ABSTRACT
Formyl peptide receptor 1 (FPR1) plays a key regulatory role in innate and adaptive immunity. Recently, we reported that the CC genotype of FPR1-E346A (rs867228, c. 1037 A > C) is an independent biomarker for patients with locally advanced rectal cancer (LARC) who received preoperative concurrent chemoradiotherapy (CCRT). Pharmacologic inhibition of FPR1 decreased the migration and infiltration of T lymphocytes into tumor microenvironment after CCRT.
KEYWORDS:
Concurrent chemoradiotherapy (CCRT) followed by total mesorectal excision (TME) surgery is the most effective treatment to improve local control, increase survival, and preserve sphincter function in patients with locally advanced rectal cancer (LARC). The CCRT regimen consists in pelvic radiotherapy (45–50.4 Gy in 5 weeks) and continuous infusion of 5-fluorouracil or oral capecitabine (fluorouracil-based) chemotherapy. Several pathologic characteristics such as tumor regression grade (TRG), lymphovascular invasion, and perineural invasion are considered prognostic factors for patients after CCRT treatment. In addition to these phenotypical pathologic characteristics, genetic and immunological biomarkers emerged as important prognostic factors for LARC patients in recent years.Citation1,Citation2
Radiation therapy is considered an ideal in situ tumor vaccination approach to elicit antitumor immunity. During treatment, the tumor microenvironment generates signals to empower crosstalk between innate and adaptive immunity and reinvigorates the immune system to recognize and kill tumor cells. As such, abscopal effects sometimes are found in non-irradiated tumors after radiation to the primary lesion.Citation3 This phenomenon occurs because the anti-tumor immune machinery is activated after CCRT not only within the irradiated lesions but also against distant metastasis. Several chemotherapeutic agents as well as radiotherapy are known to induce the immunogenic cell death (ICD) of cancer cells and promote the release of danger-associated molecular patterns (DAMPs) such as HMGB1, ATP, and ANXA1. These DAMPs interact with pattern recognition receptors (PRRs) on immune cells, in particular dendritic cells (DCs) and T lymphocytes, and initiate a series of events leading to antitumor immunity.Citation4,Citation5 Hence, CCRT not only direct destroys tumor cells but also enhances antitumor immunity by prompting ICD and releasing DAMPs to recruit immune cells to destroy residual tumor cells.Citation6 In other words, CCRT prompts immunosurveillance and immunoscavenging process to improve local control and increase survival of patients with LARC.
Formyl peptide receptor 1 FPR1 is a G-protein-coupled receptor (GPCR) mostly expressed in dendritic cell (DC) progenitor and other myeloid cells and plays several important roles in immune responses. For example, FPR1 mediates neutrophil activation and migration in innate immunity, DC positioning, and maturation in adaptive immunity and antitumor immunity.Citation7 Several single nucleotide polymorphisms (SNPs) were reported to influence the functions of FPR1. Among these SNPs, E346A (rs867228, c. 1037 A > C) is a loss-of-function SNP that affects the extreme C-terminus of FPR1, thus altering the interaction with Gi-proteins and causing defective signal transduction.Citation8,Citation9 The primary ligands for FPR1 are bacterial and mitochondrial N-formylated peptides which were actively released from dead and dying pathogens or host cells. In addition, FPR1 is the PRR for ANXA1, which is necessary for chemotherapy-induced antitumor immunity. Knockout or inhibition of FPR1 resulted in deficient interactions of DCs with dead cancer cells, leading to decreased T cell infiltration in tumor and attenuating the therapeutic efficacy of chemotherapy. FPR1-E346A was also reported to have a negative influence on the prognosis of breast patients treated with anthracycline-based chemotherapy, colorectal cancer patients treated with oxaliplatin-based chemotherapy.Citation7,Citation10
Intrigued by the critical role of FPR1 on antitumor immunity, we studied the clinical significance of FPR1-E346A genotype in LARC patients, as well as the effect of FPR1 on the therapeutic efficacy of CCRT.Citation11 Based on our data, we postulate that FPR1 reinvigorates antitumor immunity after CCRT treatment () (created with BioRender.com). Our clinical results revealed that 57% of LARC patients exhibited the CC genotype of FPR1‑E346A, which was associated with significantly worse survival outcome, poor pathologic TRG, and reduced CD8+ TILs after CCRT. Meanwhile, in a preclinical mouse model, we found that pharmacological inhibition of FPR1 dramatically reduced the therapeutic efficacy of CCRT via reducing the recruitment of cytotoxic and effector/memory T lymphocytes to the tumor bed. Therefore, it is likely that the functional influence of FPR1 may compromise the outcome CCRT treatment by influencing T lymphocyte migration and infiltration.
Figure 1. FPR1 genotype influences antitumor immunity during CCRT treatment in LARC patients. FPR1expresses on DCs and T lymphocytes and participates in DCs and T lymphocytes migration and infiltration and cytotoxic activity. Under CCRT treatment, the CC genotype of FPR1-E346A decreased the recruitment of effector and cytotoxic T lymphocytes within tumor microenvironment to lead to reduce tumor regression and the therapeutic efficacy of CCRT. (created with BioRender.com)
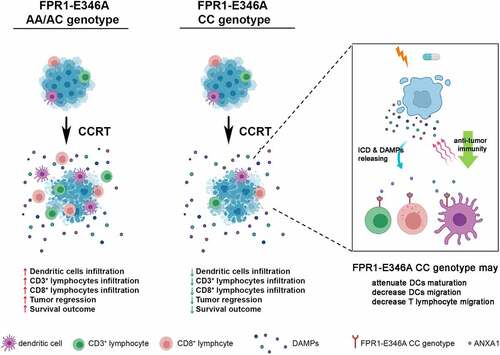
Of note, we discovered that FPR was not only expressed in DCs but also in T lymphocytes. Knockdown of FPR by siRNA or blocking FPR1 by a pharmacological antagonist (Boc-1) might inhibit the migration of either DCs or T lymphocytes toward irradiated tumor cells. However, when we cocultured T cells and DCs, only the migration ability of T lymphocytes toward irradiated tumor cells was reduced upon FPR1 blockade. Our data revealed that FPR1, in addition to participating in DCs maturation and position, might affect T lymphocyte infiltration and cytotoxic activity as well. These results may explain why the CC genotype type of FPR1-E346A correlates with less TILs, worse TRG, and poor survival outcome of CCRT-treated LARC patients (see ).
Taken together, the CC genotype type of FPR1-E346A is an independent prognostic predictor in LARC patients and, most importantly, more than half of LARC patients in our series carried CC genotype of FPR1-E346A. It will be worthwhile to validate our results in different ethnic cohorts and to explore methods to reverse the immune defect caused FPR1-E346A.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–3. doi:10.1200/JCO.2011.40.1836.
- Ryan EJ, Creavin B, Sheahan K. Delivery of personalized care for locally advanced rectal cancer: incorporating pathological, molecular genetic, and immunological biomarkers into the multimodal paradigm. Front Oncol. 2020;10:1369. doi:10.3389/fonc.2020.01369.
- Frey B, Rubner Y, Kulzer L, Werthmoller N, Weiss EM, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63(1):29–36. doi:10.1007/s00262-013-1474-y.
- Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35(46):5931–5941. doi:10.1038/onc.2016.104.
- Krombach J, Hennel R, Brix N, Orth M, Schoetz U, Ernst A, Schuster J, Zuchtriegel G, Reichel CA, Bierschenk S, et al. Priming anti-tumor immunity by radiotherapy: dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology. 2019;8(1):e1523097. doi:10.1080/2162402X.2018.1523097.
- Huang CY, Chiang SF, Ke TW, Chen TW, Lan YC, You YS, Shiau AC, Chen WT, Chao KSC. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1+ TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol Immunother. 2018;67(4):551–562. doi:10.1007/s00262-017-2109-5.
- Vacchelli E, Le Naour J, Kroemer G. The ambiguous role of FPR1 in immunity and inflammation. Oncoimmunology. 2020;9(1):1760061. doi:10.1080/2162402X.2020.1760061.
- Wenzel-Seifert K, Seifert R. Functional differences between human formyl peptide receptor isoforms 26, 98, and G6. Naunyn Schmiedebergs Arch Pharmacol. 2003;367(5):509–515. doi:10.1007/s00210-003-0714-7.
- Dorward DA, Lucas CD, Chapman GB, Haslet C, Dhaliwal K, Rossi AG. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol. 2015;185(5):1172–1184. doi:10.1016/j.ajpath.2015.01.020.
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–978. doi:10.1126/science.aad0779.
- Chiang SF, Huang KC, Chen WT, Chen TW, Ke TW, Chao KSC. Polymorphism of formyl peptide receptor 1 (FPR1) reduces the therapeutic efficiency and antitumor immunity after neoadjuvant chemoradiotherapy (CCRT) treatment in locally advanced rectal cancer. Cancer Immunol Immunother. 2021. doi:10.1007/s00262-021-02894-8.
