ABSTRACT
Anti-PD-1 monoclonal antibody is approved as an option for third-line treatment of advanced gastric and gastroesophageal junction (G/GEJ) cancer in several countries, but no anti-PD-1 monoclonal antibody treatment is yet approved for first-line treatment of advanced G/GEJ cancer. We report a phase Ib trial of HX008, a highly selective, humanized anti-programmed death-1 monoclonal antibody, plus oxaliplatin and capecitabine as first-line treatment for advanced G/GEJ cancer. Patients with previously untreated, locally advanced or metastatic G/GEJ cancer were enrolled. All patients received HX008 3 mg/kg intravenously every 3 weeks, oxaliplatin 130 mg/m2 intravenously on day 1 every 3 weeks (up to 6 cycles), and capecitabine 1000 mg/m2 orally twice daily for 14 days continuous dosing followed by a 7-day break. The primary end point was the incidence of adverse events and serious adverse events. In total, 35 patients were enrolled. Median follow-up was 12.7 months. Most frequent (>10%) grade ≥3 treatment-related adverse events were anemia (27.5%), neutropenia (20%), thrombocytopenia (17.1%), leukopenia (17.1%) and fatigue (17.3%). Objective response rate was 60.0% (95% confidence interval [CI] 42.1–76.1%). Disease control rate was 77.1% (95% CI 59.9–89.6). Median time to response and duration of response were 1.4 months (range 1.3–2.9) and 12.3 months (range 1.4–17.9+), respectively. Median PFS was 9.2 months (95% CI 5.4-not reached). These results demonstrated that HX008 combined with oxaliplatin plus capecitabine was well tolerated and demonstrated encouraging efficacy as first-line treatment for advanced G/GEJ cancer. This study was registered in china, register number was CTR20181270.
Introduction
Gastric or gastroesophageal junction (G/GEJ) cancer is the fifth most common cancer and the third leading cause of cancer death worldwide. In 2018, nearly 1,000,000 new cases and 783,000 deaths were estimated to have occurred.Citation1 Notably, almost half of the total case of G/GEJ cancer occurs in East Asian, with the age-standardized incidence rate of 32.1 per 100,000 and a mortality rate of 13.2 per 100,000.Citation1 Although the incidence rate has declined and survival has improved in recent years, G/GEJ cancer remains the second most common cancer and the second leading cause of cancer death in China, with a poor prognosis.Citation2
The standard therapy of first-line treatment for advanced G/GEJ adenocarcinoma remains to be fluoropyrimidine- and platinum-based therapy. A doublet regimen of cisplatin or oxaliplatin in combination with 5-fluorouracil or capecitabine or S-1 is preferred in Asia. In previously untreated gastric cancer, the doublet regimen demonstrated an objective response rate (ORR) of 28.8–54%, a progression-free survival (PFS) of 4.9–6.0 months, and an overall survival (OS) of 8.5–13.0 months, respectively.Citation3–5 Although several clinical trials have investigated the efficacy of targeted agents plus chemotherapy as first-line treatment, including trastuzumab,Citation6 lapatinib,Citation7 bevacizumab,Citation8 rilotumumabCitation9 and ramucirumab,Citation10 only trastuzumab significantly improved overall survival of up to 13.8 months in human epidermal growth factor receptor 2 (HER 2)-positive advanced G/GEJ cancer. Thus, the first-line treatment of advanced G/GEJ cancer is clearly unsatisfied, and potential novel agent that will improve survival in these patients is urgently needed.
Immune-checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) and PD-ligand 1 (PD-L1) have shown promising efficacy in multiple malignant diseases. PD-L1 is frequently upregulated in gastric cancer, with 12%-65% detected in tumor tissues; notably, a poorer prognosis was observed in patients with PD-L1 positive tumors.Citation11–13 Preliminary clinical data of single-agent PD-1 inhibitors in metastatic G/GEJ cancer have demonstrated anti-tumor efficacy, with response rates of 22–27% for patients with PD-L1 positive tumors and 10–17% for unselected patients.Citation14 Nivolumab and pembrolizumab have been approved as third-line treatment of advanced gastric cancer.Citation15,Citation16
Combination with immune check point inhibitors and standard chemotherapy exerts synergistic anti-tumor activity through modulation of the immune system or reshaping the tumor microenvironments (TME),Citation17–19 which has proved to improve survival in several cancer types.Citation20–24 Promising antitumor activity of combination treatment was also initially presented in advanced GC in KEYNOTE-059 studyCitation25 and ATTRACTION-4 trial,Citation26 with differential efficacy results. But the results of phase III trial KEYNOTE-062 failed to demonstrate superior efficacy of pembrolizumab plus chemotherapy in either combined positive score (CPS) ≥1 or CPS ≥10 subgroups.Citation27 However, hardly any results of combination with anti-PD-1 antibody and chemotherapy for first-line treatment in Chinese patients with advanced G/GEJ cancer has been reported.
HX008 is a highly selected, humanized, IgG4 anti-PD-1 monoclonal antibody that blocks the interaction between PD-1 and its ligand.Citation28 Results from a phase I trial of HX008 in advanced solid tumors suggested 3 mg/kg or 200 mg every 3 weeks as the recommended dose (data not published). Here, we report the safety and efficacy of HX008 with oxaliplatin plus capecitabine as first-line therapy in Chinese patients with advanced G/GEJ cancer.
Materials and methods
Eligibility criteria
Patients were ≥18 and ≤75 years of age with histologically or cytologically confirmed diagnosis of unresectable locally advanced or metastatic G/GEJ cancer, and with no exposure to previous systemic treatment for advanced or metastatic disease. Additional key eligibility criteria included: at least one measurable lesion at baseline, assessed by Response Evaluation Criteria in Advanced Solid Tumors version 1.1 (RECIST v1.1); Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; a life expectancy ≥3 months and adequate organ function. The main exclusion criteria included: active or history of autoimmune disease; active central nervous system metastases; history or current interstitial lung disease or pulmonary fibrosis; prior treatment with an agent directed against PD-1/PD-L1, CTLA-4 or another co-inhibitory T-cell receptor; history of allogeneic hematopoietic stem cell transplantation; adverse events (AEs) from previous therapy that had not recovered to grade ≤1. Patients with active gastrointestinal ulcer, intestinal obstruction, active gastrointestinal bleeding and perforation were also excluded. The full criteria are available in supplementary materials.
Study design and treatment
This study was an ongoing open-label, multi-center, single-arm randomized, phase Ib, exploratory clinical study of HX008 combined with oxaliplatin plus capecitabine as first-line therapy for patients with advanced G/GEJ cancer. Eligible patients received HX008 3 mg/kg by intravenous infusion over 60 min on day 1, oxaliplatin 130 mg/m2 by intravenous infusion over 2 hours on day 1 (for up to 6 cycles), and capecitabine 1000 mg/m2 orally twice daily for 14 days continuous dosing followed by a 7-day break of each 21-day cycle. Treatment was continued for up to one year, or until disease progression, unacceptable toxicity, or patient or investigator decision to withdraw. Patients with a durable response may receive HX008 for another year following the completion of one-year treatment. Clinically stable patients with the first radiographic progressive disease (PD) might continue treatment at the investigator’s discretion until confirmed PD. Treatment interruptions were permitted for the management of treatment-related AEs. All patients were examined at discontinuation of the protocol treatment and on day 28 post-treatment, and were followed up.
The study protocol and all amendments were approved by the Ethics Committee of each study site and conducted in accordance with the Declaration of Helsinki guidelines and applicable local laws and regulations. All patients provided written informed consent before enrollment. The study was registered in china, register number was CTR20181270.
End points and assessments
The primary endpoint was incidence of adverse events (AEs) and serious adverse events (SAEs). The secondary endpoint endpoints included ORR, duration of response (DOR) and PFS, assessed by the site investigator per RECIST v1.1, and pharmacokinetics parameters (not addressed in this article). Endpoint definitions are available in the supplementary materials.
All AEs were recorded during the study period from the initiation of treatment to 30 days after the last dose or the start date of subsequent anti-tumor therapy followed the last dose, whichever came first. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 20.0) and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 5.0). The correlation between adverse events and study drugs was evaluated.
Tumor response was assessed with chest, abdominal and pelvic computed tomography (CT) or magnetic resonance imaging (MRI) every 6 weeks until week 24, then every 12 weeks until discontinuation. For patients with available tumor samples, PD-L1 tumor expression and mismatch repair (MMR) status were determined by immunohistochemistry at a central laboratory, using anti-human PD-L1 monoclonal antibody 28–8 (Abcam, UK) and anti-MLH1, MSH2, MSH6 and PMS2 monoclonal antibodies (MXB, China), respectively. PD-L1 expression was measured using CPS, defined as the number of PD-L1-positive cells (tumor cells, lymphocytes, macrophages) as a proportion of the total number of tumor cells multiplied by 100.
Statistical analysis
This study was designed to enroll at least 15 evaluable patients, more patient could be considered if the toxicity and efficacy are acceptable. The full analysis set (FAS) consisted of patients who successfully entered the group and received at least one treatment. Safety and efficacy will be statistically analyzed based on FAS. Safety was analyzed using descriptive statistics. ORR and disease control rate (DCR) with 95% CI were calculated using the Clopper–Pearson exact method based on binomial distribution. Patients without tumor assessment data were considered nonresponders. Kaplan–Meier method was used to estimate median DOR and PFS, and their 95% CIs were estimated by Brookmeyer-Crowley method. Data analyses were conducted using SAS statistical software version 9.4.
Results
Demographics and baseline characteristics
From August 09, 2018 to June 24, 2019, 35 patients with advanced G/EGJ cancer were enrolled at 3 sites (Supplementary Table 1) in China. All patients had received ≥1 dose of HX008 combined with oxaliplatin plus capecitabine and thus were included in the FAS. Baseline characteristics are listed in . The median age was 63 (range 21–71) years and 77.1% were male. Sixty percent of patients had ECOG PS 1, and 31.4% had received prior surgery. At baseline, PD-L1 expression and MMR status detection were performed in 21 and 22 patients with available tumor samples, respectively. Among those, 12 patients (57.1%) had PD-L1 positive (CPS ≥ 1) tumors; mismatch repair deficient (dMMR) was confirmed in 2 patients (9.1%), the others were determined as mismatch repair proficient (pMMR).
Table 1. Demographics and baseline characteristics
At data cutoff (June 16, 2020), the median follow-up duration was 12.7 months (range 0.3–21.2), with a median duration of treatment of 5.7 months (range 0.3–21.2). The median number of HX008 dose administrated was 8 (range 1–26). The median cycle number of oxaliplatin and capecitabine was 6 (range 1–6) and 8 (range 1–26), respectively. A total of 26 patients (74.3%) discontinued study treatment mainly due to disease progression, and 9 patients (25.7%) were still on treatment (Supplementary Figure 1).
Safety
Most of the patients (34/35) experienced treatment-related adverse events. The most common treatment-related AEs (TRAEs) were neutropenia (65.7%), thrombocytopenia (62.9%), anemia (60.0%), leukopenia (54.3%), aspartate aminotransferase increased (42.9%) and blood bilirubin increased (40.0%) (). Grade ≥3 TRAEs occurred in 25 patients (71.4%). The most frequent (>10%) grade ≥3 TRAEs were anemia (27.5%), neutropenia (20%), thrombocytopenia (17.1%), leukopenia (17.1%) and fatigue (17.1%). Serious TRAEs including anorexia (5.7%), thrombocytopenia (5.7%), fatigue (2.9%) and small intestinal obstruction (2.9) occurred in 6 (17.1%) patients, and recovered with appropriate supportive care. Immune-related TRAEs included fatigue (22.9%), proteinuria (20.0%), hypothyroidism (14.3%), rash (11.4), hyperthyroidism (11.4), diarrhea (8.6%), arthralgia (5.7%) and pruritus (2.9%), most of which were grade 1 or 2 (). Immune-related treatment emergent AEs are listed in Supplementary Table 2.
Table 2. TRAEs of any grade occurring in ≥10% of patients
Table 3. Immune-related TRAEs
TRAEs leading to discontinuation of the protocol treatment including anorexia (2.9%), thrombocytopenia (2.9%), palmar-plantar erythrodysesthesia syndrome (2.9%), and fatigue (2.9) occurred in 4 (11.4%) patients, three of which were caused by SAEs as anorexia, thrombocytopenia and fatigue, respectively. Nearly half of patients had TRAEs leading to reduced or delayed dosing of chemotherapy and/or HX008, the most frequent (>5%) TRAEs were thrombocytopenia (17.1%), vomiting (14.3%), fatigue (11.4%), leukopenia (8.6%), nausea (8.6%), anemia (5.7%), abdominal pain (5.7%) and palmar-plantar erythrodysesthesia syndrome (5.7%). The most frequent (>5%) Grade 3 TRAEs leading to dose delay or reduction were fatigue (8.6%), thrombocytopenia (5.7%), anemia (5.7%) and palmar-plantar erythrodysesthesia syndrome (5.7%).
There was one (2.9%) patient who experienced treatment-related fatal AEs, that died from thrombocytopenia leading to upper gastrointestinal hemorrhage which was considered affirmably related to oxaliplatin and capecitabine, and unlikely related to HX008.
Efficacy
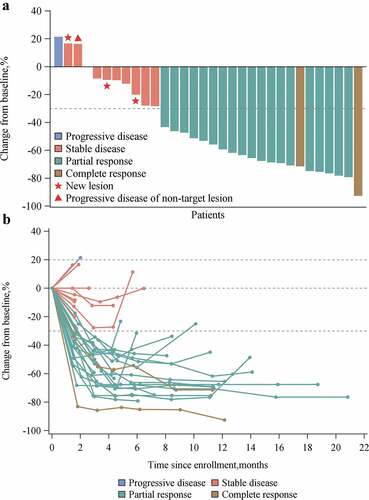
Thirty-two of 35 patients were evaluable by RECIST v1.1 criteria. Tumor evaluations by site investigators are listed in . ORR was 60.0% (95% CI 42.1–76.1), with complete response (CR) in 1 patients and partial response in 20 patients. DCR was 77.1% (95% CI 59.9–89.6). ORR and DCR in evaluable patients were 65.6% (95% CI 46.8–81.4) and 84.4% (95% CI 67.2–94.7), respectively. Most patients (28/32) with measurable disease at baseline and ≥1 evaluable postbaseline assessment experienced a reduction in target lesion size and maintained over several assessments (). Notably, tumors of two patients shrunk to be operable and received radical surgery after combination treatment. At data cutoff, nine patients remained on treatment with ongoing responses.
Table 4. Summary of response and survival data (FAS population)
Figure 1. Overall tumor responses of HX008 with oxaliplatin plus capecitabine as assessed by site investigators in patients with ≥ 1 assessable postbaseline image assessment (N = 32). (A) Best change from baseline in the size of target tumor lesion. Color code defines the best of response of target tumor lesion. (B) Percent change in the size of target tumor lesion from baseline in each patient
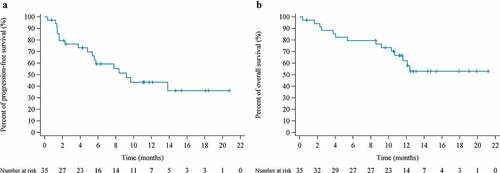
Median time to response (TTR) was 1.4 months (range 1.3–2.9). Median duration of response was 12.3 months (range 1.4–17.9+) (Supplementary figure 2). Median PFS was 9.2 months (95% CI 5.4-NR), and the 6-month PFS rate was 59.3% (95% CI 40.1–74.1). Median OS was NR (95% CI 10.7-NR), and the 12-month OS rate was 62.2% (95% CI 42.6–76.8) ().
In patients with PD-L1-positive tumors, ORR and DCR were 75% (9/12) and 83.3% (10/12), respectively, whereas in patients with PD-L1-negative tumors, ORR and DCR were 66.7% (6/9) and 100% (9/9), respectively. Nevertheless, no PFS difference (P = .19) was observed in PD-L1-positive and PD-L1-negative patients (Supplementary figure 3). Durable partial response was confirmed in two dMMR patients, both were still on treatment at the last follow-up of 12.7 and 19.9 months, respectively.
Discussion
In this single-arm, phase Ib study, HX008 combined with oxaliplatin plus capecitabine demonstrated a manageable safety profile and durable antitumor efficacy as first-line treatment in Chinese patients with advanced G/GEJ adenocarcinoma.
The incidences and severity of TRAEs with HX008 plus chemotherapy were generally consistent with those of known side effects of oxaliplatin plus capecitabineCitation29,Citation30 and anti-PD-1 antibody in combination with oxaliplatin plus capecitabine.Citation26 Most AEs were grade 1/2. Hematotoxicity, such as neutropenia and thrombocytopenia, was some of the most frequently reported, which are expected AEs associated with oxaliplatin and/or capecitabine. However, the incidences of any grade and grade ≥3 anemia and leukopenia were somewhat higher than those in ATTRACTION-4 study. Although consistent with the reported AEs of oxaliplatin or capecitabine,Citation31,Citation32 the severity might be enhanced by HX008. Furthermore, the incidences of diarrhea and nausea were relatively lower than those in ATTRACTION-4 study. Immune-related toxicities were comparable to reports with anti-PD-1 monotherapy and parallel combination therapies in similar patient populations.Citation25,Citation26,Citation33 The addition of HX008 to chemotherapy was well tolerated and did not significantly aggregate the side effect of patients with advanced G/GEJ cancer. Treatment discontinuation due to TRAEs occurred in 14.3% patients, due to fatigue (5.7%), anorexia (2.9%), thrombocytopenia (2.9%) and palmar-plantar erythrodysesthesia syndrome (2.9%), respectively.
Efficacy results of this study were generally consistent with that of ATTRACTION-4 study and KEYNOTE-059 cohort 2, which suggest that HX008 plus chemotherapy showed preliminary promising anti-tumor efficacy. In ATTRACTION-4 study, ORR evaluated by central assessment was 65.8%, median PFS was 9.7 months (95% CI 6.8–12.5), and median OS was not reached with in a median follow-up time of 13.2 months. In KEYNOTE-059 cohort 2, ORR was 60.0%, median PFS was 6.6 months (95% CI 5.9–10.6), and median OS was 13.8 months (95% CI 8.6-NR). However, in the phase III KEYNOTE-062 study, median PFS of pembrolizumab plus chemotherapy in CPS ≥ 1 patients was 6.9 months (95% CI 5.7–7.3), and median OS was 12.3 months (95% CI 9.5–14.8), which demonstrated to be noninferior to chemotherapy alone. There might be several reasons that could partially explain the different therapeutic efficacy observed in studies on advanced G/GEJ cancer. Oxaliplatin was used in our study and ATTRACTION-4 study, while cisplatin was used in KEYNOTE-059 and KEYNOTE-062 trials. It has been reported that oxaliplatin-based chemotherapy might be more efficacious and more tolerant than cisplatin-based chemotherapy in patients with advanced G/GEJ cancer.Citation34 Compared with cisplatin plus S-1, oxaliplatin plus S-1 presented significantly improved PFS (5.7 vs 4.9 months) and OS (13.0 vs 11.8 months). Indeed, compared with oxaliplatin, cisplatin possesses less activity by turning “cold” into “hot” tumors, due to its inability to trigger translocation of calreticulin to the outer leaflet of the plasma membrane of dying cells.Citation35 Furthermore, the cycle of chemotherapy administrated varied among studies, oxaliplatin was limited for up to six cycles, while capecitabine was used until progressive decrease or intolerable toxicity in our study. Lymphopenia and neutropenia, caused by long-term chemotherapy intervention especially platinum might interfere with the mechanism of the effect of anti-PD-1 antibodies by impairing clonal expansion of effector lymphocytes. On the other hand, anti-tumor efficacy of the same therapy may vary among distinct molecular subtypes. The Cancer Genome Atlas proposed molecular classification of patients with GC into four subtypes: Epstein-Barr virus (EBV), chromosomal instability (CIN), microsatellite instable (MSI) and genomically stable (GS),Citation36 while CIN and MSI subgroups had better overall survival than GS, but worse than EBV subtypes.Citation37 MSI and EBV subgroups tend to be more common in Asia than in non-Asia patients,Citation38 which has been associated with a superior response to ICIs.Citation39,Citation40 Intriguingly, immunity signature analysis between Asian and non-Asian gastric adenocarcinomas supposed an enrichment of tumor-infiltrating T-cells in non-Asian patients.Citation41 Whereas better clinical efficacy of ICIs combined with chemotherapy was affirmed in Asian patients with advanced G/GEJ cancer, which manifests the need of further mechanism development.
Although studies in several types of carcinoma have demonstrated that PD-L1 expression can be a reliable biomarker for the prediction of anti-tumor efficacy, and pembrolizumab has been approved for third-line treatment of PD-L1 positive (combined positive score ≥1) advanced G/GEJ cancer. However, no apparent association between efficacy and PD-L1 expression was determined in our exploratory analysis, which is generally unanimous with results of ATTRACTION-4 and KEYNOTE-062 studies. This result implied that PD-L1 expression might not be a robust predictive factor for anti-PD-1 antibodies combined with chemotherapy in patients with advanced G/GEJ cancer.
There are several limitations to the study. It was a single-arm study without a standard of care comparator arm, results interpreting and comparisons across trial must be cautious. The ORR was assessed by the investigators, rather than by an independent reviewer, systematic bias could be found among different investigators. The sample size was relatively small and biomarker analysis was not feasible for all patients, which made it difficult to correlate each biomarker with clinical efficacy.
In conclusion, HX008 in combination with oxaliplatin and capecitabine demonstrated an acceptable safety profile and promising anti-tumor activity as first-line treatment in Chinese patients with advanced G/GEJ cancer. Additional large-scale clinical trials are needed to further confirm the efficacy and safety of the combination treatment.
Disclosure of potential conflicts of interest
XF. Wang, Q. Jiang and YW. Dou are employees of Taizhou Hanzhong Biomedical Co., Ltd. All remaining authors declare that they have no conflict of interest.
Author contributions
Study design and conception: Jianming Xu, Nong Xu, Yuxian Bai. Clinical investigators: Jianming Xu, Nong Xu, Yuxian Bai, Rongrui Liu, Chenyu Mao, Hong Sui. Data analysis and interpretation: Jianming Xu, Xiaofei Wang, Qian Jiang, Yiwei Dou. Jianming Xu and Xiaofei Wang drafted the initial version of this manuscript. All authors participated in review and revision of the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download ()Acknowledgments
We would like to thank the patients who participated in the trial and their families. We would also like to thank the physicians, nurses, research coordinators, and other staff at each site who assisted with the study.
Supplementary materials
Supplemental data for this article can be accessed Publisher’swebsite.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–8. doi:10.3322/caac.21492.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338.
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi:10.1016/S1470-2045(08)70035-4.
- Wang J, Xu R, Li J, Bai Y, Liu T, Jiao S, Dai G, Xu J, Liu Y, Fan N, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19(1):234–244. doi:10.1007/s10120-015-0457-4.
- Lu Z, Zhang X, Liu W, Liu T, Hu B, Li W, Fan Q, Xu J, Xu N, Bai Y, et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer. 2018;21(5):782–791. doi:10.1007/s10120-018-0809-y.
- Bang Y-J, Van Cutsem E, Feyereislova A, HC C, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X.
- Hecht JR, Bang Y-J, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–A randomized phase III trial. J Clin Oncol. 2016;34(5):443–451. doi:10.1200/JCO.2015.62.6598.
- Ohtsu A, Shah MA, Van Cutsem E, SY R, Sawaki A, SR P, HY L, Yamada Y, Wu J, Langer B, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. doi:10.1200/JCO.2011.36.2236.
- Catenacci DVT, Tebbutt NC, Davidenko I, AM M, SE A-B, DH I, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017 Nov;18(11):1467–1482. doi:10.1016/S1470-2045(17)30566-1.
- Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran S-E, Van Cutsem E, Ilson DH, Alsina M, Chau I, Lacy J, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–435. doi:10.1016/S1470-2045(18)30791-5.
- Wu C, Zhu Y, Jiang J, Zhao J, Zhang X-G, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi:10.1016/j.acthis.2006.01.003.
- Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96(3):284–291. doi:10.1016/j.yexmp.2014.03.005.
- Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J, Wu C. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol. 2015;20(2):273–281. doi:10.1007/s10147-014-0701-7.
- Kelly RJ. Immunotherapy for esophageal and gastric cancer. Am Soc Clin Oncol Educ Book. 2017;37:292–300. doi:10.14694/EDBK_175231.
- Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/S0140-6736(17)31827-5.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi:10.1001/jamaoncol.2018.0013.
- Hato SV, Khong A, de Vries IJM, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 2014;20(11):2831–2837. doi:10.1158/1078-0432.CCR-13-3141.
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi:10.1016/j.immuni.2013.06.014.
- Kon E, Benhar I. Immune checkpoint inhibitor combinations: current efforts and important aspects for success. Drug Resist Updat. 2019;45:13–29. doi:10.1016/j.drup.2019.07.004.
- Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018 Dec 6;379(23):2220–2229. doi:10.1056/NEJMoa1809064.
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865.
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, CA T, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948.
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland Å, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi:10.1016/S0140-6736(19)32591-7.
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi:10.1056/NEJMoa1910549.
- Bang Y-J, Kang Y-K, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828–837. doi:10.1007/s10120-018-00909-5.
- Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee K-W, Cho H, Kang WK, Komatsu Y, Tsuda M, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30(2):250–258. doi:10.1093/annonc/mdy540.
- Tabernero J, Cutsem EV, Bang Y-J, CS F, Wyrwicz L, KW L, Kudaba I, Garrido M, HC C, Castro Salguero HR, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(18_suppl):LBA4007–LBA4007.
- Zhang J, Huang Y, Xi G, Zhang F. HX008: a humanized PD-1 blocking antibody with potent antitumor activity and superior pharmacologic properties. MAbs. 2020;12(1):1724751. doi:10.1080/19420862.2020.1724751.
- Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20(4):666–673. doi:10.1093/annonc/mdn717.
- Kim GM, Jeung H-C, Rha SY, Kim HS, Jung I, Nam BH, Lee KH, Chung HC. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48(4):518–526. doi:10.1016/j.ejca.2011.12.017.
- Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, Takeda Y, Moriwaki T, Amagai K, Sekikawa T, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer. 2014 May;50(8):1437–1445. doi:10.1016/j.ejca.2014.01.020.
- Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, Yasui H, Kim T-Y, Yamaguchi K, Fuse N, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer. 2015 Oct;18(4):824–832. doi:10.1007/s10120-014-0420-9.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi:10.1038/s41571-019-0218-0.
- Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, et al. Anti-PD-1 antibody shr-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019 Jan 15;25(2):515–523. doi:10.1158/1078-0432.CCR-18-2484.
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97-111. doi:10.1038/nri.2016.107.
- Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27(5):763–769. doi:10.1093/annonc/mdw040.
- Park C, Cho J, Lee J, Kang SY, An JY, Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, et al. Host immune response index in gastric cancer identified by comprehensive analyses of tumor immunity. Oncoimmunology. 2017;6(11):e1356150. doi:10.1080/2162402X.2017.1356150.
- Liao P, Jia F, Teer JK, Knepper TC, Zhou H-H, He Y-J, McLeod HL. Geographic variation in molecular subtype for gastric adenocarcinoma. Gut. 2019;68(7):1340–1341. doi:10.1136/gutjnl-2018-316605.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015 Jun 25;372(26):2509–2520. doi:10.1056/NEJMoa1500596.
- Kim ST, Cristescu R, Bass AJ, Kim K-M, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018 Sep;24(9):1449–1458. doi:10.1038/s41591-018-0101-z.
- Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64(11):1721–1731. doi:10.1136/gutjnl-2014-308252.