 2008;22:408–410.
- Malm I-J, Bruno TC, Fu J, Zeng Q, Taube JM, Westra W, Pardoll D, Drake CG, Kim YJ. Expression profile and in vitro blockade of programmed death-1 in human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck. 2015;37(8):1088–1095. doi:10.1002/hed.23706.
- Mattox AK, Lee J, Westra WH, Pierce RH, Ghossein R, Faquin WC, Diefenbach TJ, Morris LG, Lin DT, Wirth LJ, et al. PD-1 expression in head and neck squamous cell carcinomas derives primarily from functionally anergic CD4+ TILs in the presence of PD-L1+ TAMs. Cancer Res. 2017;77(22):6365–6374. doi:10.1158/0008-5472.CAN-16-3453.
- Yu G-T, Bu -L-L, Huang C-F, Zhang W-F, Chen W-J, Gutkind JS, Kulkarni AB, Sun Z-J. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPα axis in HPV negative head and neck squamous cell carcinoma. Oncotarget. 2015;6(39):42067–42080. doi:10.18632/oncotarget.5955.
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi:10.1038/nature22396.
- Wang J, Cao Z, Zhang X-M, Nakamura M, Sun M, Hartman J, Harris RA, Sun Y, Cao Y. Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res. 2015;75(2):306–315. doi:10.1158/0008-5472.CAN-14-2819.
- Parsa R, Andresen P, Gillett A, Mia S, Zhang X-M, Mayans S, Holmberg D, Harris RA. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61(11):2881–2892. doi:10.2337/db11-1635.
- Solomon B, Young RJ, Bressel M, Urban D, Hendry S, Thai A, Angel C, Haddad A, Kowanetz M, Fua T, et al. Prognostic significance of PD-L1+ and CD8+ immune cells in HPV+ oropharyngeal squamous cell carcinoma. Cancer Immunol Res. 2018 Jan 29. doi:10.1158/2326-6066.CIR-17-0299.
- Tsiara A, Liontos M, Kaparelou M, Zakopoulou R, Bamias A, Dimopoulos M-A. Implementation of immunotherapy in the treatment of advanced non-small cell lung cancer (NSCLC). Ann Transl Med. 2018;6(8). doi:10.21037/atm.2018.02.07.
- Daste A, de Mones E, Digue L, François L, Domblides C, Dupin C, Bigourdan A, Ravaud A. Immunotherapy in head and neck cancer: need for a new strategy? Rapid progression with nivolumab then unexpected response with next treatment. Oral Oncol. 2017;64:e1–e3. doi:10.1016/j.oraloncology.2016.10.020.
