ABSTRACT
As an adverse survival prognosticator, chemokine (C-X-C motif) ligand 13 (CXCL13) has been studied in several types of malignancies. The secretion and physiological roles of CXCL13 in follicular helper T cells (TFH) cells have been well described, while the clinical significance of CD8+ tumor-infiltrating lymphocytes (TILs)-associated CXCL13 remains unknown. This study aims to investigate the clinical significance of CXCL13+CD8+ T cells in survival and chemotherapeutic responsiveness prediction in gastric cancer. In this study, 440 patients enrolled from Zhongshan Hospital with tumor microarray (TMA) specimens were randomly divided into testing set (n = 220) and validation set (n = 220) for analysis. CXCL13+CD8+ T cells were detected by multicolor immunohistochemistry. Fresh tumor tissue samples from another 60 gastric cancer patients were collected to detect CXCL13+CD8+ T cells functional status by flow cytometry (FCM). We found that high intratumoral CXCL13+CD8+ T cells infiltration predicted poor overall survival and inferior chemotherapeutic responsiveness in gastric cancer. CXCL13+CD8+T cells were associated with immunoevasive contexture with increased regulatory T (Treg) cells and dysfunctional cytotoxic T lymphocytes (CTLs). Moreover, the combinational analysis of CXCL13+CD8+ T cells and CD8+ T cells infiltration stratified patients into distinct risk groups with different clinical outcomes and chemotherapeutic responsiveness. Conclusively, intratumoral CXCL13+CD8+ T cells infiltration could be an independent prognostic and predictive marker for gastric cancer patients. CXCL13+CD8+ T cells represented an exhausted CD8+ T cell subset, and might be a potential immunotherapeutic target in gastric cancer.
Introduction
Gastric cancer ranks the fifth most frequently diagnosed malignancy and the third leading cause of cancer-associated mortality in the world.Citation1,Citation2 Radical gastrectomy is the most effective treatment for gastric cancer patients.Citation3 Additionally, for patients with TNM stage II and III tumors, postoperative fluorouracil-based adjuvant chemotherapy (ACT) is a common first-line adjuvant therapy.Citation4 However, gastric cancer is a heterogeneous disease with distinct molecular phenotypes and immune contexture, which attenuates the effect of chemotherapeutic agents and makes it difficult to predict patient clinical outcomes and therapeutic responsiveness to ACT.Citation5,Citation6
In recent years, immune checkpoint inhibitors (ICIs), which aim at reactivating antitumor immune responses, have proven highly effective in a series of solid malignancies.Citation7 Unfortunately, the total response rate of gastric cancer patients to immunotherapy is less than 15%.Citation8,Citation9 Multiple factors may affect immunotherapeutic responsiveness, including interferon signaling,Citation10 programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) interaction,Citation11 and the degree of cytotoxic T lymphocyte (CTL) infiltration.Citation12
CD8+ cytotoxic T cells in response to tumor antigens undergo substantial functional and phenotypic alternations, including reduced secretion of effector chemokines and elevated expression of inhibitory receptors.Citation13 Inhibitory receptors such as PD-1 constitute critical immune checkpoints in T cell activation, and their expression represents a major mechanism by which T cell proliferation and function are regulated and limited. Blockade of PD-1 and other inhibitory receptors reinvigorates CD8+ T cell-mediated immunity and has revolutionized our approach to the treatment of cancers.Citation14 However, despite the exact clinical benefits of ICIs in many patients, the underlying molecular mechanisms that lead to T cell re-activation during immune checkpoints inhibition are not fully understood, and it remains to be elucidated why some patients respond yet others do not. Consequently, identification of immunotherapeutic responsiveness biomarkers and immune evasion regulators is of urgent need, and further classification of gastric cancer based on T cell functional phenotype might add more information to patient prognosis and decision-making for treatment strategies.
Chemokine (C-X-C motif) ligand 13 (CXCL13) is a chemokine predominantly involved in the recruitment of B cells to lymph nodes and in the formation of germinal centers.Citation15 As a tumor-associated chemokine, CXCL13 plays an important part in tumor proliferation and migration.Citation16 Meanwhile, CXCL13 has been demonstrated as an adverse survival prognosticator in various cancer types, including oral squamous cell carcinoma,Citation17 non-small cell lung cancer,Citation18 gastric cancer,Citation19 hepatocellular carcinoma,Citation20 prostate cancer,Citation21 advanced colorectal cancer,Citation22 lymphoma,Citation23 and neuroblastic tumor.Citation24 CXCL13 is principally produced by stromal cells resident in B cell follicles, in murine secondary lymphoid organs.Citation25,Citation26 However, recent studies have reported that CXCL13 can also be secreted by T cells, especially CD4+ follicular helper T (TFH) cellsCitation27–29 and CD8+ tumor-infiltrating lymphocytes (TILs).Citation30 The secretion and physiological roles of CXCL13 in TFH cells have been well described,Citation27,Citation31 while CD8+ TILs-associated CXCL13 still requires further investigation.Citation32 Interestingly, although intratumoral CXCL13 expression was an adverse survival prognosticator, CXCL13+ TFH cell density paradoxically indicated better survival,Citation27 indicating the function and the potential effect of CXCL13 on tumor immune microenvironment might be more complicated than we thought. In our previous study, we have demonstrated that CXCL13 could predict poor survival outcomes and therapeutic responsiveness in gastric cancer.Citation19 However, the source of CXCL13 and the underlying mechanism for CXCL13-associated adverse clinical outcomes remain unknown in gastric cancer, and require further investigation.
In the current study, we inspected the clinical significance and functional features of CXCL13+CD8+ T cells in gastric cancer. CXCL13+CD8+ T cells represented a dysfunctional CD8+ T cell subset, and were associated with immunoevasive tumor microenvironment, indicating that CXCL13+CD8+ T cells might be a potential immunotherapy target in gastric cancer.
Materials and methods
Patients and gastric tissue samples
The study was primarily based on two independent patient cohorts. Cohort 1 includes 496 patients from Zhongshan hospital, Fudan University. However, 56 patients were excluded due to data missing, dot loss or suffering from metastatic diseases. Consequently, we enrolled 440 patients in the current study. These patients received radical gastrectomy and standard D2 lymphadenectomy between August 2007 and December 2008. Four hundred and forty gastric tissue samples of Cohort 1 were formalin-fixed and paraffin-embedded (FFPE). Clinical data including sex, age, tumor size, tumor location, Lauren classification,Citation33 tumor grade, T classification, N classification, TNM stage, and application of fluorouracil-based ACT were retrospectively collected. Tumor stages were determined according to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM classification. The 440 gastric cancer patients of Cohort 1 were randomly divided into two independent patient sets (Testing set, n = 220; Validation set, n = 220). After surgery, routine fluorouracil-based chemotherapy was administered to the patients with TNM stage II and III tumors. As the endpoint of this study, the overall survival (OS) was counted from the date of gastrectomy to the date of death or the last follow-up. All patients were observed until April 2014. Cohort 2 enrolled additional 60 gastric cancer patients from the Zhongshan hospital, Fudan University. The patients of Cohort 2 underwent radical gastrectomy and standard D2 lymphadenectomy between August 2018 and November 2018. Fresh tissue samples of these patients were collected during surgery and performed with flow cytometry (FCM). Corresponding 60 FFPE tissue blocks were also retrospectively acquired and constructed as an independent tissue microarray (TMA). Detailed patients characteristics were presented (). Written informed consent was obtained from each patient from Zhongshan hospital, Fudan University, and the study was approved by the institutional review board and ethics committee of Zhongshan hospital, Fudan University.
Table 1. Association between CXCL13+CD8+ T cells infiltration and clinicopathological features in gastric cancer
Pathological analysis on gastric cancer subtypes
The Lauren classification, first addressed by P LAUREN in 1965, was an attempt to classify gastric cancer into two histological main types: diffuse-type and intestinal-type carcinoma.Citation33 In this study, the Lauren classification was reviewed by the two pathologists (Dr. Lingli Chen and Dr. Peipei Zhang). In 2015, the Asian Cancer Research Group (ACRG) established clinically relevant molecular subtypes (ACRG classification) that encompassed the heterogeneity and provided useful clinical information for gastric cancer. The ACRG used gene expression data to describe four molecular subtypes linked to distinct patterns of molecular alterations, disease progression, and prognosis in gastric cancer, which included microsatellite instability (MSI) subtype, microsatellite stable/epithelial-to-mesenchymal transition (MSS/EMT) subtype, microsatellite stable/tumor protein 53 (TP53)-active (MSS/TP53+) subtype and microsatellite stable/TP53-inactive (MSS/TP53−) subtype.Citation34 In this study, we used the ACRG Gastric cohort GSE62254 downloaded from NCBI Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/). The ACRG classification of gastric cancer was defined in GSE62254. In GSE62254, the CXCL13+CD8+ T cell signature comprised CD2, CD3E, CD5, CD7, CD8A, CD8B, CD27, CD28, HIF-1α, TGF-β1, TGF-β2, TGF-β3, CTGF, SMAD2, SMAD3, CXCL13 and CXCR5.Citation35,Citation36 Signature score was defined as the mean of normalized expression of related genes [log2 (FPKM +1)]. Gene signature was listed in Supplementary Table S1.
Immunohistochemistry
The TMAs of cohort 1 and cohort 2 were constructed as previously described.Citation37 Double-staining immunohistochemistry was performed and each entire set of TMAs was stained at the same time to ensure an objective comparison between different samples. The slides were baked at 60°C for 6 hours, deparaffinized in xylene for three times, 15 minutes each time and rehydrated in graded alcohol. Then, they were heated in sodium citrate buffer (0.01 M sodium citrate buffer, pH = 6) for antigen retrieval and blocked with 3% H2O2 in methanol at 37°C for 30 minutes. Next, 10% normal goat serum was incubated at 37°C to eliminate nonspecific reactions and then applied with anti-CD8A antibody (Abcam, ab199016, diluted at 1:500, Supplementary Table S2) for 2 hours at 37°C. Subsequently, anti-CXCL13 antibody (ThermoFisher, PA5-28827, diluted at 1:1000, Supplementary Table S2) was applied to incubate the slides overnight at 4°C. Next day, TMA slides were washed, incubated with HRP/Mouse and AP/Rabbit secondary antibody with 3,30-diaminobenzidine (DAB) and Vector Blue to visualize the reaction products, respectively, and mounted with aqueous mounting media. In our study, two pathologists (Dr. Peipei Zhang and Dr. Lingli Chen) who were blinded to the clinicopathological data scored all samples separately. The IHC staining sections were scanned at high magnification (×200) and captured by NIS-Elements F3.2 to identify the three independent microscopic fields with the densest infiltration of immune cells to ensure representativeness and homogeneity. Identical settings were used for each photograph. The mean count of their evaluation was adopted. Variations in the enumeration, exceeding 5 cells, were reevaluated separately by both pathologists to acquire consensus.
Flow cytometry
Fresh gastric cancer tissues were collected as soon as the tumors were resected during surgery. Single cells were isolated with the use of collagenase IV and then stained with appropriate monoclonal antibodies (mAbs) for 30 min at 4°C. If necessary, staining of intracellular molecules was performed with Fixation/Permeabilization Solution Kit (BD Biosciences) according to the manufacturer’s instructions. Stained cells were washed and resuspended in phosphate-buffered saline/0.1% bovine serum albumin coupled with azide. Subsequently, flow cytometry was performed with FACSCelesta flow cytometer (BD Biosciences) and analyzed by FlowJo software (Tree Star). Antibodies utilized in the current study were listed (Supplementary Table S2).
Statistical analysis
In Cohort 1, we chose the median value 4 cells/HPF (at ×200 magnification) as the cutoff value. The tumors infiltrated with CXCL13+CD8+ T cells <4 cells/HPF were defined as CXCL13+CD8+ T low subgroup, while the tumors infiltrated with CXCL13+CD8+ T cells ≥4 cells/HPF were defined as CXCL13+CD8+ T high subgroup. As to Cohort 2, the cutoff value was also the median value. Continuous variables were analyzed with the use of Student’s t test or Mann–Whitney U-test. The Kruskal-Wallis test followed by Dunn’s multiple comparisons test were performed to compare CXCL13+CD8+ T cell signature within distinct ACRG molecular subtypes. The relationship between CXCL13+CD8+ T cell density and patient characteristics was evaluated by chi-squared test or Fisher’s exact test. Survival outcomes were analyzed through Kaplan–Meier curves, log-rank test, and multivariate analysis based on Cox proportional hazards method. All statistical analyses were 2-sided and P-value of < 0.05 was considered as statistically significant. Illustration and statistical analyses were conducted by GraphPad Prism (Version 8.00), Medcalc (Version 12.7.0), IBM SPSS Statistics (Version 25.0) or R software (Version 3.6.1).
Results
Intratumoral CXCL13+CD8+T cells predict poor prognosis in gastric cancer
In this study, we enrolled two independent gastric cancer patient cohorts (). Immunohistochemistry (IHC) was performed (. a and Supplementary Figure S1. A). Compared with peritumor tissues, intratumor tissues showed significantly higher infiltration of CXCL13+CD8+ T cells (P< .001; Supplementary Figure S1. B). Consequently, we were predominantly focused on intratumoral CXCL13+CD8+ T cells in our following study.
Figure 1. Intratumoral CXCL13+CD8+T cells predict poor prognosis in gastric cancer. (a) Immunohistochemical double-staining of CD8 (brown) and CXCL13 (blue) in gastric cancer tissues. Black arrowhead showed CXCL13+CD8+ T cells (left panel). Immunohistochemistry (IHC) staining for CXCL13 (median panel), CD8 (right panel) and corresponding negative control were also shown. Neg ctrl refers to negative control. (b) Association between CXCL13+CD8+ T cell signature and different ACRG classifications. Kruskal-Wallis test followed by Dunn’s multiple comparisons test, *P< .05, **P< .01, ***P < .001, ns refers to not significant. (c) Association between the number of CXCL13+CD8+ T cells and patient survival outcomes. Mann-Whitney U test, *P< .05, **P< .01, ***P < .001, ns refers to not significant. (d-e) Kaplan-Meier curves (d) for overall survival (OS) according to the number of intratumor CXCL13+CD8+ T cells and multivariate analysis (e) based on clinicopathological characteristics in Testing set (n = 220) and Validation set (n = 220), respectively. The OS was compared between CXCL13+CD8+ T high and CXCL13+CD8+ T low subgroups. Log-rank test was performed for Kaplan-Meier curves. HR refers to hazard ratio, CI refers to confidence interval
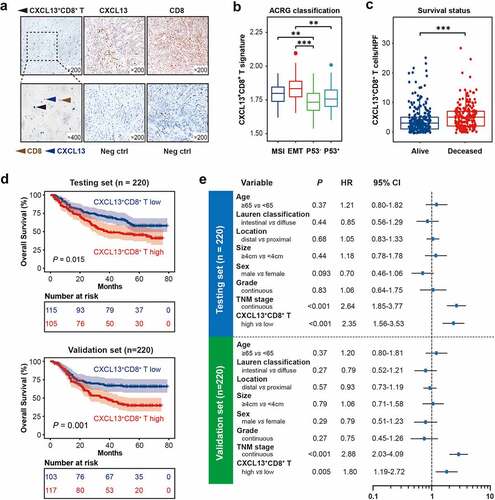
Gastric cancer is a heterogeneous disease. In 2015, the Asian Cancer Research Group (ACRG) established clinically relevant molecular subtypes that encompassed this heterogeneity and provided useful clinical information. The ACRG gastric cancer molecular subtypes included MSI subtype, MSS/EMT subtype, MSS/TP53+ subtype and MSS/TP53− subtype.Citation34 In this study, we inspected the infiltration of CXCL13+CD8+ T cells in 4 ACRG gastric cancer subtypes, respectively. However, no certain type displayed significant accumulation of CXCL13+CD8+ T cells (. b). Therefore, we assumed that CXCL13+CD8+ T cells might indicate a novel subtype of gastric cancer. The association between CXCL13+CD8+ T cells infiltration and clinicopathological parameters was also inspected (). Altogether, these data underline that there exists an abnormally increased infiltration of CXCL13+CD8+T cells in tumor microenvironment, which might indicate a novel subtype of gastric cancer.
To further investigate the clinical significance of intratumoral CXCL13+CD8+ T cells in gastric cancer, we inspected the association between CXCL13+CD8+ T cell infiltration and survival status. Notably, we found that the patients who had dismal survival outcomes tended to have significantly more CXCL13+CD8+ T cells infiltrating into the tumor site (. c). Furthermore, we applied Kaplan–Meier curves and log-rank test to compare the overall survival (OS) between CXCL13+CD8+ T high and CXCL13+CD8+ T low subgroups. In both Testing set and Validation set, higher infiltration of CXCL13+CD8+ T cells predicted significantly poorer OS (. d). Multivariate Cox regression analysis indicated that CXCL13+CD8+ T cells could serve as a potential independent prognostic factor for survival outcomes in both Testing set and Validation set (. e). Collectively, these results indicate that the infiltration of CXCL13+CD8+ T cells could be an independent adverse prognosticator in gastric cancer.
Intratumoral CXCL13+CD8+T cells indicate inferior responsiveness to fluorouracil-based adjuvant chemotherapy in gastric cancer
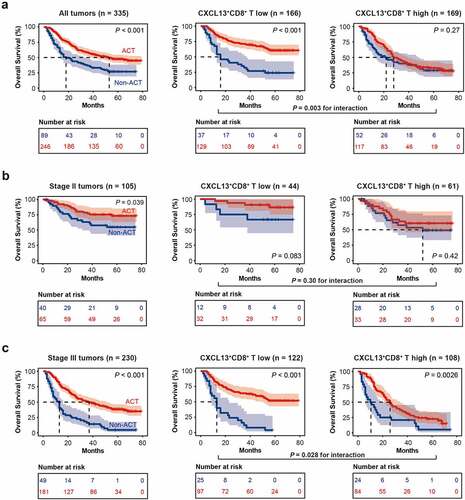
Subsequently, to discover whether CXCL13+CD8+ T cells could contribute to the chemoresistance to fluorouracil, we investigated the association between CXCL13+CD8+ T cell infiltration and responsiveness to adjuvant chemotherapy, based on the overall survival outcomes of the patients with TNM stage II/III tumors. Notably, the CXCL13+CD8+ T cell high subgroup conferred significantly inferior responsiveness to fluorouracil-based ACT, regarding OS (P= .003 for interaction, . a). The same result was also observed when we further stratified patients according to TNM stage. In TNM stage III tumors, CXCL13+CD8+ T cell high subgroup also had inferior responsiveness to fluorouracil-based ACT (P= .028 for interaction, . c). In TNM stage II tumors, although no significant results was observed according to interaction test, there was a trend toward better OS in CXCL13+CD8+ T cell low subgroup (. b). And we assumed that the negative results of interaction test in TNM stage II tumors might result from the relatively small cohort of stage II patients (n = 105). Conclusively, these results suggest that intratumoral CXCL13+CD8+ T cells could potentially indicate inferior therapeutic responsiveness to fluorouracil-based ACT in gastric cancer.
Figure 2. Intratumoral CXCL13+CD8+T cells indicate inferior responsiveness to fluorouracil-based adjuvant chemotherapy in gastric cancer. (a) Kaplan-Meier curves for TNM stage II/III patients with or without adjuvant chemotherapy (ACT) treatment in CXCL13+CD8+ T high/low subgroups. (b) Kaplan-Meier curves for TNM stage II patients with or without ACT treatment in CXCL13+CD8+ T high/low subgroups. (c) Kaplan-Meier curves for TNM stage III patients with or without ACT treatment in CXCL13+CD8+ T high/low subgroups. Log-rank test was performed for Kaplan-Meier curves
CXCL13+CD8+T cells potentially indicate a dysfunctional T cell phenotype in gastric cancer
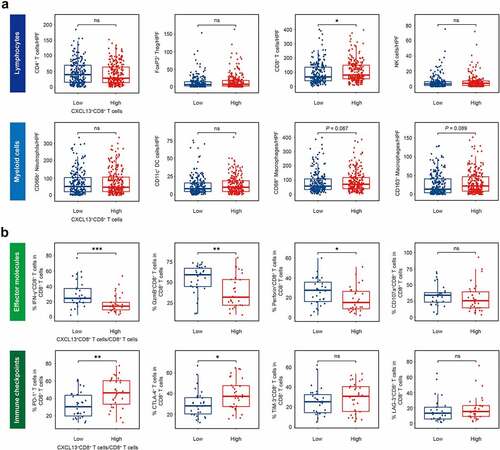
Since we have highlighted the clinical significance of CXCL13+CD8+ T cells, and found CXCL13+CD8+ T cells could predict poor OS and inferior chemotherapeutic responsiveness in gastric cancer ( and ), we wondered whether this subgroup of CD8+ T cells were associated with immunoevasive tumor microenvironment. To validate this hypothesis, we conducted IHC staining on several significant tumor-infiltrating immune cells, including CD4+ T cells, forkhead box P3 positive (FoxP3+) regulatory T (Treg) cells, CD8+ T cells, CD66b+ neutrophils, CD56+ Natural Killer (NK) cells, CD68+ macrophages, CD163+ M2-polarized macrophages and CD11c+ dentritic cells (DC, . a and Supplementary Figure S2). Notably, only CD8+ T cells showed elevated infiltration in CXCL13+CD8+ T high subgroup (. a). We assumed if CXCL13+CD8+ T cells could potentially represent a subset of CD8+ T cells featured by a certain functional phenotype. Interestingly, we observed that a high ratio of CXCL13+CD8+ T cells/CD8+ T cells indicated decreased expression of effector molecules interferon-γ (IFN-γ), granzyme B (GzmB) and perforin, yet elevated expression of immune checkpoints, including PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) within CD8+ T cells (. b and Supplementary Figure S3). Conclusively, these data suggest that CXCL13+CD8+ T cells potentially indicate a dysfunctional T cell phenotype in gastric cancer.
Figure 3. Intratumoral CXCL13+CD8+T cells are associated with a dysfunctional T-cell phenotype in gastric cancer. (a) Association between the intratumoral infiltration of CXCL13+CD8+ T cells and significant immune cells, including CD4+ T cells, FoxP3+ Treg cells, CD8+ T cells, CD66b+ neutrophil cells, CD56+ NK cells, CD68+ macrophages, CD163+ macrophages and CD11c+ DC cells. Unpaired t test (CD4, CD8, CD66b, CD56, CD68, CD163, CD11c), Mann-Whitney U test (FoxP3), *P< .05, **P< .01, ***P < .001, ns refers to not significant. (b) Flow cytometry to detect the expression of effector/activated molecules (IFN-γ, granzyme B, perforin, CD107a) and immune checkpoints (PD-1, CTLA-4, TIM-3, LAG-3) on CD8+ T cells in CXCL13+CD8+ T cells/CD8+ T cells high or low subgroups. Mann-Whitney U test, *P< .05, **P< .01, ***P < .001, ns refers to not significant
Stratification of patients based on CXCL13+CD8+T cells and CD8+T cells infiltration predicts prognosis and chemotherapeutic responsiveness in gastric cancer
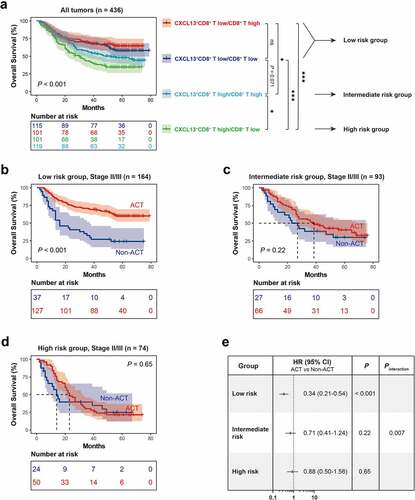
Since CXCL13+CD8+ T cells potentially indicated a dysfunctional T cell phenotype in gastric cancer, we combined CXCL13+CD8+ T cells and CD8+ T cells infiltration and assumed if we could provide a new stratification system to predict patient survival and therapeutic responsiveness more precisely. The cutoff value for CD8+ T high/low subgroup was also the median value. Four patients were excluded due to the dot loss after IHC staining for CD8. Interestingly, we found that CD8+ T cells could only stratify patient survival outcome in CXCL13+CD8+ T high subgroup, and CXCL13+CD8+ T low subgroup experienced better OS than CXCL13+CD8+ T high/CD8+ T high subgroup or CXCL13+CD8+ T high/CD8+ T low subgroup (. a), we trichotomized the patients into three risk subgroups, defined as low-risk group (CXCL13+CD8+ T low), intermediate risk group (CXCL13+CD8+ T high/CD8+ T high), and high-risk group (CXCL13+CD8+ T high/CD8+ T low). Consistent with our hypothesis, low-risk group showed superior responsiveness to fluorouracil-based ACT (P = .007 for interaction, . b-e). Consequently, these results indicate that the combination of CXCL13+CD8+ T cells and CD8+ T cells could be applied to stratify patients into various risk subgroups, thus predicting distinct prognosis and responsiveness to fluorouracil-based chemotherapy. Conclusively, our risk classification model could be a reliable prognostic and predictive factor in gastric cancer.
Figure 4. Stratification of patients based on CXCL13+CD8+T cells and CD8+T cells infiltration predicts prognosis and chemotherapeutic responsiveness in gastric cancer. (a) CD8+ T cells could only stratify patient survival outcome in CXCL13+CD8+ T high subgroup, while CXCL13+CD8+ T low subgroup experienced better OS than CXCL13+CD8+ T high/CD8+ T high subgroup or CXCL13+CD8+ T high/CD8+ T low subgroup. The patients were further trichotomized into three risk subgroups, defined as low risk group (CXCL13+CD8+ T low), intermediate risk group (CXCL13+CD8+ T high/CD8+ T high), and high risk group (CXCL13+CD8+ T high/CD8+ T low). (b-e) Low risk group showed superior responsiveness to fluorouracil-based ACT (P = .007 for interaction). HR refers to hazard ratio, CI refers to confidence interval, ACT refers to adjuvant chemotherapy
Discussion
It is generally believed that CD8+ T cells act as a positive prognosticator in most cancer types, as they could attack cancer cells directly.Citation38 However, emerging studies have reported that several certain subtypes of intratumoral CD8+ T cells are associated with poor clinical outcomes,Citation39 which attributes to T cell dysfunction.Citation32 Previous studies, which investigate T cells in human melanoma and hepatocellular carcinoma, have identified CXCL13 as a significantly upregulated gene in dysfunctional CD8+ T cells.Citation28,Citation29,Citation40 Until now, few studies have investigated the mechanism of CXCL13 up-regulation in malignancies. Some studies indicated that CXCL13 expression might be regulated by phosphatidylinositol 3-kinase (PI3K)-AKT pathwayCitation41 or Wnt/β-Catenin signal pathway.Citation42 The up-regulated CXCL13 could interact with its specific receptor C-X-C motif chemokine receptor 5 (CXCR5), and form the CXCL13-CXCR5 axis, which plays an important role in tumor proliferation and migration.Citation16 Although previous studies reported that the expression of CXCL13 receptor CXCR5 was significantly up-regulated in gastric cancer,Citation43 and CXCR5 could be expressed on CD8+ T cell,Citation44 CD4+ TFH cellsCitation45 and myeloid-derived suppressor cells (MDSCs),Citation46 the clinical significance and the potential impact of CXCR5 on gastric cancer was still obscure, and would be further investigated in our following studies.
Immune checkpoint blockade (ICB) which reactivates tumor-specific T cells through targeting the PD-1/PD-L1 axis has emerged as a promising treatment strategy for various malignancies.Citation47 However, very few patients (13%) responded to ICB in gastric cancer,Citation8,Citation9 and efforts to improve ICB treatment efficacy were confounded by a lack of understanding of the underlying mechanisms of immune evasion.Citation48 Notably, prior studies have reported that not all PD-1+ cells may respond to anti-PD-1 treatment equally,Citation49,Citation50 and not all PD-1+ T cells indicated exhausted or dysfunctional T cells, since PD-1 expression might begin to rise once activated.Citation51 A prior study has described PD-1hi CD8+ T cells as a highly distinct cellular pool. Exhausted PD-1hi T cells were characterized by high levels of CXCL13 production, and CXCL13+PD-1hiCD8+ T cells could mediate the tolerance to immunotherapy.Citation30 Notably, three recent studies that investigated T cells in human malignancies have identified CXCL13 as one of the significantly up-regulated genes in highly exhausted TILs,Citation28,Citation29,Citation40 and CXCL13 mRNA expression was accompanied by constitutive protein secretion from CD8+ T cells,Citation27,Citation30 suggesting a possible involvement of these cells in the formation of immune evasion and resistance to ICB.
In this study, we described a subset of intratumoral CD8+ T cells which expresses CXCL13 in gastric cancer, and analyzed the prognostic and predictive value of CXCL13+CD8+ T cells in gastric cancer. We found that higher infiltration of CXCL13+CD8+ T cells could indicate poor prognosis and inferior therapeutic responsiveness to fluorouracil-based adjuvant chemotherapy in gastric cancer. Furthermore, we found that CXCL13+CD8+ T cells represented a dysfunctional phenotype of CD8+ T cells, with decreased IFN-γ, granzyme B, and perforin level yet elevated PD-1 and CTLA-4 expression. These results indicated that higher infiltration of CXCL13+CD8+ T cells might be correlated with an immunoevasive tumor microenvironment, which provided an explanation for the poor survival and inferior responsiveness to ACT in gastric cancer.
However, our study was retrospective and required a further validation to confirm our findings within the framework of larger and multi-centered clinical cases. Besides, the underlying mechanism that CXCL13+CD8+ T cells orchestrated immune evasion was poorly understood and needed further investigation and elucidation in the following studies. In conclusion, our study suggested that intratumoral CXCL13+CD8+ Tcells could be an independent prognosticator for poor OS and inferior responsiveness to ACT in gastric cancer. Moreover, CXCL13+CD8+ Tcells indicated adysfunctional phenotype of CD8+ Tcells, and could identify an immunoevasive subtype gastric cancer, indicating that CXCL13+CD8+ Tcells might be apotential immunotherapy target in gastric cancer.
Authors’ contributions
K. Jin, Y. Cao, Y. Gu and H. Fang for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; Y. Fei, J. Wang, X. Liu, K. Lv, X. He, C. Lin, H. Liu, H. Li and H. He for technical and material support; R. Li, H. Zhang and J. Xu for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Availability of data and material
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Xu upon reasonable request.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Consent for publication
All authors provide their consent for publication of the manuscript.
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent was obtained from each patient included and this study was performed in accordance with the Declaration of Helsinki.
Supplemental Material
Download ()Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27:1.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–11. doi:10.3322/caac.21492.
- Songun I, Putter H, Kranenbarg EM, Sasako M, Van De Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–449. doi:10.1016/S1470-2045(10)70070-X.
- Nishida T. Adjuvant therapy for gastric cancer after D2 gastrectomy. Lancet. 2012;379(9813):291–292. doi:10.1016/S0140-6736(11)61928-4.
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi:10.1038/nrc1074.
- Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350–1357. doi:10.1200/JCO.2015.63.7215.
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584.
- Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–2844. doi:10.1200/JCO.2017.76.6212.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal S, Shah M, Metges JP, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013.
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi:10.1172/JCI91190.
- Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14(11):655–668. doi:10.1038/nrclinonc.2017.88.
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi:10.1126/science.aad0095.
- Kallies A, Zehn D, Utzschneider DT. Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol. 2020;20(2):128–136. doi:10.1038/s41577-019-0223-7.
- Speiser DE, Utzschneider DT, Oberle SG, Münz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014;14(11):768–774. doi:10.1038/nri3740.
- Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104(7):1952–1960. doi:10.1182/blood-2004-03-1206.
- Biswas S, Sengupta S, Roy Chowdhury S, Jana S, Mandal G, Mandal PK, Saha N, Malhotra V, Gupta A, Kuprash DV, et al. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat. 2016;155(3):615–616. doi:10.1007/s10549-016-3713-3.
- Sambandam Y, Sundaram K, Liu A, Kirkwood KL, Ries WL, Reddy SV. CXCL13 activation of c-Myc induces RANK ligand expression in stromal/preosteoblast cells in the oral squamous cell carcinoma tumor-bone microenvironment. Oncogene. 2013;32(1):97–105. doi:10.1038/onc.2012.24.
- Singh R, Gupta P, Kloecker GH, Singh S, Lillard JW. Expression and clinical significance of CXCR5/CXCL13 in human nonsmall cell lung carcinoma. Int J Oncol. 2014;45(6):2232–2240. doi:10.3892/ijo.2014.2688.
- Wei Y, Lin C, Li H, Xu Z, Wang J, Li R, Liu H, Zhang H, He H, Xu J, et al. CXCL13 expression is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Cancer Immunol Immunother. 2018;67(2):261–269. doi:10.1007/s00262-017-2083-y.
- Duan Z, Gao J, Zhang L, Liang H, Huang X, Xu Q, Zhang Y, Shen T, Lu F. Phenotype and function of CXCR5+CD45RA-CD4+ T cells were altered in HBV-related hepatocellular carcinoma and elevated serum CXCL13 predicted better prognosis. Oncotarget. 2015;6(42):44239–44253. doi:10.18632/oncotarget.6235.
- Singh S, Singh R, Sharma PK, Singh UP, Rai SN, Chung LWK, Cooper CR, Novakovic KR, Grizzle WE, Lillard JW, et al. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett. 2009;283(1):29–35. doi:10.1016/j.canlet.2009.03.022.
- Qi XW, Xia SH, Yin Y, Jin L-F, Pu Y, Hua D, Wu H-R. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014;18:1916–1924.
- Kim SJ, Ryu KJ, Hong M, Ko YH, Kim WS. The serum CXCL13 level is associated with the glasgow prognostic score in extranodal NK/T-cell lymphoma patients. J Hematol Oncol. 2015;8:49. doi:10.1186/s13045-015-0142-4.
- Del Grosso F, Coco S, Scaruffi P, Stigliani S, Valdora F, Benelli R, Salvi S, Boccardo S, Truini M, Croce M, et al. Role of CXCL13-CXCR5 crosstalk between malignant neuroblastoma cells and Schwannian stromal cells in neuroblastic tumors. Mol Cancer Res. 2011;9(7):815–823. doi:10.1158/1541-7786.MCR-10-0367.
- Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181(9):6189–6200. doi:10.4049/jimmunol.181.9.6189.
- Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20(1):14–25. doi:10.1016/j.smim.2007.12.001.
- Gu-Trantien C, Migliori E, Buisseret L, De Wind A, Brohée S, Garaud S, Noël G, C.v. LD, Lodewyckx J-N, Naveaux C, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2(11):11. doi:10.1172/jci.insight.91487.
- Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi:10.1172/JCI46102.
- Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356. e16. doi:10.1016/j.cell.2017.05.035.
- Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004. doi:10.1038/s41591-018-0057-z.
- Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. doi:10.1146/annurev-immunol-031210-101400.
- Thommen DS, Schumacher TN, Cell T. Dysfunction in Cancer. Cancer Cell. 2018;33(4):547–562. doi:10.1016/j.ccell.2018.03.012.
- Lauren P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi:10.1111/apm.1965.64.1.31.
- Cristescu R, Lee J, Nebozhyn M, Kim K-M, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449. doi:10.1038/nm.3850.
- Ammirante M, Shalapour S, Kang Y, Jamieson CAM, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc National Acad Sci. 2014;111(41):14776–14781. doi:10.1073/pnas.1416498111.
- Dai S, Zeng H, Liu Z, Jin K, Jiang W, Wang Z, Lin Z, Xiong Y, Wang J, Chang Y, et al. Intratumoral CXCL13+CD8+ T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J ImmunoTher Cancer. 2021;9(2):e001823. doi:10.1136/jitc-2020-001823.
- Cao Y, Liu H, Li H, Lin C, Li R, Wu S, Zhang H, He H, Zhang W, Xu J. Association of O6-methylguanine-DNA methyltransferase protein expression with postoperative prognosis and adjuvant chemotherapeutic benefits among patients with stage II or III gastric cancer. JAMA Surg. 2017;152(11):e173120. doi:10.1001/jamasurg.2017.3120.
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi:10.1038/nrclinonc.2017.101.
- Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66(5):794–801. doi:10.1136/gutjnl-2015-310839.
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352(6282):189–196. doi:10.1126/science.aad0501.
- Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 2015;400(1–2):287–295. doi:10.1007/s11010-014-2285-y.
- Li C, Kang D, Sun X, Liu Y, Wang J, Gao P. The effect of C-X-C Motif Chemokine 13 on Hepatocellular Carcinoma Associates with Wnt Signaling. Biomed Res Int. 2015;2015:345413.
- Yu C, Zhang Y. Characterization of the prognostic values of CXCR family in gastric cancer. Cytokine. 2019;123:154785. doi:10.1016/j.cyto.2019.154785.
- Zhong C, Song Z, Li M. Gastric cancer patients display a distinctive population of IFNg+IL10+ double positive CD8 T cells, which persists longer during prolonged activation. Exp Cell Res. 2019;382(2):111487. doi:10.1016/j.yexcr.2019.06.032.
- Meng X, Yu X, Dong Q, Xu X, Li J, Xu Q, Ma J, Zhou C. Distribution of circulating follicular helper T cells and expression of interleukin-21 and chemokine C-X-C ligand 13 in gastric cancer. Oncol Lett. 2018;16(3):3917–3922. doi:10.3892/ol.2018.9112.
- Ding Y, Shen J, Zhang G, Chen X, Wu J, Chen W. CD40 controls CXCR5-induced recruitment of myeloid-derived suppressor cells to gastric cancer. Oncotarget. 2015;6(36):38901–38911. doi:10.18632/oncotarget.5644.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4–328rv4. doi:10.1126/scitranslmed.aad7118.
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723. doi:10.1016/j.cell.2017.01.017.
- Thommen DS, Schreiner J, Müller P, Herzig P, Roller A, Belousov A, Umana P, Pisa P, Klein C, Bacac M, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3(12):1344–1355. doi:10.1158/2326-6066.CIR-15-0097.
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc National Acad Sci. 2008;105(39):15016–15021. doi:10.1073/pnas.0801497105.
- Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–674. doi:10.1038/s41577-019-0221-9.
